Domain III of Cry1Ac Is Critical to Binding and Toxicity against Soybean Looper (Chrysodeixis includens) but Not to Velvetbean Caterpillar (Anticarsia gemmatalis)
Abstract
:1. Introduction
2. Results
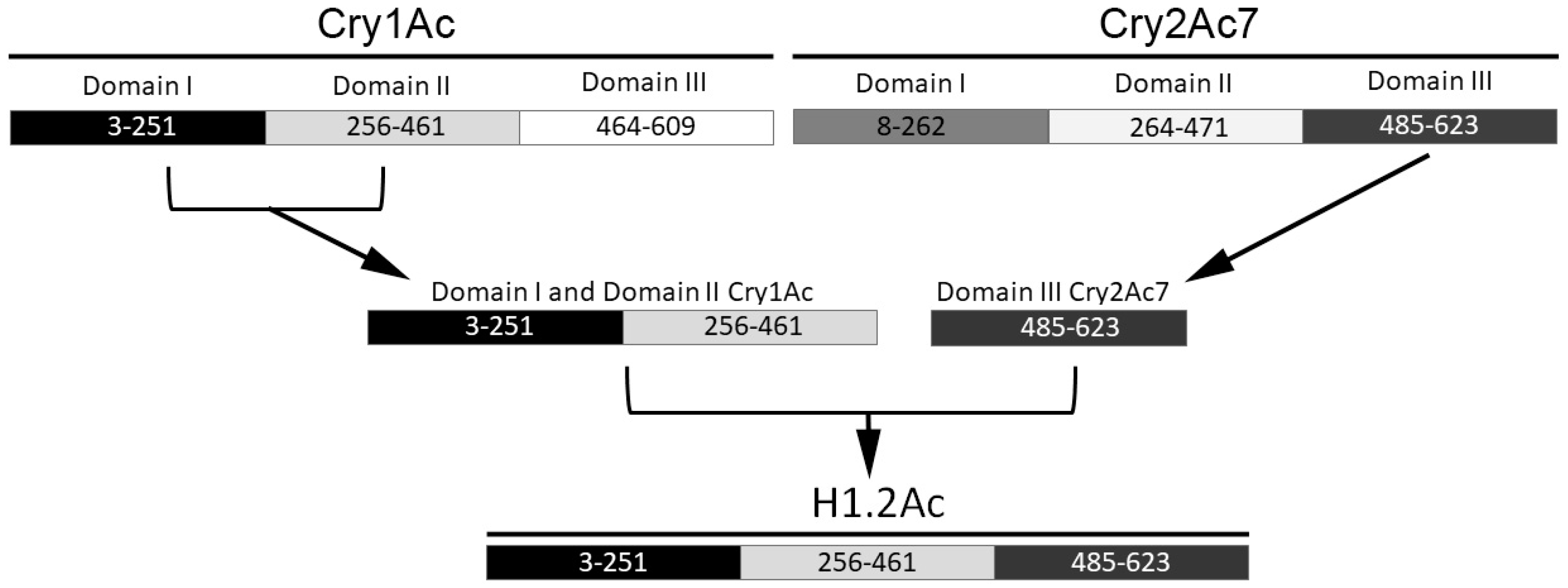
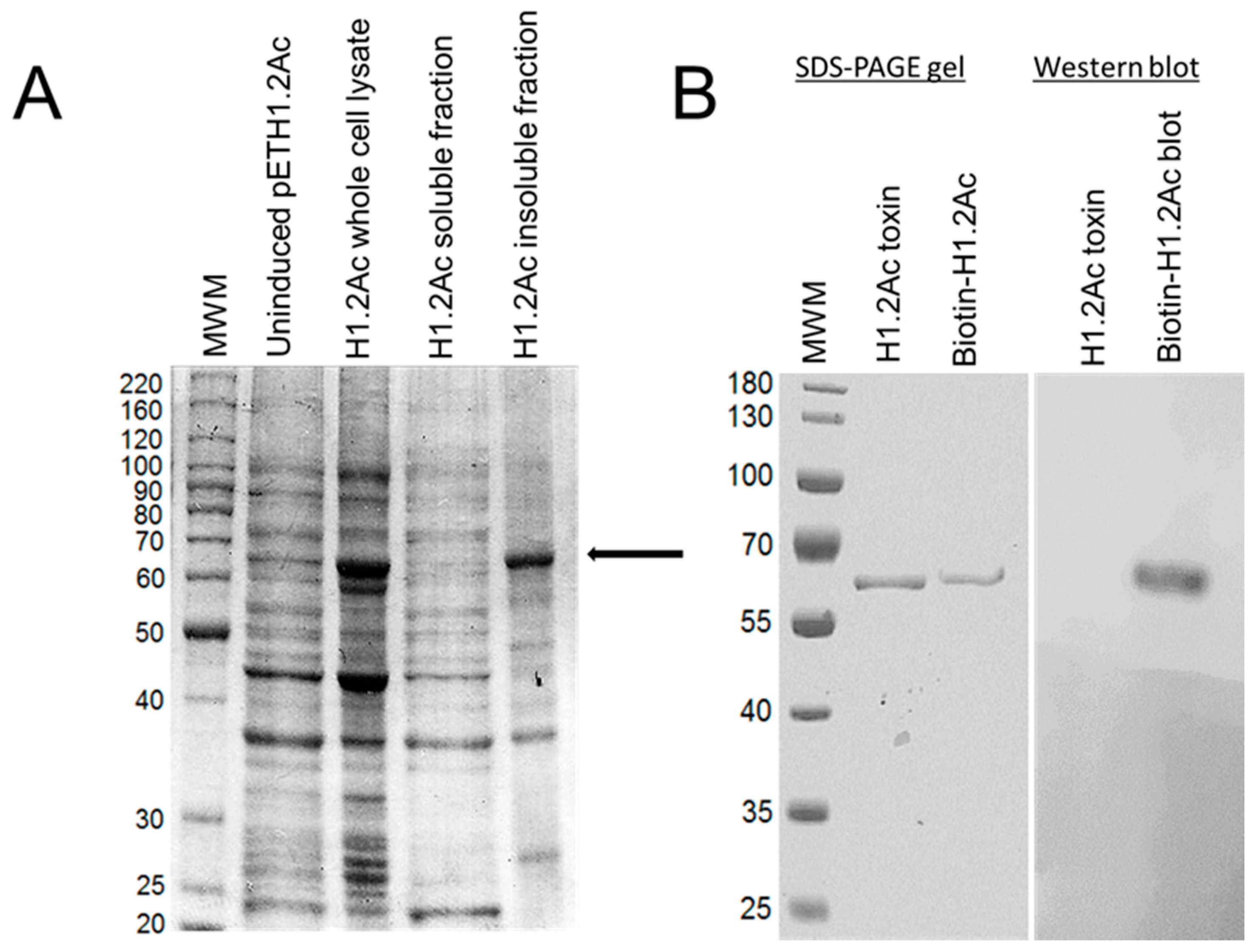
2.1. Hybrid Protein Construction and Production
2.2. Insecticidal Activity
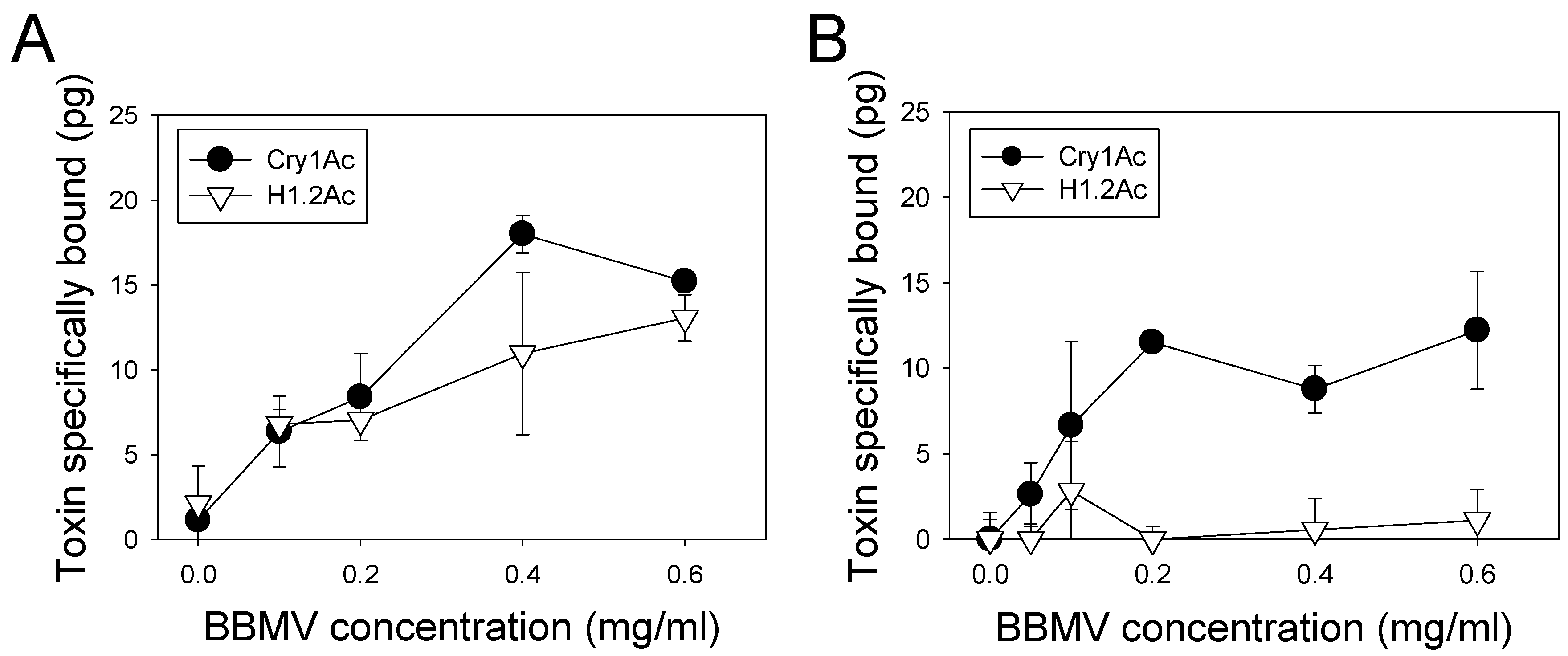
2.3. Toxin Binding Assays
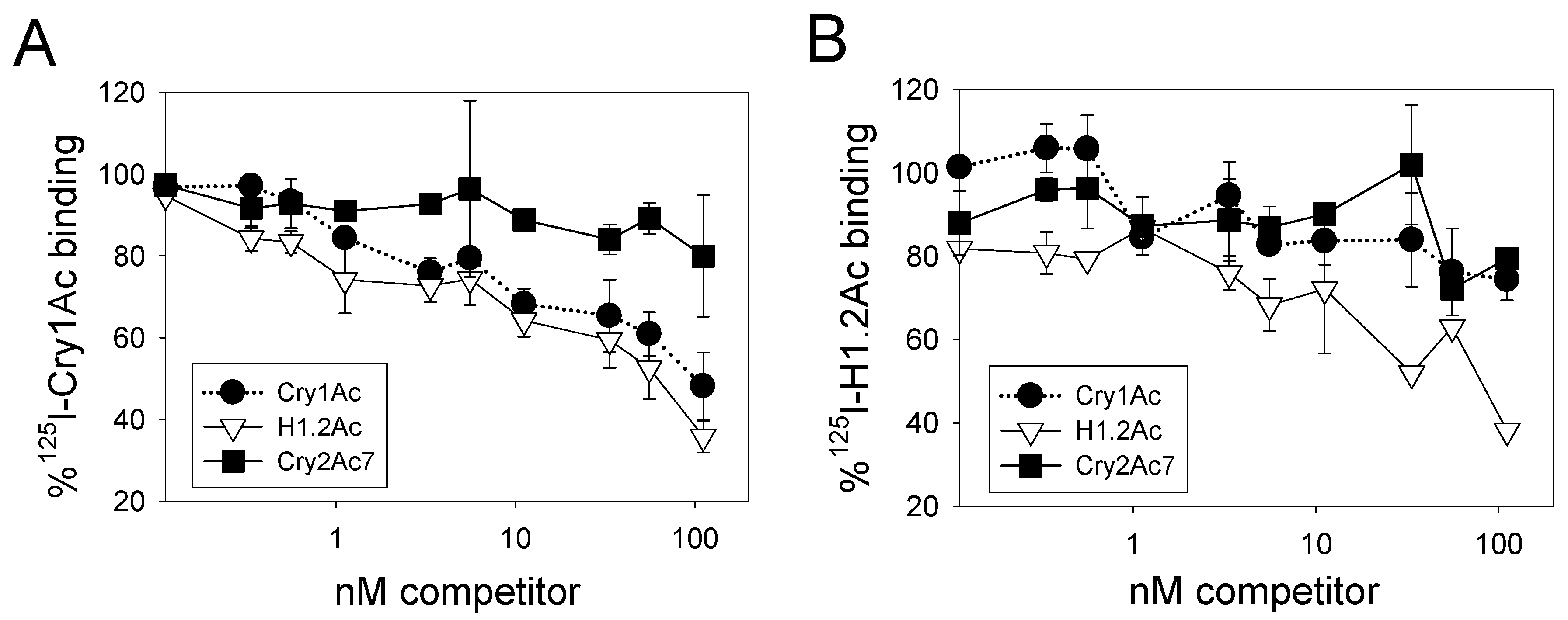
2.4. Competition Binding Assays
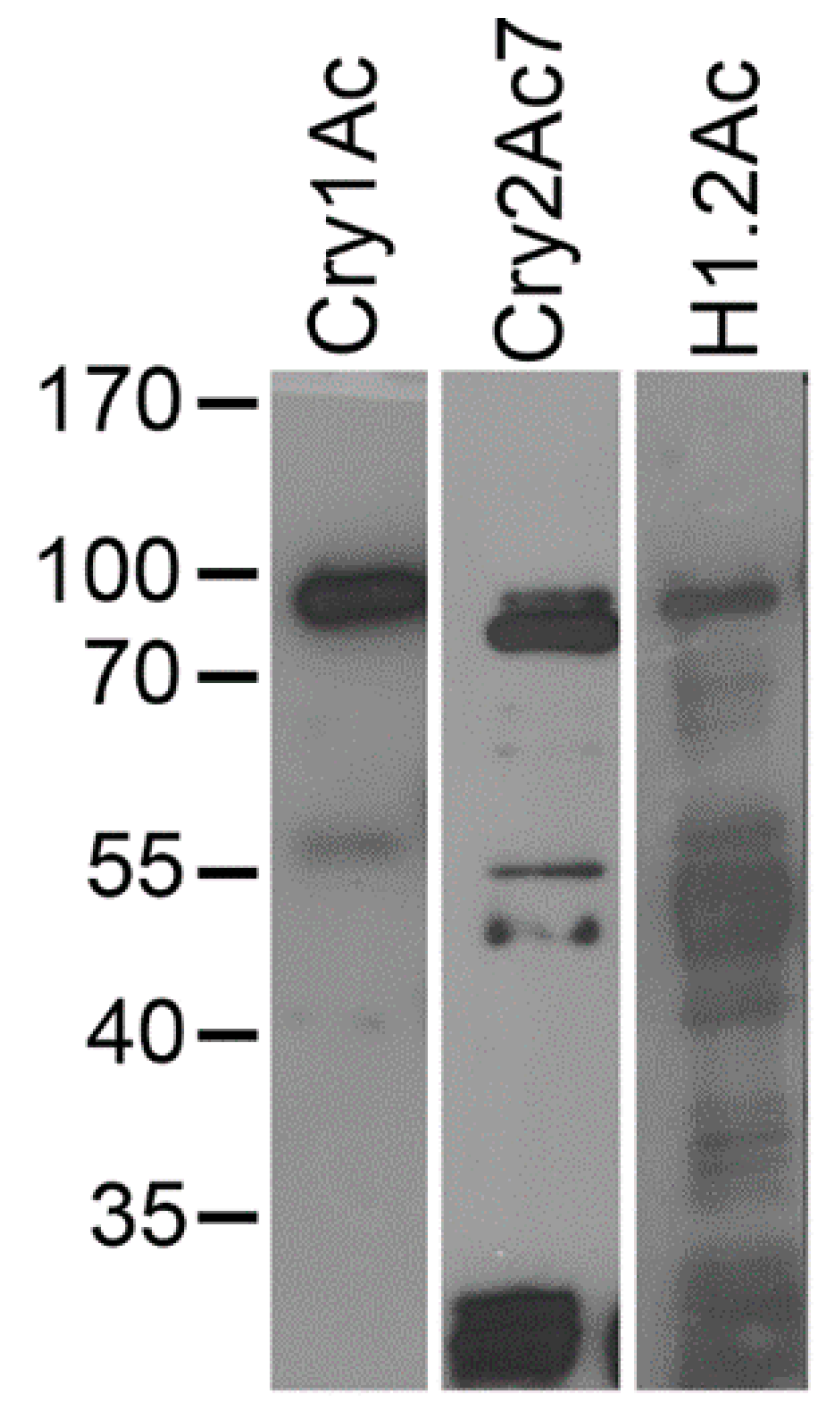
2.5. Ligand Blots of Biotinylated Toxins
3. Discussion
4. Materials and Methods
4.1. Cloning of H1.2Ac Hybrid
4.2. Over-Expression and Purification of Recombinant Protoxins
4.3. Activation and Purification of the Recombinant Toxins
4.4. Insect Bioassays
4.5. Preparation of Brush Border Membrane Vesicles
4.6. Radiolabeling and Biotinylation of Cry Proteins
4.7. Binding and Competition Assays
4.8. Ligand and Western Blotting
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Betz, F.S.; Hammond, B.G.; Fuchs, R.L. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharmacol. 2000, 32, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Adang, M.; Crickmore, N.; Jurat-Fuentes, J.L. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. In Advances in Insect Physiology Vol. 47: Insect Midgut and Insecticidal Proteins; Dhadialla, T.S., Gill, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 39–87. [Google Scholar]
- Dean, D.H.; Rajamohan, F.; Lee, M.K.; Wu, S.J.; Chen, X.J.; Alcantara, E.; Hussain, S.R. Probing the mechanism of action of Bacillus thuringiensis insecticidal proteins by site-directed mutagenesis—A minireview. Gene 1996, 179, 111–117. [Google Scholar] [CrossRef]
- De Maagd, R.A.; Bakker, P.L.; Masson, L.; Adang, M.J.; Sangadala, S.; Stiekema, W.; Bosch, D. Domain III of the Bacillus thuringiensis delta-endotoxin Cry1Ac is involved in binding to Manduca sexta brush border membranes and to its purified aminopeptidase N. Mol. Microbiol. 1999, 31, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rang, C.; Vachon, V.; de Maagd, R.A.; Villalon, M.; Schwartz, J.L.; Bosch, D.; Frutos, R.; Laprade, R. Interaction between functional domains of Bacillus thuringiensis insecticidal crystal proteins. Appl. Environ. Microbiol. 1999, 65, 2918–2925. [Google Scholar] [PubMed]
- De Maagd, R.A.; Weemen-Hendriks, M.; Stiekema, W.; Bosch, D. Bacillus thuringiensis delta-endotoxin Cry1C domain III can function as a specificity determinant for Spodoptera exigua in different, but not all, Cry1-Cry1C hybrids. Appl. Environ. Microbiol. 2000, 66, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- De Maagd, R.A.; Weemen-Hendriks, M.; Molthoff, J.W.; Naimov, S. Activity of wild-type and hybrid Bacillus thuringiensis delta-endotoxins against Agrotis ipsilon. Arch. Microbiol. 2003, 179, 363–367. [Google Scholar] [CrossRef] [PubMed]
- De Maagd, R.A.; Kwa, M.S.; van der Klei, H.; Yamamoto, T.; Schipper, B.; Vlak, J.M.; Stiekema, W.J.; Bosch, D. Domain III substitution in Bacillus thuringiensis delta-endotoxin CryIA(b) results in superior toxicity for Spodoptera exigua and altered membrane protein recognition. Appl. Environ. Microbiol. 1996, 62, 1537–1543. [Google Scholar] [PubMed]
- Karlova, R.; Weemen-Hendriks, M.; Naimov, S.; Ceron, J.; Dukiandjiev, S.; de Maagd, R.A. Bacillus thuringiensis delta-endotoxin Cry1Ac domain III enhances activity against Heliothis virescens in some, but not all Cry1-Cry1Ac hybrids. J. Invertebr. Pathol. 2005, 88, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Ayra, C.; Rodríguez, L.; Trujillo, D.; Fernández, Y.; Ponce, M.; Morán, I.; Téllez, P. Broadening the target host range of the insecticidal Cry1Ac1 toxin from Bacillus thuringiensis by biotechnological means. Biotecnol. Apl. 2008, 25, 270–275. [Google Scholar]
- Walters, F.S.; de Fontes, C.M.; Hart, H.; Warren, G.W.; Chen, J.S. Lepidopteran-active variable-region sequence imparts coleopteran activity in eCry3.1Ab, an engineered Bacillus thuringiensis hybrid insecticidal protein. Appl. Environ. Microbiol. 2010, 76, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, O.; Malvestiti, G.S.; Dourado, P.M.; Oliveira, W.S.; Martinelli, S.; Berger, G.U.; Head, G.P.; Omoto, C. Assessment of the high-dose concept and level of control provided by MON 87701 × MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag. Sci. 2012, 68, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, R.; Behle, R.; Liu, R.; Niu, L.; Song, P.; Shakoori, A.R.; Jurat-Fuentes, J.L. Activity of Bacillus thuringiensis Cry1Ie2, Cry2Ac7, Vip3Aa11 and Cry7Ab3 proteins against Anticarsia gemmatalis, Chrysodeixis includens and Ceratoma trifurcata. J. Invertebr. Pathol. 2017, 150, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Burton, S.L.; Ellar, D.J.; Li, J.; Derbyshire, D.J. N-acetylgalactosamine on the putative insect receptor aminopeptidase N is recognised by a site on the domain III lectin-like fold of a Bacillus thuringiensis insecticidal toxin. J. Mol. Biol. 1999, 287, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.R.; All, J.N.; McPherson, R.M.; Boerma, H.R.; Parrott, W.A. Field evaluation of soybean engineered with a synthetic cry1Ac transgene for resistance to corn earworm, soybean looper, velvetbean caterpillar (Lepidoptera: Noctuidae), and lesser cornstalk borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 2000, 93, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Jurat-Fuentes, J.L.; Adang, M.J. Characterization of a Cry1Ac-receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Eur. J. Biochem. 2004, 271, 3127–3135. [Google Scholar] [CrossRef] [PubMed]
- Pardo-López, L.; Gómez, I.; Rausell, C.; Sanchez, J.; Soberón, M.; Bravo, A. Structural changes of the Cry1Ac oligomeric pre-pore from Bacillus thuringiensis induced by N-acetylgalactosamine facilitates toxin membrane insertion. Biochemistry 2006, 45, 10329–10336. [Google Scholar] [CrossRef] [PubMed]
- Bel, Y.; Sheets, J.J.; Tan, S.Y.; Narva, K.E.; Escriche, B. Toxicity and binding studies of Bacillus thuringiensis Cry1Ac, Cry1F, Cry1C and Cry2A proteins in the soybean pests Anticarsia gemmatalis and Chrysodeixis (Pseudoplusia) includens. Appl. Environ. Microbiol. 2017, 83, e00326-17. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Zhang, Y.; Ding, X.; Hu, S.; Sun, Y.; Yu, Z.; Liu, S.; Zhu, Z.; Xia, L. A Cry1Ac toxin variant generated by directed evolution has enhanced toxicity against lepidopteran insects. Curr. Microbiol. 2011, 62, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.; Tabashnik, B.E.; Mazza, A.; Préfontaine, G.; Potvin, L.; Brousseau, R.; Schwartz, J.L. Mutagenic analysis of a conserved region of domain III in the Cry1Ac toxin of Bacillus thuringiensis. Appl. Environ. Microbiol. 2002, 68, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; You, T.H.; Gould, F.L.; Dean, D.H. Identification of residues in domain III of Bacillus thuringiensis Cry1Ac toxin that affect binding and toxicity. Appl. Environ. Microbiol. 1999, 65, 4513–4520. [Google Scholar] [PubMed]
- Daniel, A.; Sangadala, S.; Dean, D.H.; Adang, M.J. Denaturation of either Manduca sexta aminopeptidase N or Bacillus thuringiensis Cry1A toxins exposes binding epitopes hidden under nondenaturing conditions. Appl. Environ. Microbiol. 2002, 68, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Gouffon, C.; Van Vliet, A.; Van Rie, J.; Jansens, S.; Jurat-Fuentes, J.L. Binding sites for Bacillus thuringiensis Cry2Ae toxin on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A toxin. Appl. Environ. Microbiol. 2011, 77, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- LeOra, S. Polo-Plus, POLO for Windows; LeOra Software: Petaluma, CA, USA, 1987. [Google Scholar]
- Wolfersberger, M.; Luthy, P.; Maurer, A.; Parenti, P.; Sacchi, V.F.; Giordana, B.; Hanozet, G.M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1987, 86A, 301–308. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Gould, F.L.; Adang, M.J. Altered Glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. Appl. Environ. Microbiol. 2002, 68, 5711–5717. [Google Scholar] [CrossRef] [PubMed]
- Jurat-Fuentes, J.L.; Adang, M.J. Importance of Cry1 delta-endotoxin domain II loops for binding specificity in Heliothis virescens (L.). Appl. Environ. Microbiol. 2001, 67, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jakka, S.R.; Gong, L.; Hasler, J.; Banerjee, R.; Sheets, J.J.; Narva, K.; Blanco, C.A.; Jurat-Fuentes, J.L. Field-evolved Mode 1 resistance of the fall armyworm to transgenic Cry1Fa-expressing corn associated with reduced Cry1Fa toxin binding and midgut alkaline phosphatase expression. Appl. Environ. Microbiol. 2015, 82, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
| Toxin | A. gemmatalis | C. includens | Source b |
|---|---|---|---|
| H1.2Ac | 10.20 (1.57–20.80) | NA c | this study |
| Cry1Ac | 20.31 (11.09–27.90) | 109.48 (52.85–179.50) | [13] |
| Cry2Ac7 | 46.53 (5.70–102.60) | 214.18(109.71–315.91) | [13] |
| Primer | Sequence (5′-3′) | Restriction Enzyme | Tm |
|---|---|---|---|
| 1AcFD1 | TGGAGGTCATATGGATAACAATCCGAACATC | NdeI | 69.7 |
| 1AcRD2 | GTAGTCGACTTCAGCACTACGATGTATCCA | SalI | 70.8 |
| 2AcFD3 | TCTGTCGACTTCACCGTATCTCCAATACATGCC | SalI | 73.0 |
| 2AcRD3 | TGTCTCGAGTTAATACAGTGGTGGAAGGTTAG | XhoI | 71.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushtaq, R.; Shakoori, A.R.; Jurat-Fuentes, J.L. Domain III of Cry1Ac Is Critical to Binding and Toxicity against Soybean Looper (Chrysodeixis includens) but Not to Velvetbean Caterpillar (Anticarsia gemmatalis). Toxins 2018, 10, 95. https://doi.org/10.3390/toxins10030095
Mushtaq R, Shakoori AR, Jurat-Fuentes JL. Domain III of Cry1Ac Is Critical to Binding and Toxicity against Soybean Looper (Chrysodeixis includens) but Not to Velvetbean Caterpillar (Anticarsia gemmatalis). Toxins. 2018; 10(3):95. https://doi.org/10.3390/toxins10030095
Chicago/Turabian StyleMushtaq, Rubina, Abdul Rauf Shakoori, and Juan Luis Jurat-Fuentes. 2018. "Domain III of Cry1Ac Is Critical to Binding and Toxicity against Soybean Looper (Chrysodeixis includens) but Not to Velvetbean Caterpillar (Anticarsia gemmatalis)" Toxins 10, no. 3: 95. https://doi.org/10.3390/toxins10030095





