An On-Site Simultaneous Semi-Quantification of Aflatoxin B1, Zearalenone, and T-2 Toxin in Maize- and Cereal-Based Feed via Multicolor Immunochromatographic Assay
Abstract
:1. Introduction
2. Results and Discussion
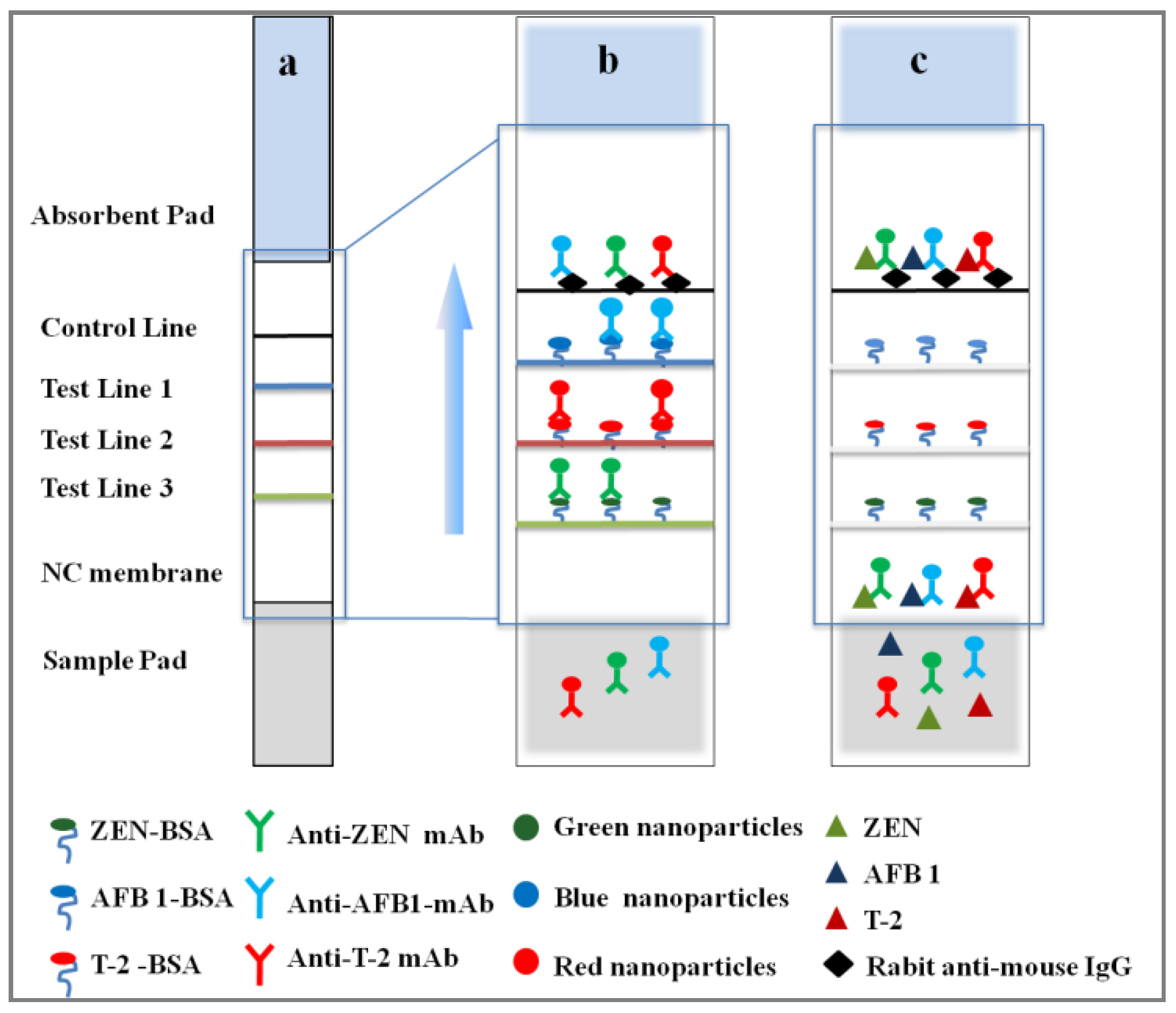
2.1. Design Principle
2.2. ICS Parameters Optimization
2.3. ICS Performance Evaluation
2.4. Comparison between ICS and HPLC-MS/MS
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Instrumentation
4.3. AuNPs Synthesis
4.4. Monoclonal Antibodies (mAb) Production
4.5. mAb-Nanoparticles (mAb-NPs) Conjugation
4.5.1. Preparation of mAb-BNPs (Blue Nanoparticles) for AFB1 Detection
4.5.2. Preparation of mAb-AuNPs Conjugates for T-2 Toxin Detection
4.5.3. Preparation of mAb-GNPs (Green Nanoparticles) for ZEN Detection
4.6. Preparation of ICS
4.6.1. Immobilization of Antigens
4.6.2. ICS Assembly
4.7. Sample Preparation
4.8. Sensitivity, Specificity and Accuracy Evaluation of the ICS
4.9. Validation via HPLC-MS/MS Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. Nutr. 2013, 53, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Zhang, Z.; Ding, X.; Tang, X. Multi-component immunochromatographic assay for simultaneous detection of aflatoxin B1, ochratoxin A and zearalenone in agro-food. Biosens. Bioelectron. 2013, 49, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, K.; Nawaz, S.; Hetmanski, M. Mycotoxins in ingredients of animal feeding stuffs: Ii. Determination of mycotoxins in maize and maize products. Food Addit. Contam. 1998, 15, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed-focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Yavuz, O.; Das, Y.K.; Guvenc, D.; Muglali, O.H. Occurrence of aflatoxin B1, T-2 toxin and zearalenone in compound animal feed. J. Anim. Vet. Adv. 2009, 8, 403–407. [Google Scholar]
- International Agency for Research on Cancer and International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; World Health Organization: Geneva, Switzerland, 1987; Volume 7. [Google Scholar]
- Gong, Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, west Africa. Environ. Health Perspect. 2004, 1334–1338. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Zhang, Y.; Yang, J.; Wang, F.; Wang, Y.; Deng, R.; Zhang, G. Development of an immunochromatographic strip test for the rapid detection of zearalenone in corn. J. Agric. Food Chem. 2014, 62, 11116–11121. [Google Scholar] [CrossRef] [PubMed]
- Hervas, M.; Lopez, M.A.; Escarpa, A. Integrated electrokinetic magnetic bead-based electrochemical immunoassay on microfluidic chips for reliable control of permitted levels of zearalenone in infant foods. Analyst 2011, 136, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, W.Y.; Chen, M.M.; Xiong, H.Y.; Zhang, X.H.; Wang, S.F. A high sensitivity electrochemical sensor based on fe3+-ion molecularly imprinted film for the detection of T-2 toxin. Electroanalysis 2014, 26, 2739–2746. [Google Scholar] [CrossRef]
- Lai, X.-W.; Sun, D.-L.; Ruan, C.-Q.; Zhang, H.; Liu, C.-L. Rapid analysis of aflatoxinsB-1, B-2, and ochratoxinA in rice samples using dispersive liquid-liquid microextraction combined with HPLC. J. Sep. Sci. 2014, 37, 92–98. [Google Scholar] [CrossRef] [PubMed]
- GarridoFrenich, A.; Martinez Vidal, J.L.; Romero-Gonzalez, R.; del Aguilera-Luiz, M. Simple and high-throughput method for the multi mycotoxin analysis in cereals and related foods by ultra-high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2009, 117, 705–712. [Google Scholar]
- Leszczynska, J.; Maslowska, J.; Owczarek, A.; Kucharska, U. Determination of aflatoxins in food products by the ELISA method. Czech J. Food Sci. 2001, 19, 8–12. [Google Scholar] [CrossRef]
- Wu, S.J.; Duan, N.; Zhu, C.Q.; Ma, X.Y.; Wang, M.; Wang, Z.P. Magnetic nanobead-based immunoassay for the simultaneous detection of aflatoxin b-1 and ochratoxin A using upconversion nanoparticles as multicolor labels. Biosens. Bioelectron. 2011, 30, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kim, G.; Lee, S. A gold nanoparticle and aflatoxin b1-bsa conjugates based lateral flow assay method for the analysis of aflatoxin B1. Materials 2012, 5, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Chen, Q.; Han, M.M.; Zhou, J.Y.; Gong, L.; Niu, Y.M.; Zhang, Y.; He, L.D.; Zhang, L.Y. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, A.Y.; De Saeger, S.; Sibanda, L.; Verheijen, R.; Van Peteghem, C. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of zearalenone and deoxynivalenol. Anal. Bioanal. Chem. 2007, 389, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Taranova, N.A.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. ‘Traffic light’ immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens. Bioelectron. 2015, 63, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Li, P.W.; Zhang, Q.; Yang, Y.; Zhang, W.; Guan, D.; Ding, X.X. Extract-free immunochromatographic assay for on-site tests of aflatoxin M1 in milk. Anal. Methods 2012, 4, 3307–3313. [Google Scholar] [CrossRef]
- Molinelli, A.; Grossalber, K.; Fuhrer, M.; Baumgartner, S.; Sulyok, M.; Krska, R. Development of qualitative and semiquantitative immunoassay-based rapid strip tests for the detection of T-2 toxin in wheat and oat. J. Agric. Food Chem. 2008, 56, 2589–2594. [Google Scholar] [CrossRef] [PubMed]

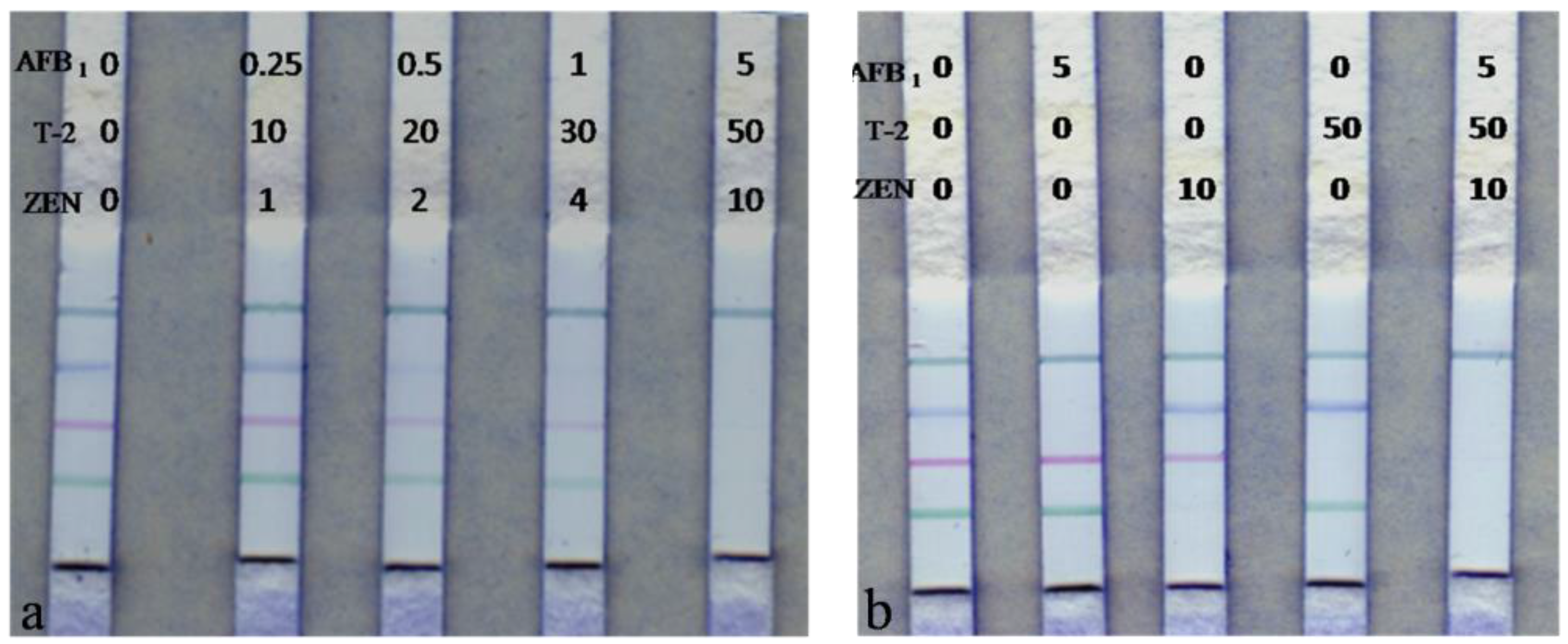
| AFB1/T-2/ZEN (ng/mL) | ICS Visual Result (n = 5) | |||
|---|---|---|---|---|
| Test Line 1 | Test Line 2 | Test Line 3 | Control Line | |
| 0/0/0 | − | − | − | − |
| 0.25/10/1 | − | − | − | − |
| 0.5/20/2 | + | − | + | − |
| 1/30/4 | ++ | + | + | − |
| 5/50/10 | ++ | ++ | ++ | − |
| No. | Sample | ICA (n = 4) | Result of LC-MS/MS (ng/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | T-2 | ZEN | Found ± SD (ng/mL) | |||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | AFB1 | T-2 | ZEN | ||
| 1 | maize | − | − | + | + | − | − | − | − | ++ | ++ | ++ | ++ | 0.55 ± 0.07 | 1.73 ± 0.46 | 45.72 ± 9.11 |
| 2 | maize | + | + | ++ | ++ | + | + | + | ++ | − | − | − | − | 1.33 ± 0.35 | 47.67 ± 6.40 | ND |
| 3 | maize | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | − | − | − | 1.62 ± 0.45 | 76.02 ± 7.19 | ND |
| 4 | feedstuff | − | − | − | − | − | − | − | − | + | + | + | + | ND | 16.86 ± 0.73 | 8.53 ± 0.46 |
| 5 | feedstuff | − | − | − | − | − | − | − | − | ++ | ++ | ++ | ++ | ND | ND | 18.63 ± 1.81 |
| 6 | feedstuff | ++ | ++ | ++ | ++ | − | − | − | − | ++ | ++ | ++ | ++ | 2.56 ± 0.32 | 11.07 ± 0.99 | 11.87 ± 0.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhang, Z.; Zhang, Q.; Zhang, W.; Yu, L.; Wang, D.; Li, H.; Li, P. An On-Site Simultaneous Semi-Quantification of Aflatoxin B1, Zearalenone, and T-2 Toxin in Maize- and Cereal-Based Feed via Multicolor Immunochromatographic Assay. Toxins 2018, 10, 87. https://doi.org/10.3390/toxins10020087
Xu L, Zhang Z, Zhang Q, Zhang W, Yu L, Wang D, Li H, Li P. An On-Site Simultaneous Semi-Quantification of Aflatoxin B1, Zearalenone, and T-2 Toxin in Maize- and Cereal-Based Feed via Multicolor Immunochromatographic Assay. Toxins. 2018; 10(2):87. https://doi.org/10.3390/toxins10020087
Chicago/Turabian StyleXu, Lin, Zhaowei Zhang, Qi Zhang, Wen Zhang, Li Yu, Du Wang, Hui Li, and Peiwu Li. 2018. "An On-Site Simultaneous Semi-Quantification of Aflatoxin B1, Zearalenone, and T-2 Toxin in Maize- and Cereal-Based Feed via Multicolor Immunochromatographic Assay" Toxins 10, no. 2: 87. https://doi.org/10.3390/toxins10020087







