1. Introduction
Globally more frequent and intense cyanobacterial blooms are occurring [
1,
2,
3] with climate change being viewed as the most plausible cause [
4,
5]. Both direct warming effects on growth and indirect effects on water column stability are expected to promote cyanobacteria over freshwater eukaryotic phytoplankton at elevated temperatures [
4,
5,
6,
7]. Moreover, synergism between temperature and nutrients in eutrophic and hyper-eutrophic lakes [
8,
9] and warming-enhanced nutrient loading with rising temperatures are also predicted to intensify cyanobacterial blooms [
3,
5,
10,
11].
Such blooms may come with risks for the aquatic ecosystem causing anoxia related fish kills and food web alterations [
12,
13]. Cyanobacterial blooms may be a threat to animal and public safety as many cyanobacteria produce a variety of potent toxins [
14,
15]. For instance, 458 suspected human illnesses and 175 animal deaths have been linked with bloom events in the U.S.A. during 2007–2011 [
16]. Cyanobacteria can produce a variety of toxins of which the microcystins (MCs) are most abundant globally. The main producer of MCs is the cosmopolitan cyanobacterium
Microcystis [
17]. Despite a wealth of information in the literature on cyanobacterial blooms and their toxins [
18], consequences for the toxicity of cyanobacteria under global warming are less well studied. Laboratory experiments [
19,
20] as well as field observations [
21,
22] suggest that toxic strains in a bloom could be benefitting more from warming than the non-toxic ones, yet not always correlations with temperature are found [
23]. Nonetheless, toxin quota likely decrease with higher temperatures as has been reported for MC producing organisms based on toxicity measurements [
24,
25] and direct quantification of the toxin quota [
26,
27,
28,
29,
30]. The information for tropical strains is even more limited [
28] and to date no indication exist on temperature affected toxicity of cyanobacteria in Southern Vietnam.
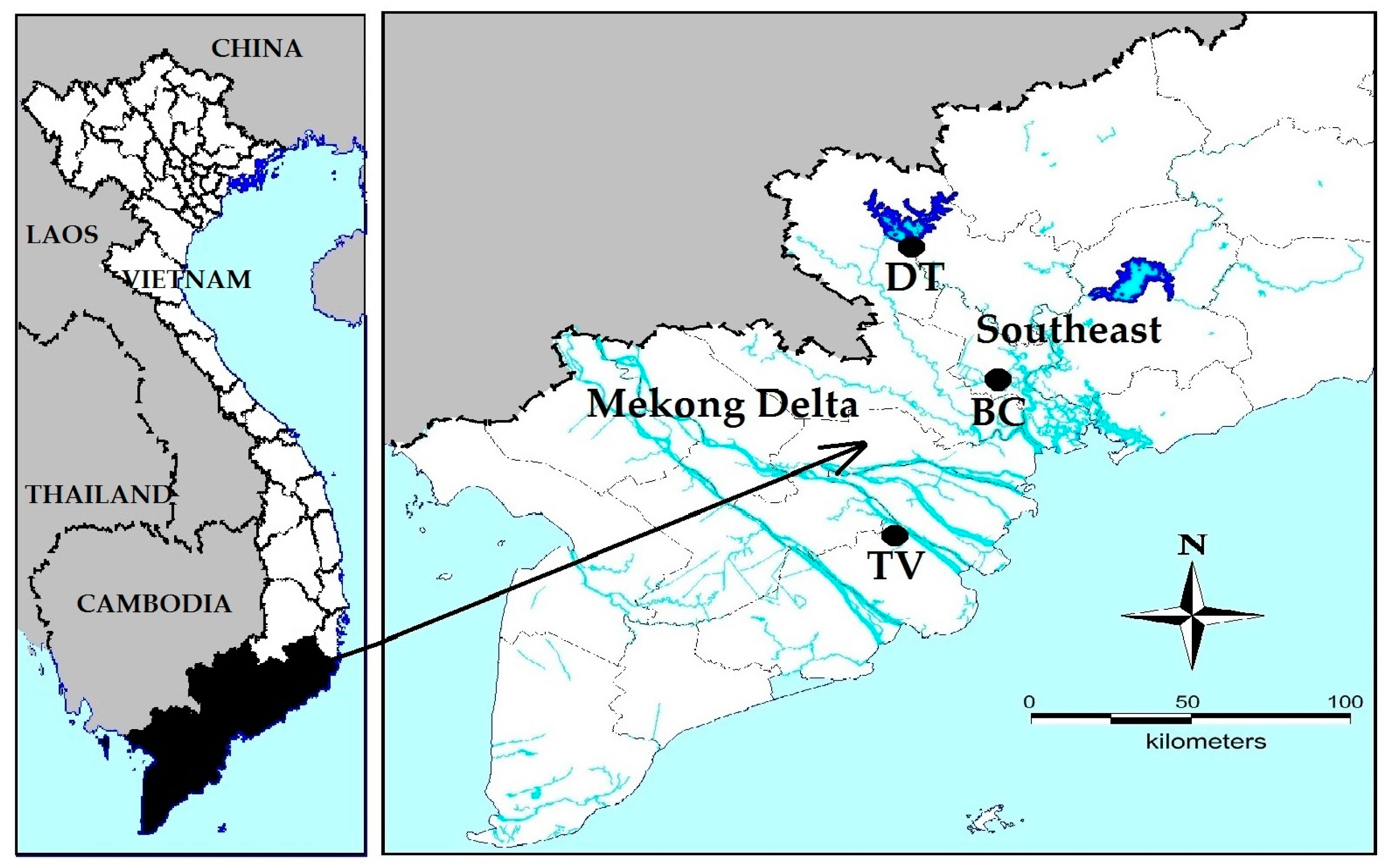
Southern Vietnam, including the Mekong Delta, is expected to be one of the most extremely impacted regions by climate change [
31,
32]. This region is the food basket of Vietnam with ongoing rapid development of intensive agriculture and aquaculture, which are main sources of pollution through discharging pesticides [
33] and nutrients [
34]. However, there is a clear knowledge gap on occurrence, dominance, distribution, toxins and toxicity of cyanobacteria in Southern Vietnam. A few studies have focused on toxin producing cyanobacteria in natural lakes and reservoirs in Vietnam [
35,
36,
37], of which Pham et al. [
38] detected MC at maximally 2.5 µg L
−1 in Dau Tieng Reservoir. The heat waves in the Indochina peninsula in recent years [
39] may be an indication of near future conditions. To get more insight in how one of the most abundant cyanobacteria,
Microcystis [
17] will respond to different temperatures, we isolated six
Microcystis strains from fish ponds and a reservoir in South Vietnam to test the hypothesis that temperature would affect growth rates and that MC cell quota would decline with rising temperature.
3. Discussion
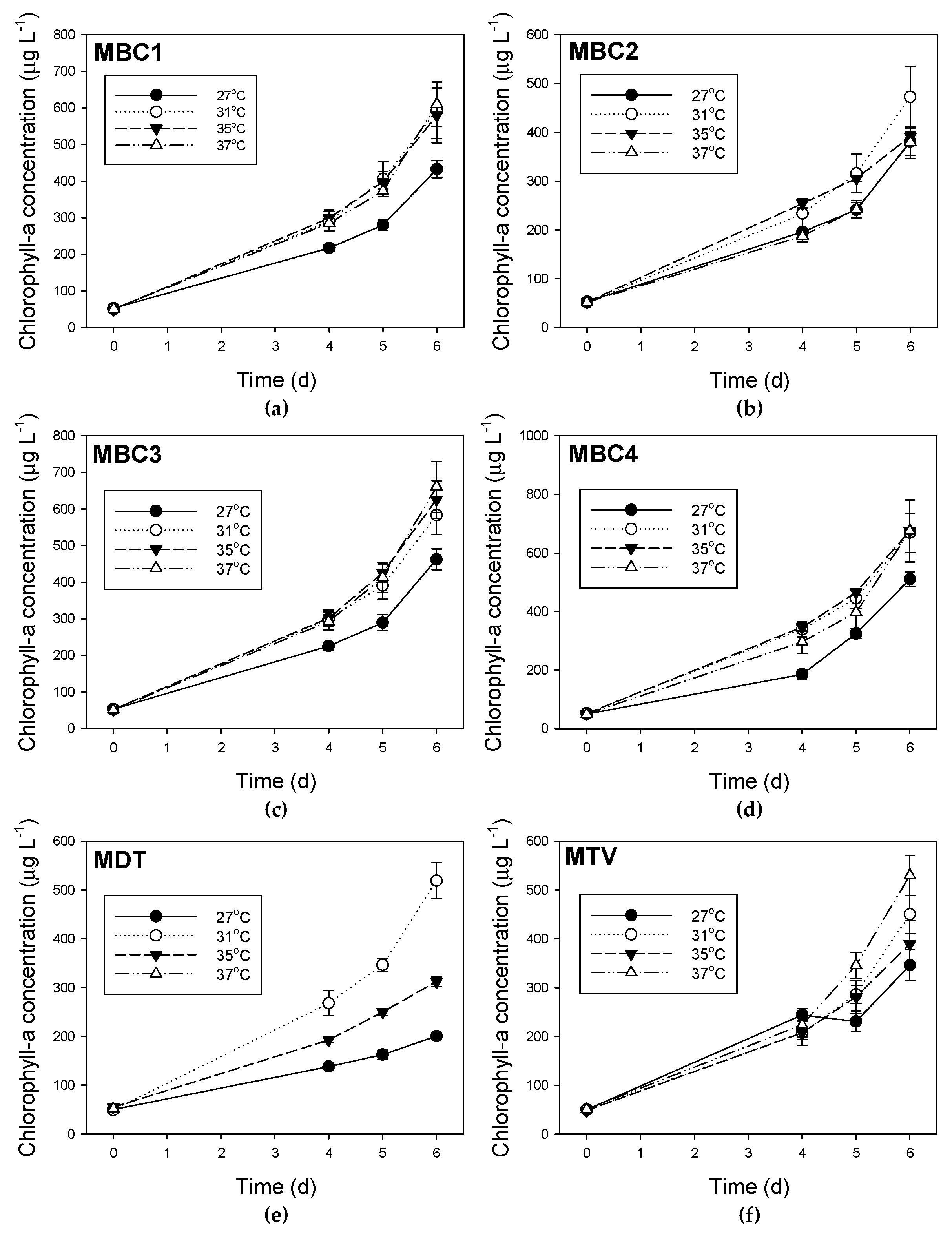
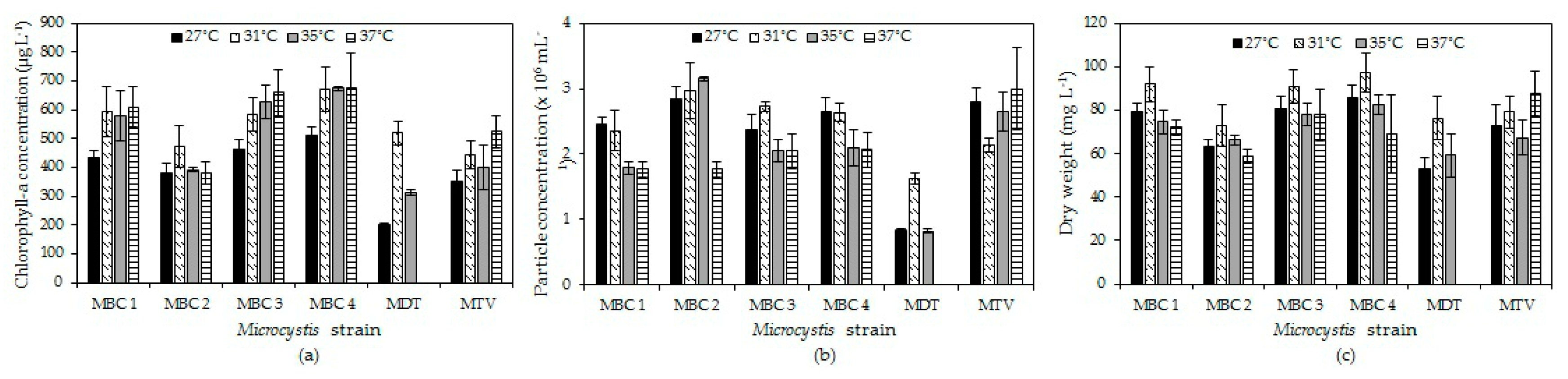
Our results support the hypothesis that elevated temperature may directly promote growth of tropical
Microcystis but the temperature effect is strain dependent. All strains yielded higher biomass in terms of chlorophyll-a concentration and dry-weight at 31 °C compared to 27 °C and then either stabilised, slightly increased or declined with higher temperature. Growth rates were comparable to those found in the literature for
Microcystis (
Appendix E,
Table A3). In general, growth rates showed optima around and above 31 °C that yielded an overall average optimum growth temperature of 34.3 °C (±2.3 °C) for the six strains and three endpoints used. This optimum growth temperature is somewhat higher than usually found in
Microcystis (
Appendix E,
Table A3) but Thomas and Litchman [
41] reported a similar optimum of 34.1 °C in one of their
M. aeruginosa strains (Bear AC-02), Geada et al. [
42] found the highest growth of
M. aeruginosa at 35 °C and Mowe et al. [
28] at 36 °C. For some of the strains we have tested the optimum growth rate might even be above 37 °C. That optima are higher than the temperatures these species may encounter in nature is regularly observed and explained from the asymmetry in the growth-temperature reaction norm, where growth rates decline faster beyond the optimum temperature than below it [
41].
Our
Microcystis strains were selected from sites that had water temperatures between 33.1 °C and 37.7 °C. The single strain that could not survive prolonged periods at 37 °C was strain MDT selected from the DauTieng reservoir that experienced a temperature of 33.5 °C at the moment of sampling. The average temperature in this reservoir was 31.05 °C with the maximal and minimal temperatures of 34 °C and 27.5 °C, respectively [
38]. This indicates that the
Microcystis strain MDT may have never been experienced at temperatures of 35 °C and 37 °C in the field as in our experiment. The highest temperature level 37 °C in our experiment, therefore, was too high for the
Microcystis strain MDT to survive. Hence, five out of our six strains were able to grow at 37 °C. Likewise, in the literature considerable variability in the upper temperature limit
Microcystis may tolerate is reported; four out of five tropical
Microcytis were able to grow at 36 °C [
28], one out of four
Microcystis was able to grow at 35 °C but not at 40 °C, while another
Microcystis even grew up to 40 °C [
42]. Evidently, several
Microcystis strains are well adapted to thrive under high temperature.
In the Southern region of Vietnam, the mean annual temperature increases from around 27 °C in January to above 30 °C in April; the warming temperature in April in recent years was contributed by heat waves in the Indochina peninsula [
39]. This is in line with the prediction that the Mekong Delta will be one of the regions that will be most extremely influenced by climate change [
31,
32]. Particularly, dry season rainfall is projected to decrease [
43], leading to a drier and longer dry season with heat waves pushing water temperatures up to 37 °C. This condition may be an appropriate recipe for more frequent and larger blooms of tropical
Microcystis strains in the Southern region of Vietnam.
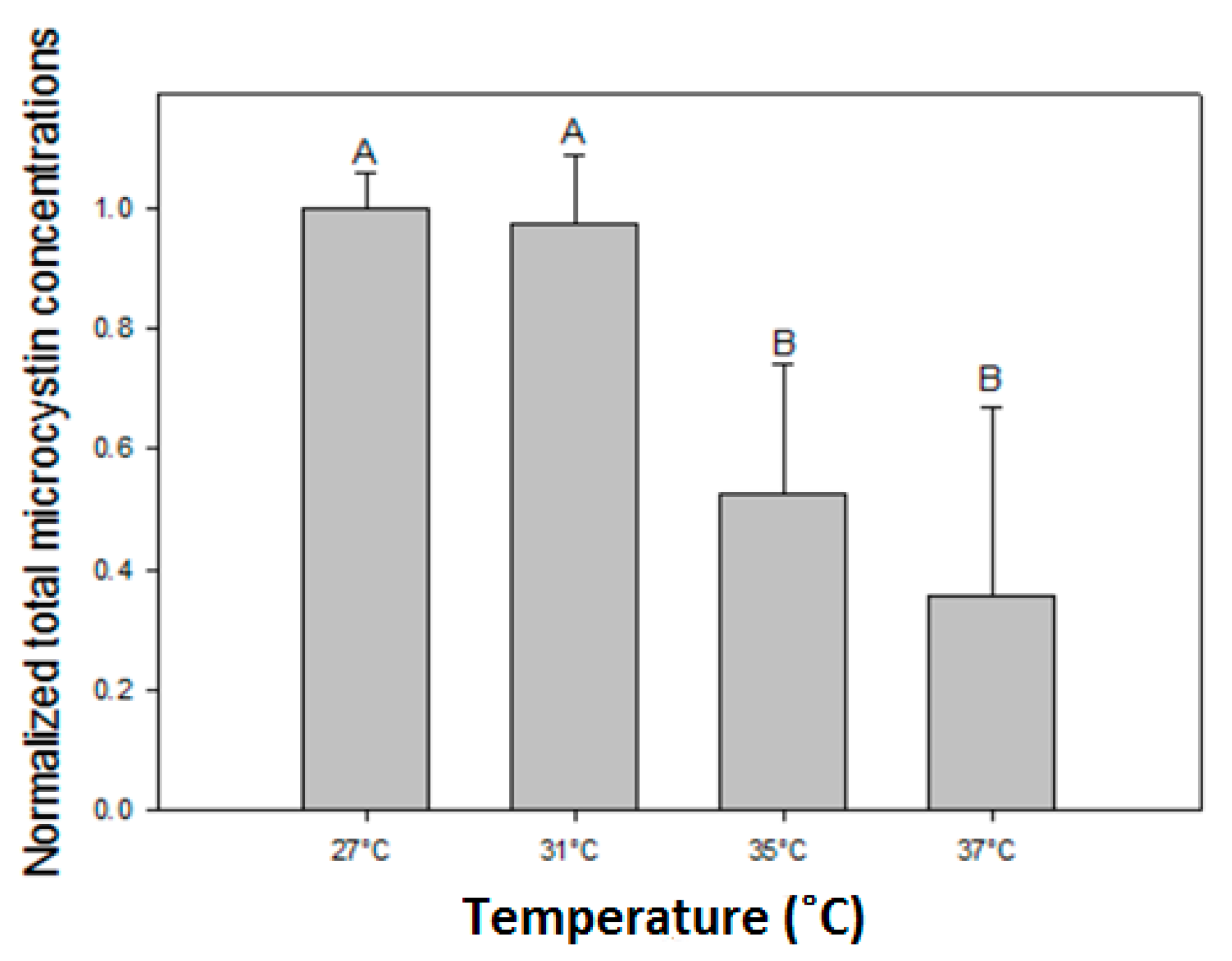
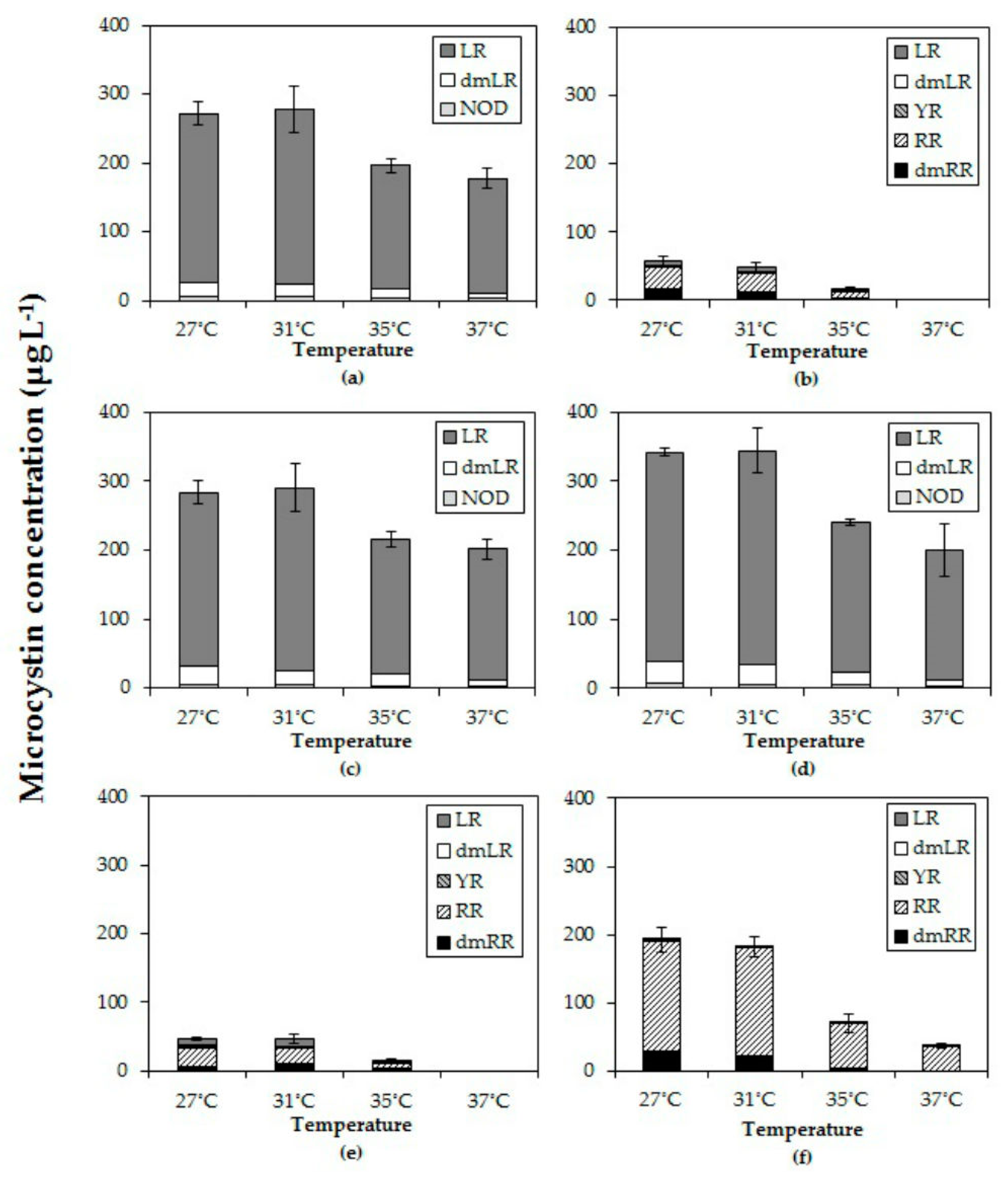
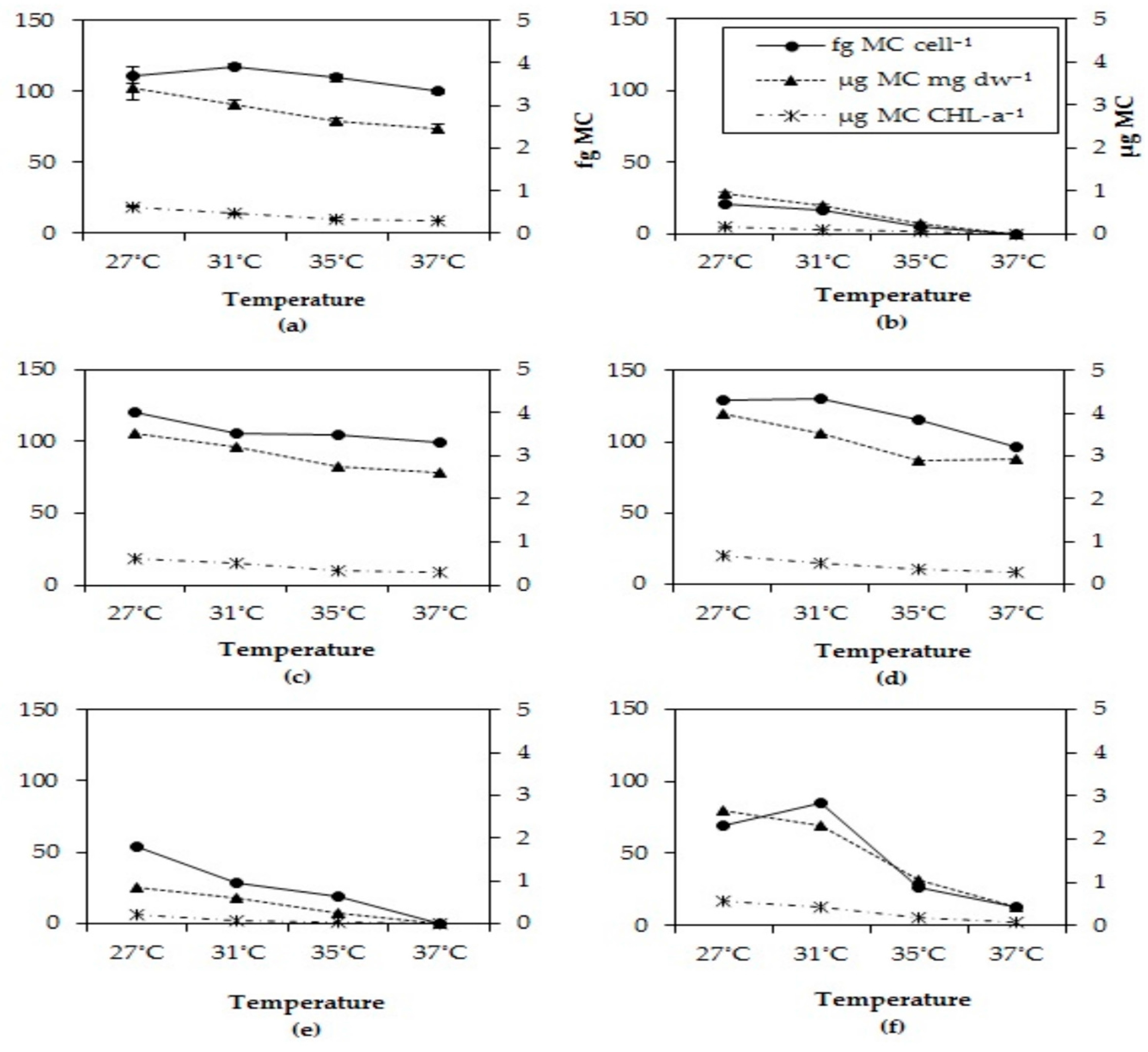
More blooms or higher biomass, however does not necessarily imply higher health risk. Our results evidently show that in all strains total MC concentrations declined, toxicity expressed as MC-LR equivalents declined and that toxin quota declined with elevated temperatures. Similarly, [
D-Leu
1]-MC-LR concentrations in
M. aeruginosa grown at 26 °C, 28 °C, 30 °C and 35 °C were 950, 500, 365 and 100 µg L
−1, respectively, which was due to lower cell quota [
44]. In two other
M. aeruginosa strains total MC concentrations declined from 178 µg L
−1 and 79 µg L
−1 in cultures reared at 20 °C and 25 °C, respectively to 3 and to 2 µg L
−1 in cultures at 35 °C [
30]. Also in these strains MC quota dropped sharply with increasing temperature [
30]. Lower MC quota at higher temperatures seems a more general pattern, because MC cell quota were lower in
M. aeruginosa grown at 29 °C than at 26 °C [
26], lower at 30 °C than at 20 °C [
27] and were lower in five tropical
Microcystis species grown at 36 °C than at lower temperatures [
28]. A possible explanation for lower MC cell quota at higher temperatures is that MC may be a protectant against oxidative stress by binding to proteins [
45]. Inasmuch as higher temperatures may increase oxidative stress in
Microcystis, lower MC cell quota may be the results of binding of MCs to proteins [
26]. The hot methanol extraction we have used will not liberate these bound MCs, which makes follow up research including the bound MC pool a logical step. For risk assessment, however, it remains to be determined if the rather irreversible thioether bond between the Mdha methylene group of MC and the thiol group of cysteine [
46] can be broken easily.
The hot methanol (MeOH) extraction in our study uses 75% MeOH, which is far above the recommended >40% to avoid adsorption to laboratory tools when working with MC-containing solutions [
47]. Lack of or acceptable loss was also confirmed by recovery determinations using spiked cyanobacterial matrix for the MC variants [
48]. In addition to MCs, NOD was also included in the analysis. Low and trace amounts of NOD were found in the three most toxic strains. These strains were isolated from brackish water but no
Nodularia—the most common NOD producer—was present in these waters. Recently, more reports of NOD from habitats that lacked
Nodularia have appeared, including from lakes that were dominated by
Microcystis [
49,
50]. Meanwhile, NOD has been shown being produced by
Nostoc endosymbionts of Macrozamia [
51], by free-living Nostoc from Brazil [
52] and in a novel species
Iningainema pulvinus that was isolated from a freshwater wetland spring [
53].
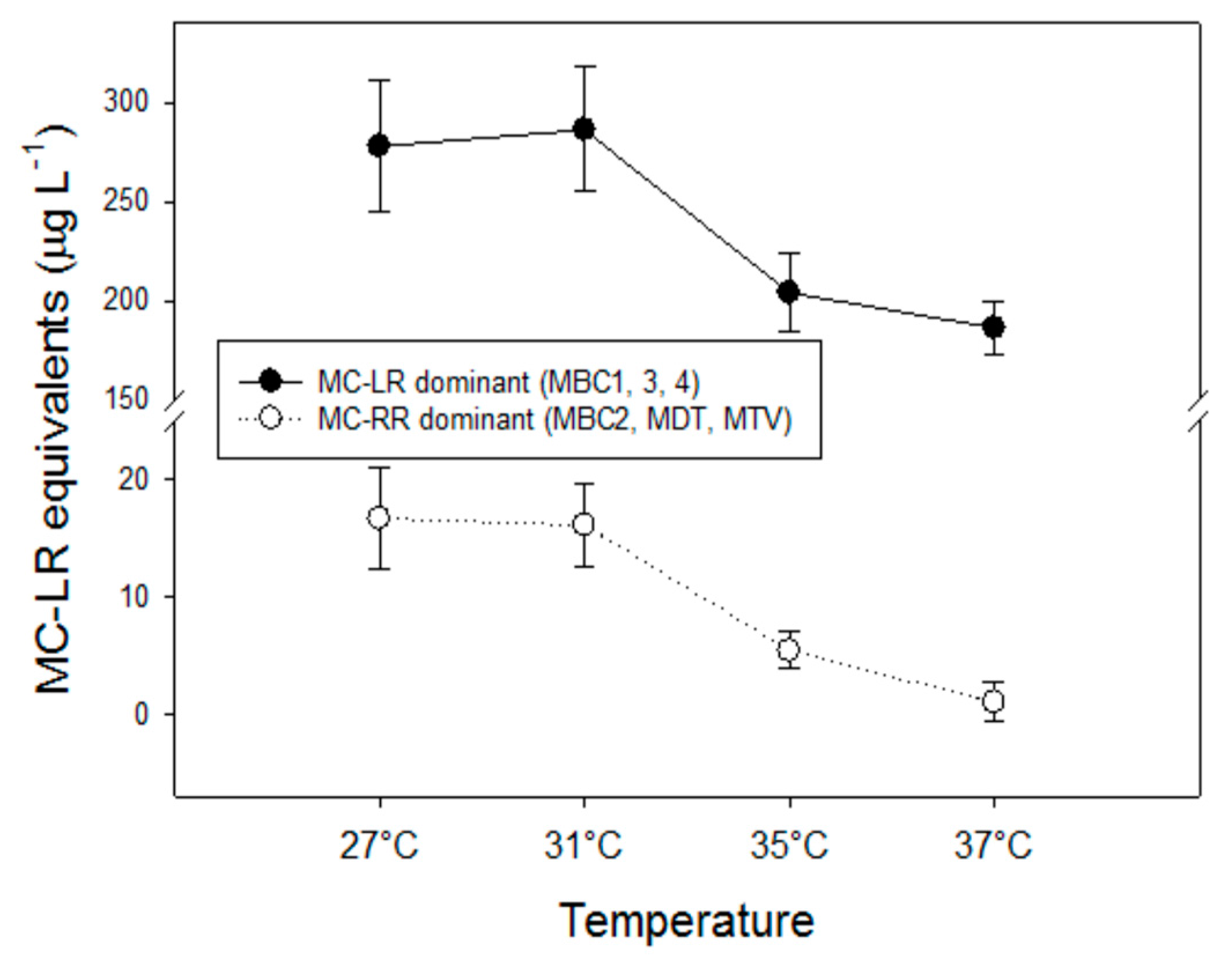
The observed decline of total MC concentrations with higher temperatures differed in strains with deviating MC profiles. In the strains with predominantly MC-LR (MBC1,3,4), total MC concentrations were 27% (±3%) lower at 35 °C and 35% (±6%) lower at 37 °C than 27 °C but in strains with MC-RR as dominant variant total MC concentrations were 67% (±5%) lower at 35 °C and 94% (±11%) lower at 37 °C than at 27 °C. Hence, the response to temperature is strain dependent and seems more pronounced in strains producing less toxic MC variants (dmMC-RR, MC-RR). Since arginine (R) contains three more bound nitrogen atoms than leucine (L), a stronger decrease in MC-RR might be caused by lower arginine cell content under enhanced nitrogen limitation [
54]. However, we have no indications that there would have been strong N limitation in the higher temperatures, whilst not at lower temperature. Nitrogen limitation will impair amino acid synthesis [
54] but also leading to chlorosis [
55] and reduced growth rates [
56]. All cultures expressed exponential growth, except of course MDT at 37 °C, were quite greenish from appearance and no loss of pigments was indicated from PHYTO-PAM analyses (
Appendix G,
Figure A5 and
Figure A6).
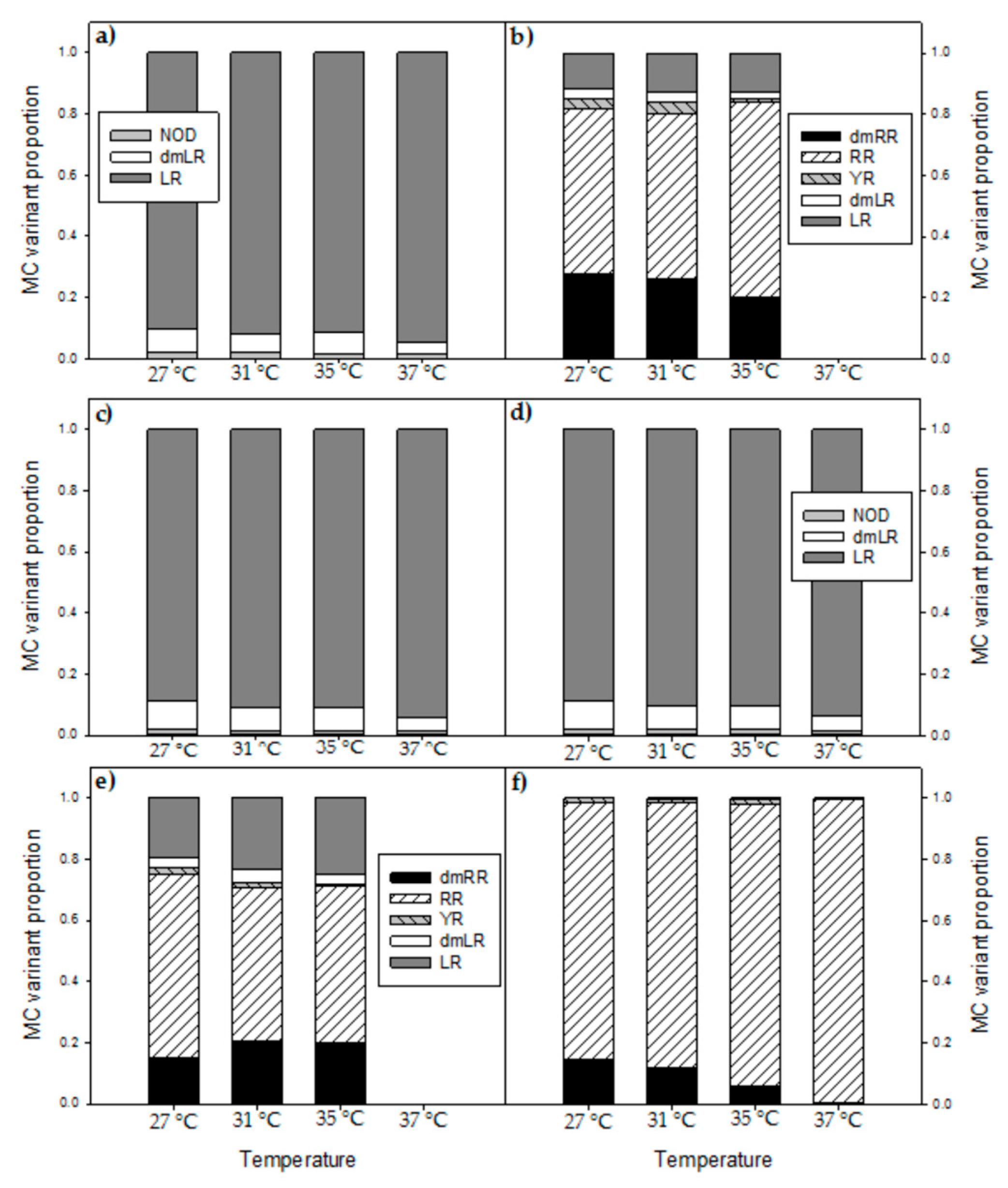
In addition to clearly declined absolute amounts and cell quota of MCs with elevated temperatures, also the MC profiles were changed. In five out of six strains the proportion of the most toxic variant MC-LR increased with higher temperatures, which has also been observed by [
26]. The response of MC-RR was less clear and either its proportion increased with temperature or decreased, again illustrating strain specific responses to increased temperature.
Dolichospermum sp. showed relatively more MC-RR at higher temperatures [
57]. A similar response was found by Maliaka et al. [
58] who observed that the share of MC-RR in the total MC-pool of incubated lake seston at 20 °C was 47% (±5)% but increased to on average 62% (±3)% at 25 °C and 66% (±8)% at 30 °C. In contrast, Amé and Wunderlin [
59] found a higher proportion of MC-LR at higher temperature (28 °C) than at lower temperature (20 °C) and the opposite for MC-RR. These authors also noted higher MC-LR cell quota and lower MC-RR cell quota, also when expressed per protein biomass [
59]. Those studies show that the MC-profile in
Microcystis may be changed with elevated temperature, which could be caused by altered availability of certain amino acids [
54] but such hypothesis needs further investigation. In the studies with incubated field samples [
58,
59], however, also competitive replacement of strains varying in toxin profile could have occurred. In our experiment, the altered MC profiles can be ascribed to changes in the production and/or fate of MC variants produced by each isolate. Whether these isolates are fully representative for the actual field locations is difficult to decipher. Nonetheless, the MC profiles of field samples collected at the same sites where the strains, MBC4, MDT and MTV were isolated showed high similarity to the MC profiles of the corresponding strains (
Appendix H,
Figure A7). The profile of MBC2, however, deviated from the field sample and thus this isolate might not have been the main toxin producer during the bloom event. Field data (MC profiles) from locations MBC1 and MBC3 are not available.
In field populations, higher temperatures may promote growth rates of toxigenic
Microcystis cells leading to a larger proportion of toxic cells [
19]. The different MC profiles and MC quota as well as strain specific response to higher temperatures make it difficult to predict what will be the expected toxicity, also since “chance is the pacemaker of evolution of toxin production” [
60]. However, there is little doubt that aggravated eutrophication will lead to more cyanobacterial biomass and since the MC concentration is determined by the biomass of toxigenic strains and their toxin quota, despite MC quota seem to decline with higher temperatures, elevated in situ MC concentrations may occur [
30,
58]. Also in Southern Vietnam surface accumulations of cyanobacteria are occurring, which is an important biomass concentrating event especially during heat wave episodes, in which extremely high MC concentrations have been measured [
61]. i.e. MC concentration of field samples collected in TraVinh at 33.1 °C was 4033 µg g
−1 dry-weight that was 1.5; 1.8; 3.9 and 9.6 times higher than that of the
Microcystis strain MTV grown at 27 °C, 31 °C, 35 °C and 37 °C, respectively. Hence, although cells may become less toxic in a near future Southern Vietnam, the expected surface accumulations and increased biomass under predicted climate change, urge for measures to reduce the cyanobacterial biomass drastically.