Botulinum Toxin B Affects Neuropathic Pain but Not Functional Recovery after Peripheral Nerve Injury in a Mouse Model
Abstract
:1. Introduction
2. Results
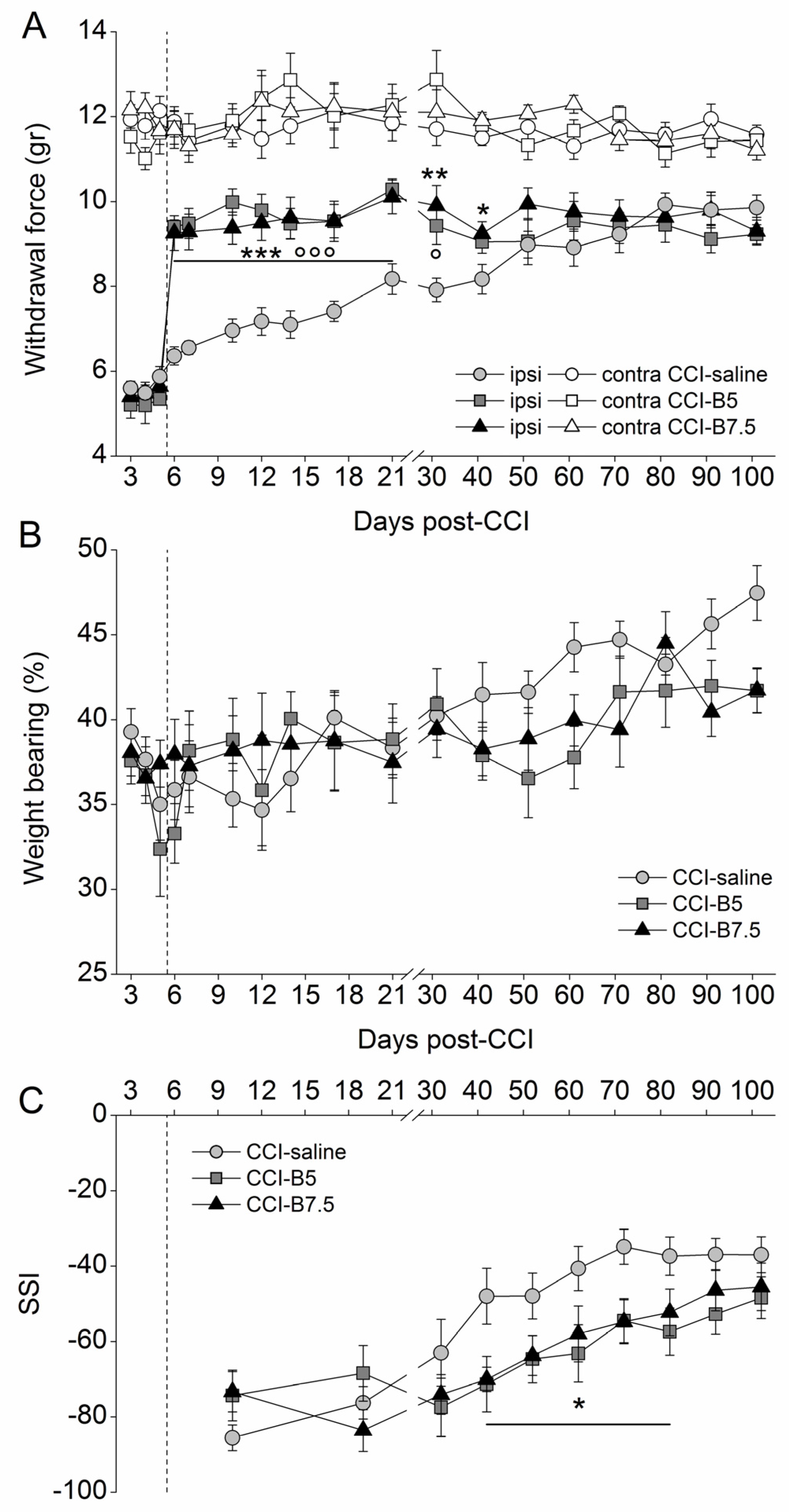
2.1. Effects of BoNT/B on Mechanical Nociceptive Threshold in CCI Mice
2.2. Effects of BoNT/B on Functional Recovery after CCI
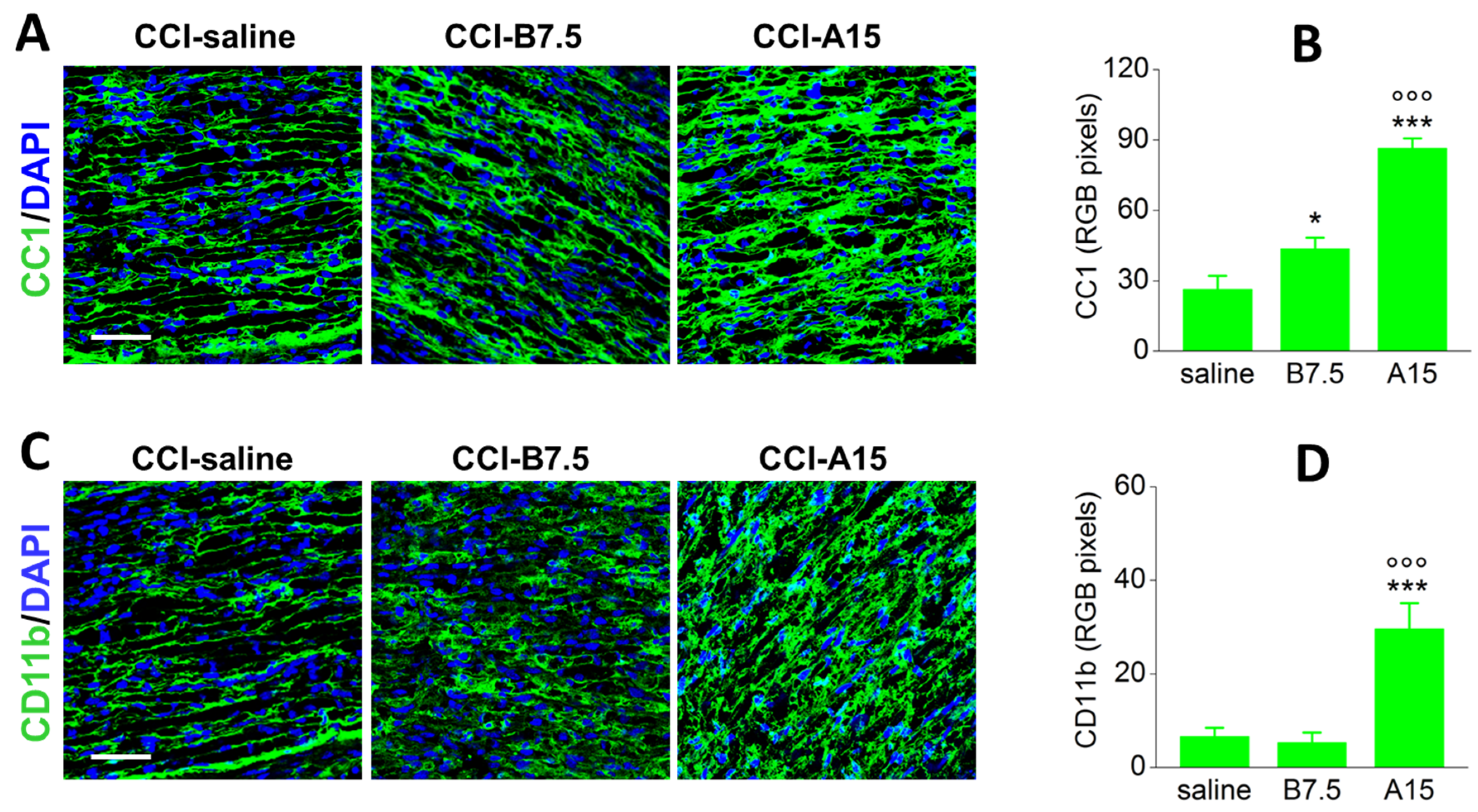
2.3. Effect of BoNTs on Cytoskeleton and Myelin Sheath of Injured Sciatic Nerve
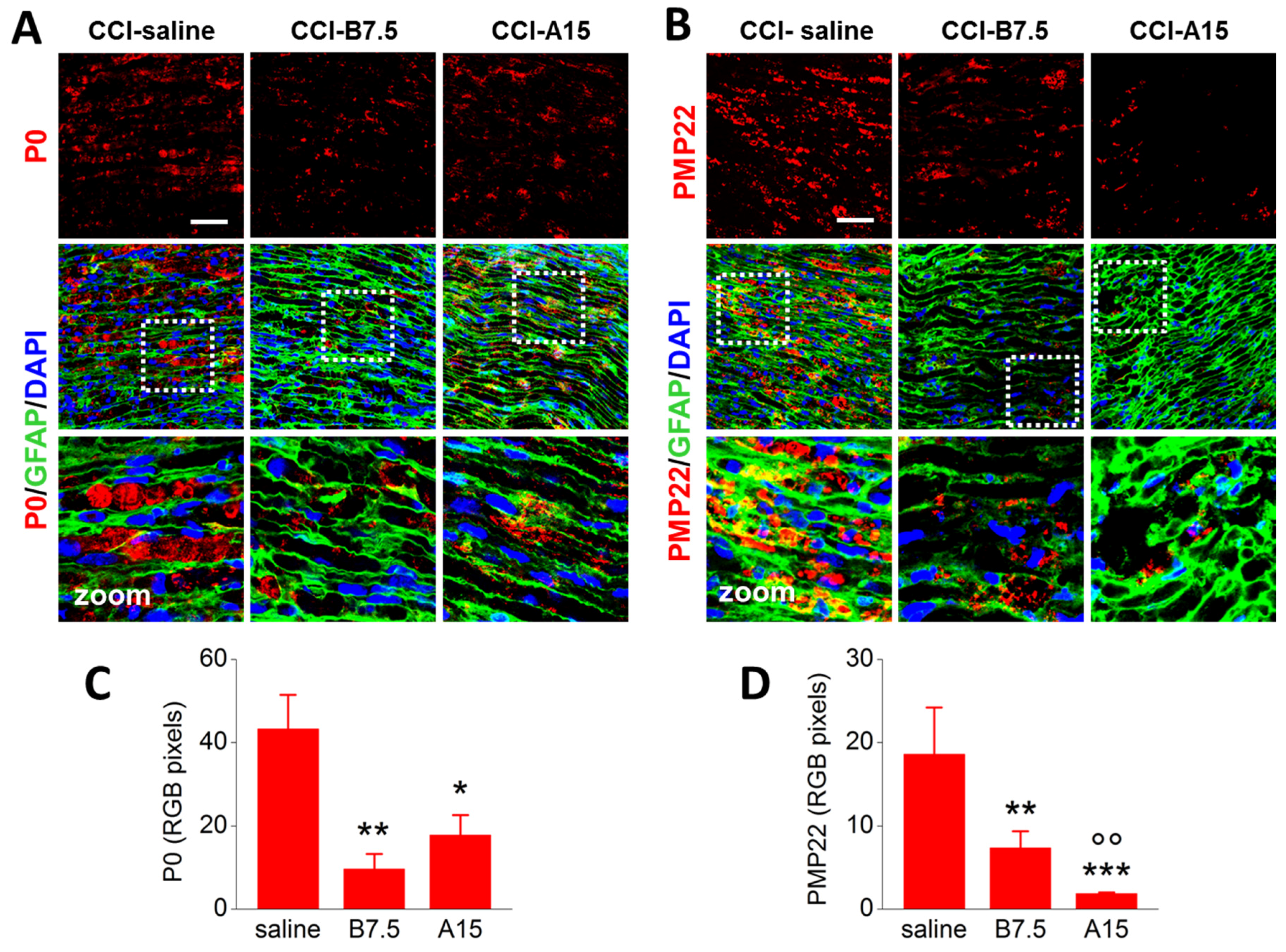
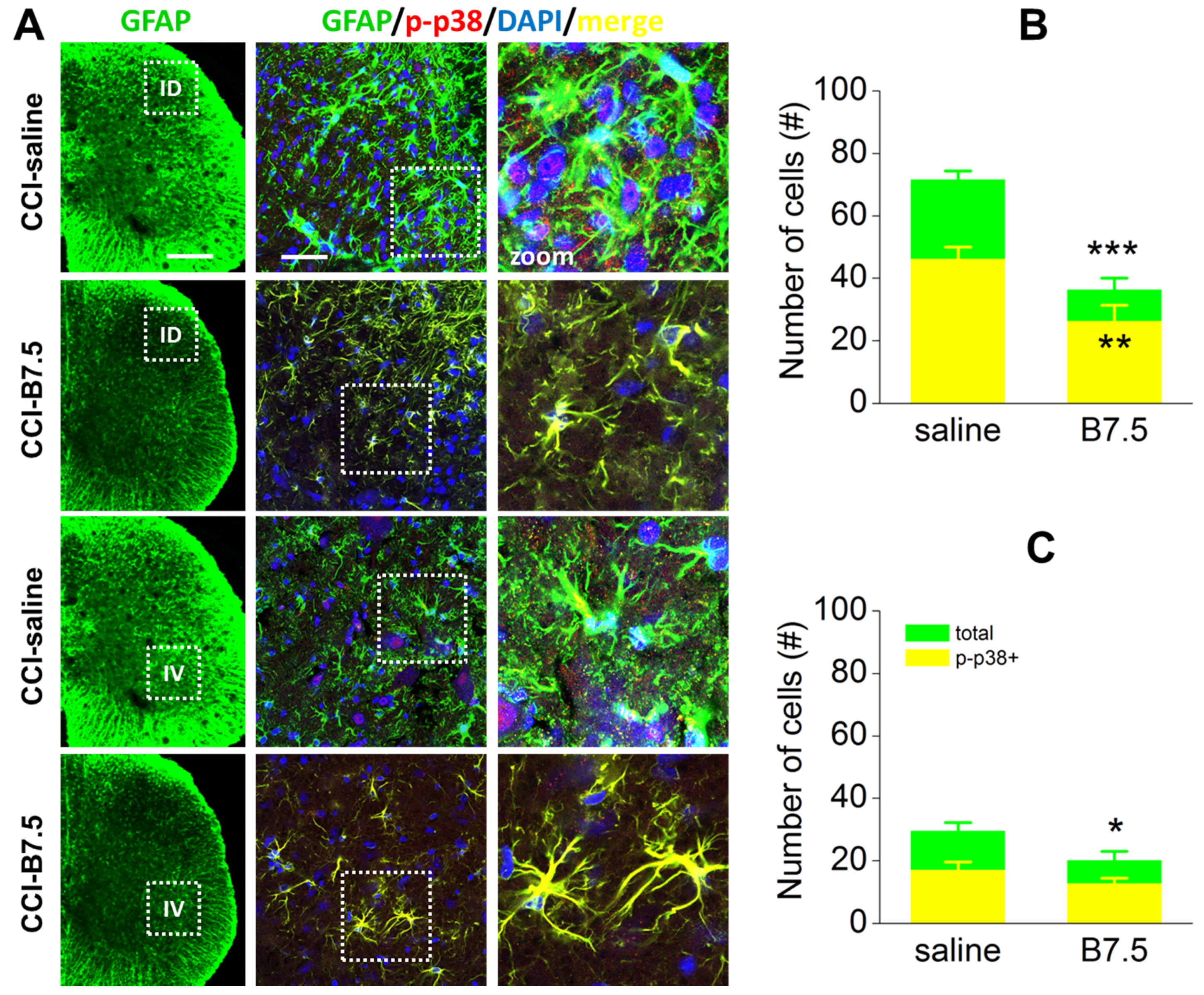
2.4. Effects of BoNTs on Myelin-Associated Protein Expressed by SCs
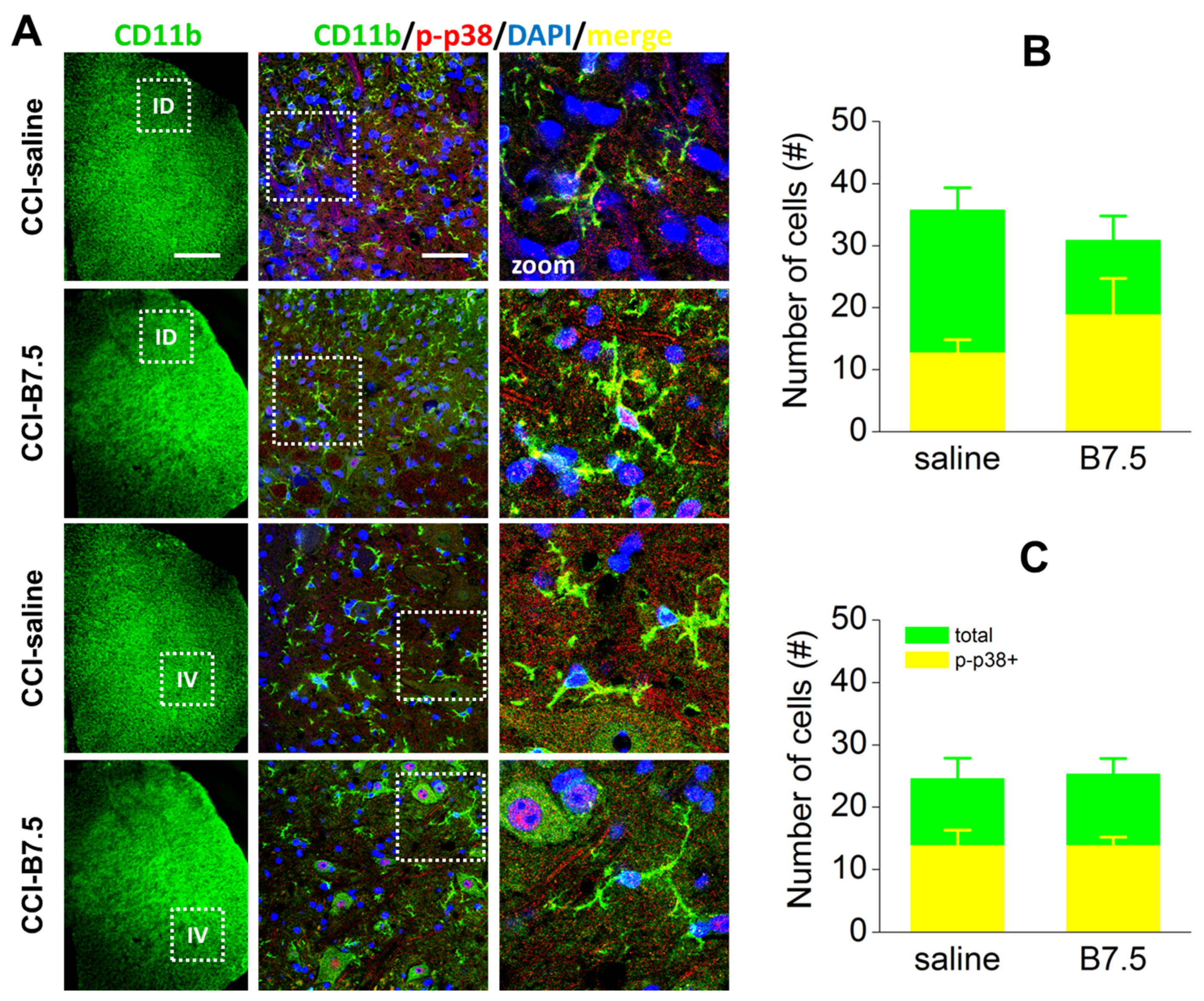
2.5. Effect of BoNTs on the Immune Cells after CCI
2.6. Effect of BoNT/B on Spinal Cord after CCI
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure
4.3. Pharmacological Treatments and Experimental Groups
4.4. Behavioral Tests
4.4.1. Measurement of Mechanical Nociceptive Threshold
4.4.2. Measurement of Weight Bearing
4.4.3. Walking Footprint Analysis and Sciatic Static Index
4.5. Immunohistochemistry Assays
4.5.1. Immunostaining of Sciatic Nerve
4.5.2. Immunostaining of Spinal Cord
4.5.3. Confocal IF Analysis
4.6. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cairns, B.E.; Gazerani, P. Botulinum neurotoxin A for chronic migraine headaches: Does it work and how? Pain Manag. 2014, 4, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Luvisetto, S.; Gazerani, P.; Cianchetti, C.; Pavone, F. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins 2015, 7, 3818–3844. [Google Scholar] [CrossRef] [PubMed]
- Matak, I.; Lackovic, Z. Botulinum toxin A, brain and pain. Prog. Neurobiol. 2014, 119–120, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S.; Yaksh, T.L.; Ramachandran, R. Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins 2015, 7, 4519–4563. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Current gaps in basic science knowledge of botulinum neurotoxin biological actions. Toxicon 2015, 107, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Rasotto, M.B. On botulinum neurotoxin variability. MBio 2015, 6, e02131-14. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Pluhackova, K.; Böckmann, R.A. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front. Physiol. 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [CrossRef] [PubMed]
- Luvisetto, S.; Marinelli, S.; Cobianchi, S.; Pavone, F. Anti-allodynic efficacy of botulinum neurotoxin A in a model of neuropathic pain. Neuroscience 2007, 145, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Luvisetto, S.; Cobianchi, S.; Makuch, W.; Obara, I.; Mezzaroma, E.; Caruso, M.; Straface, E.; Przewlocka, B.; Pavone, F. Botulinum neurotoxin type A counteracts neuropathic pain and facilitates functional recovery after peripheral nerve injury in animal models. Neuroscience 2010, 171, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Rojewska, E.; Makuch, W.; Korostynski, M.; Luvisetto, S.; Marinelli, S.; Pavone, F.; Przewlocka, B. The effect of botulinum neurotoxin A on sciatic nerve injury-induced neuroimmunological changes in rat dorsal root ganglia and spinal cord. Neuroscience 2011, 175, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Zychowska, M.; Rojewska, E.; Makuch, W.; Luvisetto, S.; Pavone, F.; Marinelli, S.; Przewlocka, B.; Mika, J. Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain. Eur. J. Pharmacol. 2016, 791, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Clinical uses of botulinum neurotoxins: Current indications, limitations and future developments. Toxins 2012, 4, 913–939. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, A.R.; Del Grande, A.; Petracca, M.; Ialongo, T.; Ricciardi, L. Clinical differences between botulinum neurotoxin type A and B. Toxicon 2015, 107, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, S.; Gustavsson, Y.; Marino, M.J.; Dickenson, A.H.; Yaksh, T.L.; Sorkin, L.S.; Ramachandran, R. Effects of intraplantar botulinum toxin-B on carrageenan-induced changes in nociception and spinal phosphorylation of GluA1 and Akt. Eur. J. Neurosci. 2016, 44, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.J.; Terashima, T.; Steinauer, J.J.; Eddinger, K.A.; Yaksh, T.L.; Xu, Q. Botulinum toxin B in the sensory afferent: Transmitter release, spinal activation, and pain behavior. Pain 2014, 155, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Luvisetto, S.; Marinelli, S.; Lucchetti, F.; Marchi, F.; Cobianchi, S.; Rossetto, O.; Montecucco, C.; Pavone, F. Botulinum neurotoxins and formalin-induced pain: Central vs. peripheral effects in mice. Brain Res. 2006, 1082, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.P.; Khan, I.; Suhail, M.S.; Malkmus, S.; Yaksh, T.L. Spinal botulinum neurotoxin B: Effects on afferent transmitter release and nociceptive processing. PLoS ONE 2011, 6, e19126. [Google Scholar]
- Park, H.J.; Marino, M.J.; Rondon, E.S.; Xu, Q.; Yaksh, T.L. The effects of intraplantar and intrathecal botulinum toxin type B on tactile allodynia in mono and polyneuropathy in the mouse. Anesth. Analg. 2015, 121, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Pavone, F.; Ueda, I. Is BoNT/B useful for pain treatment? Pain 2014, 155, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Dubový, P. Wallerian Degeneration and Peripheral Nerve Conditions for Both Axonal Regeneration and Neuropathic Pain Induction. Ann. Anat. 2011, 193, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.E.; Barres, B.A. Why Is Wallerian Degeneration in the CNS So Slow? Annu. Rev. Neurosci. 2007, 30, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.A.; Clark, A.K.; Marchand, F.; McMahon, S.B. Pathophysiology of peripheral neuropathic pain: Immune cells and molecules. Anesth. Analg. 2007, 105, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Pavone, F.; Luvisetto, S. Botulinum neurotoxin for pain management: Insights from animal models. Toxins 2010, 2, 2890–2913. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Brown, M.C. Macrophages and nerve regeneration. Curr. Opin. Neurobiol. 1992, 2, 679–682. [Google Scholar] [CrossRef]
- Barrette, B.; Hébert, M.A.; Filali, M.; Lafortune, K.; Vallières, N.; Gowing, G.; Julien, J.P.; Lacroix, S. Requirement of Myeloid Cells for Axon Regeneration. J. Neurosci. 2008, 28, 9363–9376. [Google Scholar] [CrossRef] [PubMed]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Jaramillo, J.; Luvisetto, S.; Pavone, F.; Navarro, X. Botulinum neurotoxin A promotes functional recovery after peripheral nerve injury by increasing regeneration of myelinated fibers. Neuroscience 2017, 359, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Bennett, D.L. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp. Neurol. 2012, 234, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The Neuropathic Pain Triad: Neurons, Immune Cells and Glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Vacca, V.; Marinelli, S.; Luvisetto, S.; Pavone, F. Botulinum toxin A increases analgesic effects of morphine, counters development of morphine tolerance and modulates glia activation and μ opioid receptor expression in neuropathic mice. Brain Behav. Immun. 2013, 32, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, G.; Piehl, F.; Meister, B. VAMP-1 and VAMP-2 Gene Expression in Rat Spinal Motoneurones: Differential Regulation after Neuronal Injury. Eur. J. Neurosci. 1998, 10, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Edelmann, L.; Jahn, R.; Dahlström, A. Axonal transport and distribution of synaptobrevin I and II in the rat peripheral nervous system. J. Neurosci. 1996, 16, 137–147. [Google Scholar] [PubMed]
- Jeftinija, S.D.; Jeftinija, K.V.; Stefanovic, G. Cultured astrocytes express proteins involved in vesicular glutamate release. Brain Res. 1997, 750, 41–47. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Luvisetto, S.; Rossetto, O.; Montecucco, C.; Pavone, F. Toxicity of botulinum neurotoxins in central nervous system of mice. Toxicon 2003, 41, 475–481. [Google Scholar] [CrossRef]
- Luvisetto, S.; Marinelli, S.; Rossetto, O.; Montecucco, C.; Pavone, F. Central injection of botulinum neurotoxins: Behavioural effects in mice. Behav. Pharmacol. 2004, 15, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.R. A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon 2001, 39, 1815–1820. [Google Scholar] [CrossRef]
- Baptista, A.F.; Gomes, J.R.; Oliveira, J.T.; Santos, S.M.; Vannier-Santos, M.A.; Martinez, A.M. A New Approach to Assess Function after Sciatic Nerve Lesion in the Mouse—Adaptation of the Sciatic Static Index. J. Neurosci. Meth. 2007, 161, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lam, A.; Sato, I.; Okabe, T.; Sato, Y. Reflectance and Fluorescence Spectral Recovery via Actively Lit RGB Images. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 38, 1313–1326. [Google Scholar] [CrossRef] [PubMed]

| Target | Marker | CCI-A15 | CCI-B7.5 |
|---|---|---|---|
| Non-myelinating SCs | GFAP | ↑ | ↓ |
| Myelinating SCs | S100b | = | = |
| Peripheral myelin | P0 | ↓ | ↓↓ |
| Peripheral myelin | PMP22 | ↓↓↓ | ↓↓ |
| Mast cells | CC1 | ↑↑↑ | ↑ |
| Macrophages | CD11b | ↑↑↑ 1 | = |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finocchiaro, A.; Marinelli, S.; De Angelis, F.; Vacca, V.; Luvisetto, S.; Pavone, F. Botulinum Toxin B Affects Neuropathic Pain but Not Functional Recovery after Peripheral Nerve Injury in a Mouse Model. Toxins 2018, 10, 128. https://doi.org/10.3390/toxins10030128
Finocchiaro A, Marinelli S, De Angelis F, Vacca V, Luvisetto S, Pavone F. Botulinum Toxin B Affects Neuropathic Pain but Not Functional Recovery after Peripheral Nerve Injury in a Mouse Model. Toxins. 2018; 10(3):128. https://doi.org/10.3390/toxins10030128
Chicago/Turabian StyleFinocchiaro, Alba, Sara Marinelli, Federica De Angelis, Valentina Vacca, Siro Luvisetto, and Flaminia Pavone. 2018. "Botulinum Toxin B Affects Neuropathic Pain but Not Functional Recovery after Peripheral Nerve Injury in a Mouse Model" Toxins 10, no. 3: 128. https://doi.org/10.3390/toxins10030128






