Monocyte Response to Different Campylobacter jejuni Lysates Involves Endoplasmic Reticulum Stress and the Lysosomal–Mitochondrial Axis: When Cell Death Is Better Than Cell Survival
Abstract
:1. Introduction
2. Results
2.1. Morphological Features, Cell Death, and Absolute Count
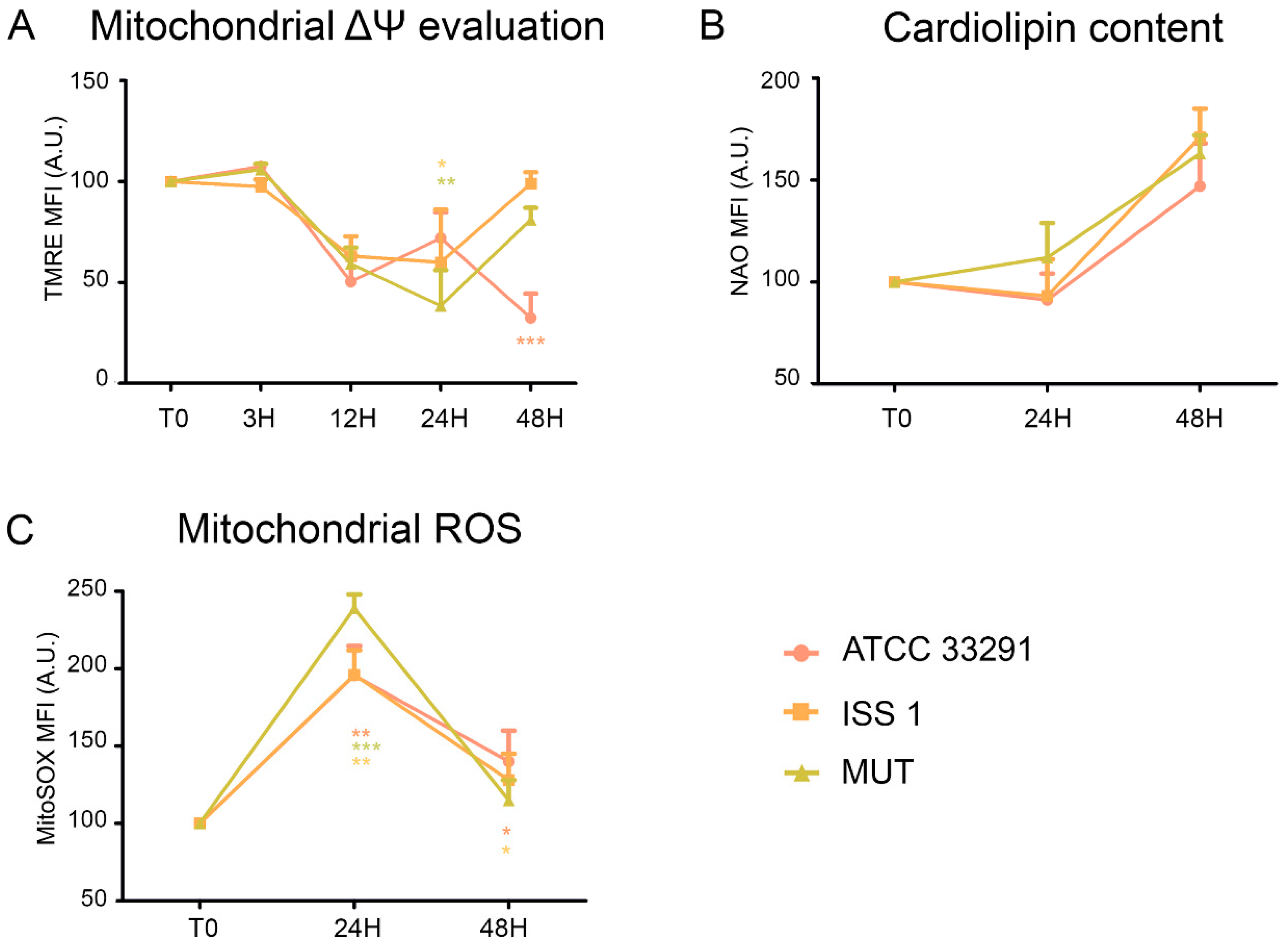
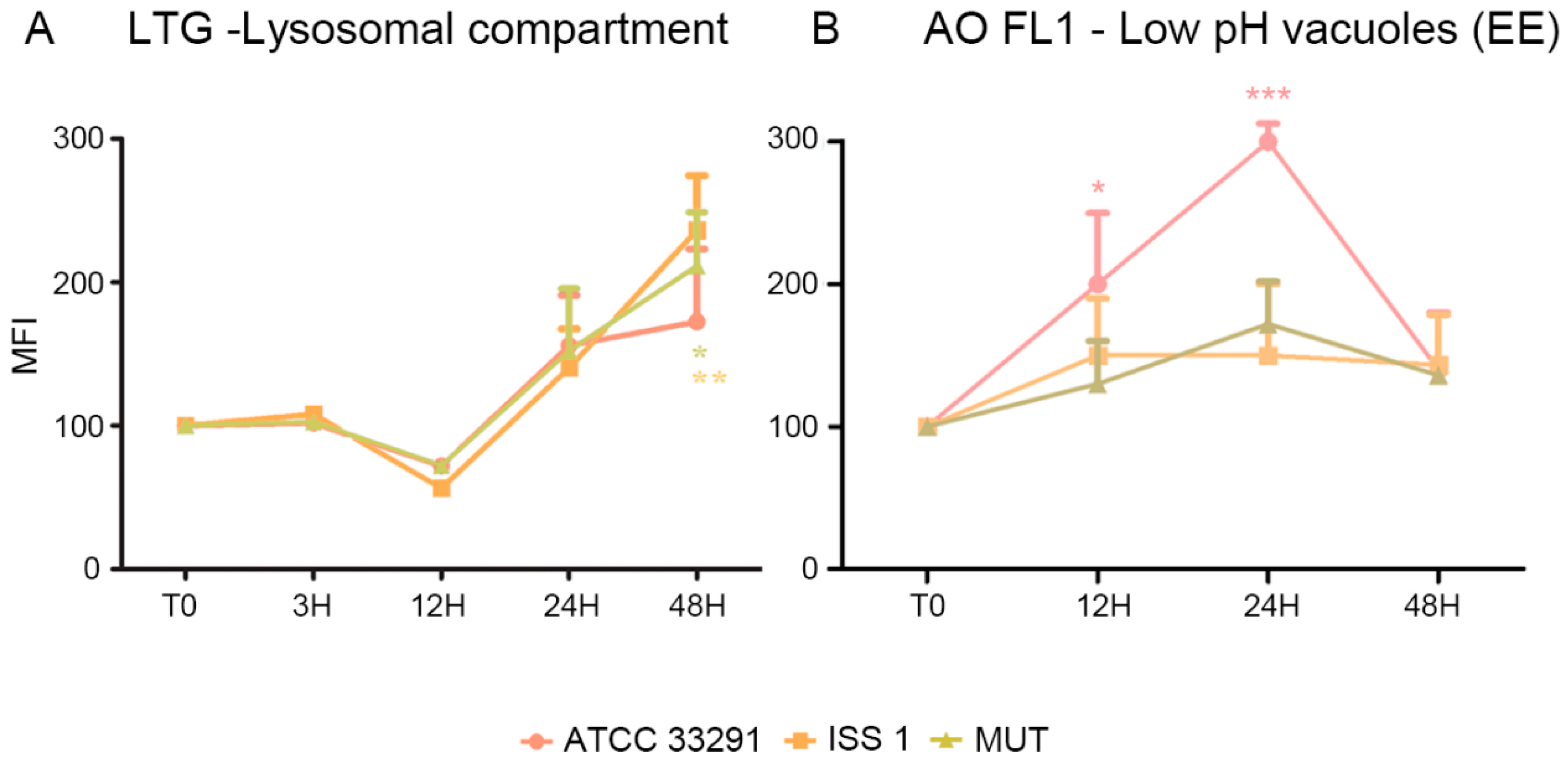
2.2. Mitochondrial and Lysosomal Alterations
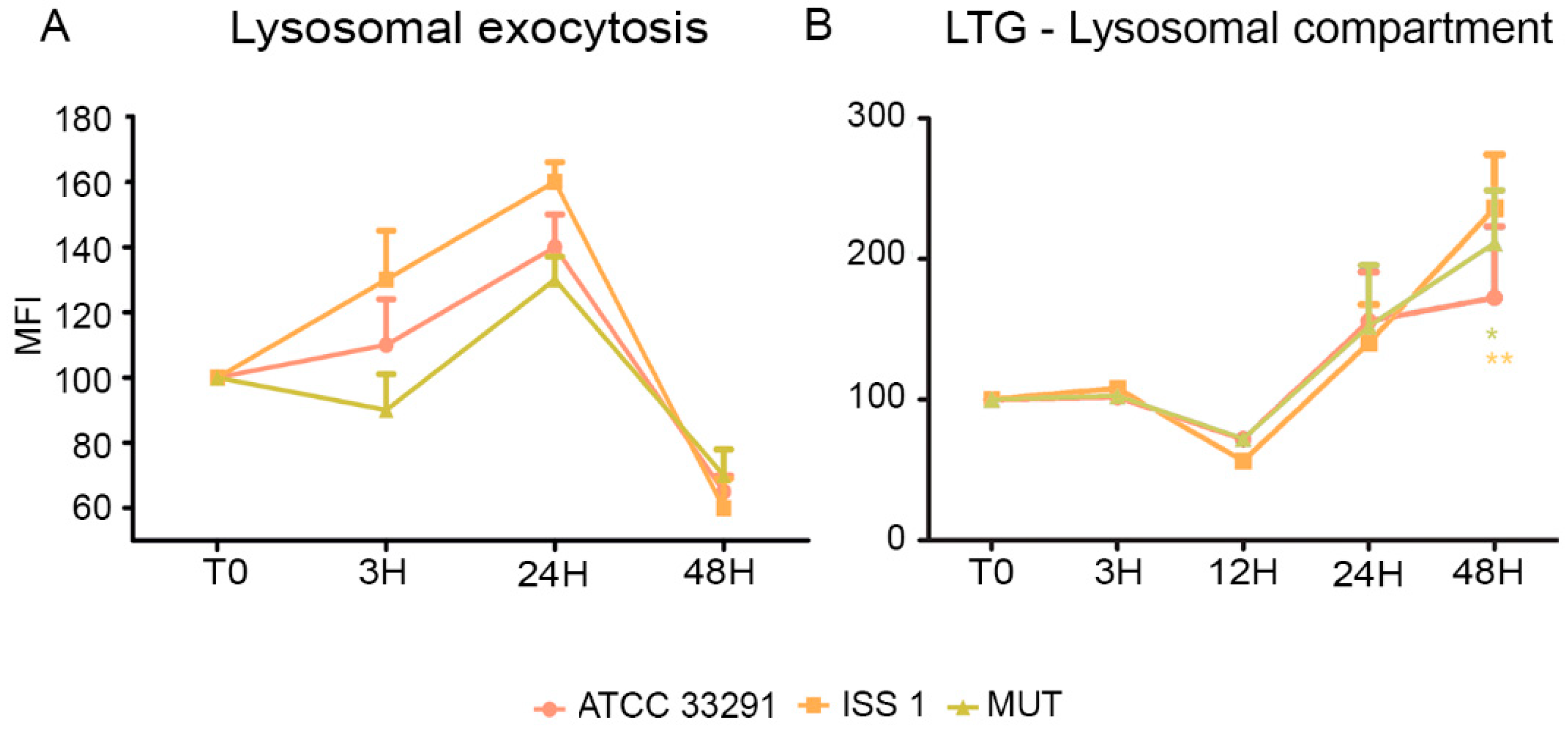
2.3. Surface LAMP-1 Expression: Lysosomal Exocytosis
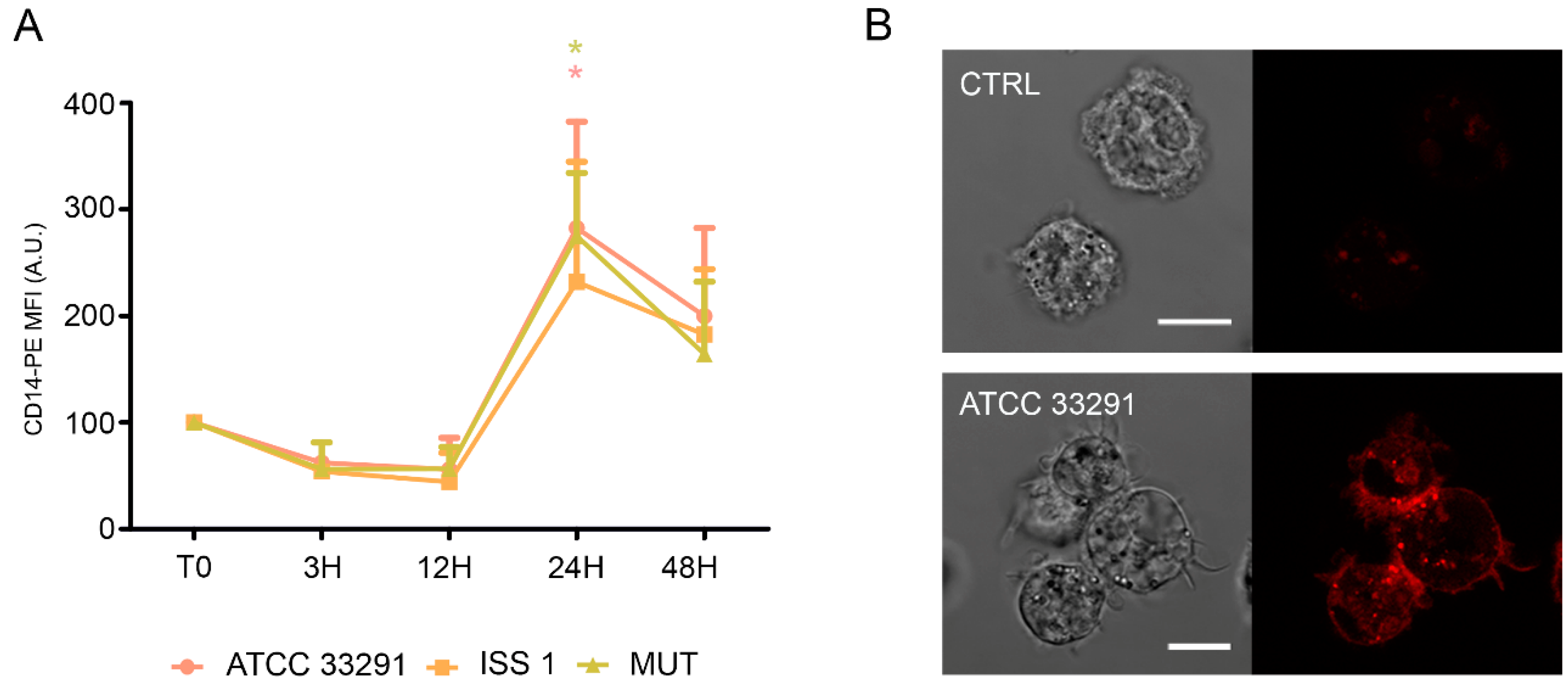
2.4. CD14 and ICAM-1 (CD54) Variations
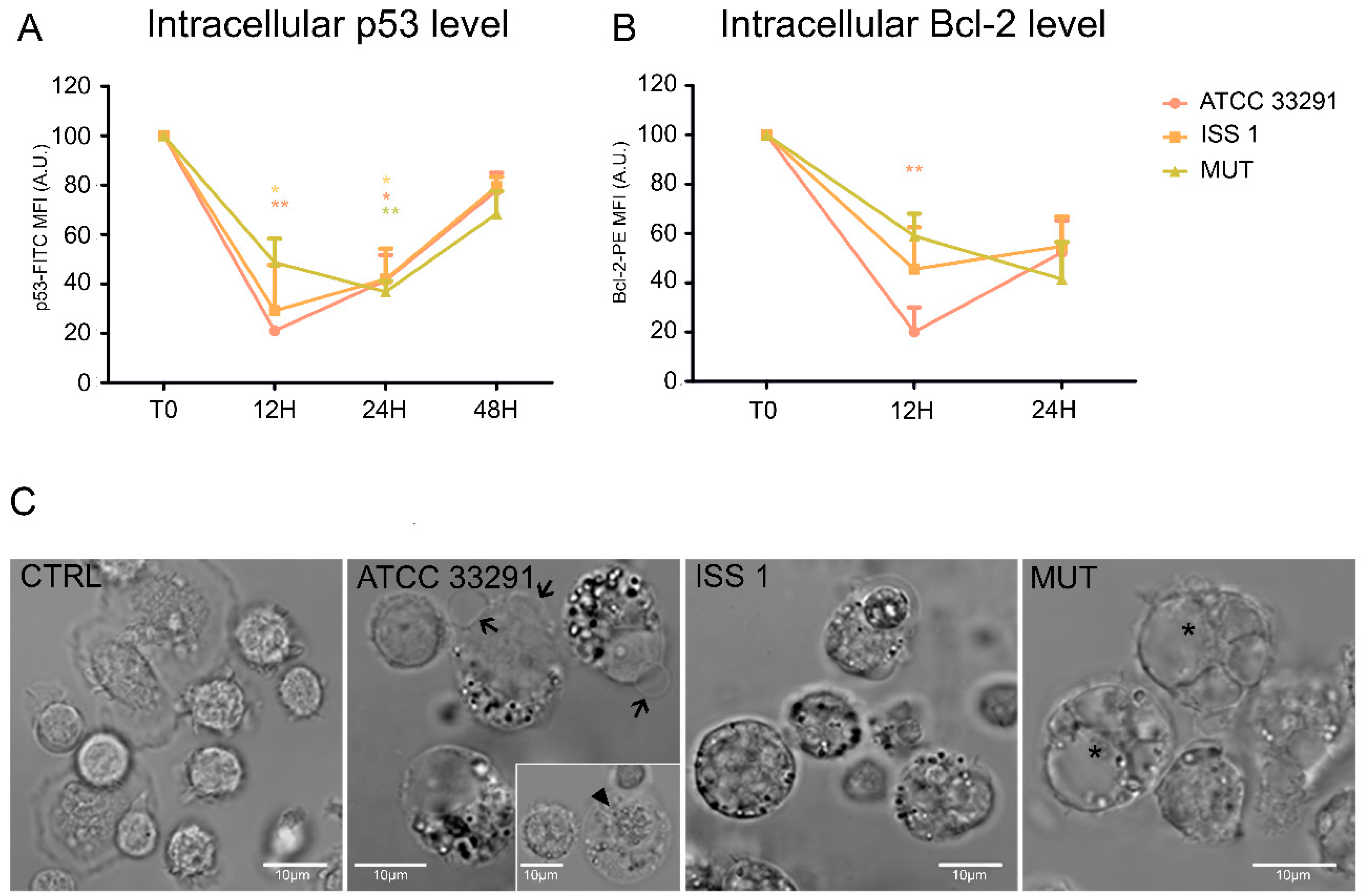
2.5. p53 and Bcl-2 Detection
2.6. ER Stress and Modulation of Intracellular Ca2+ Levels
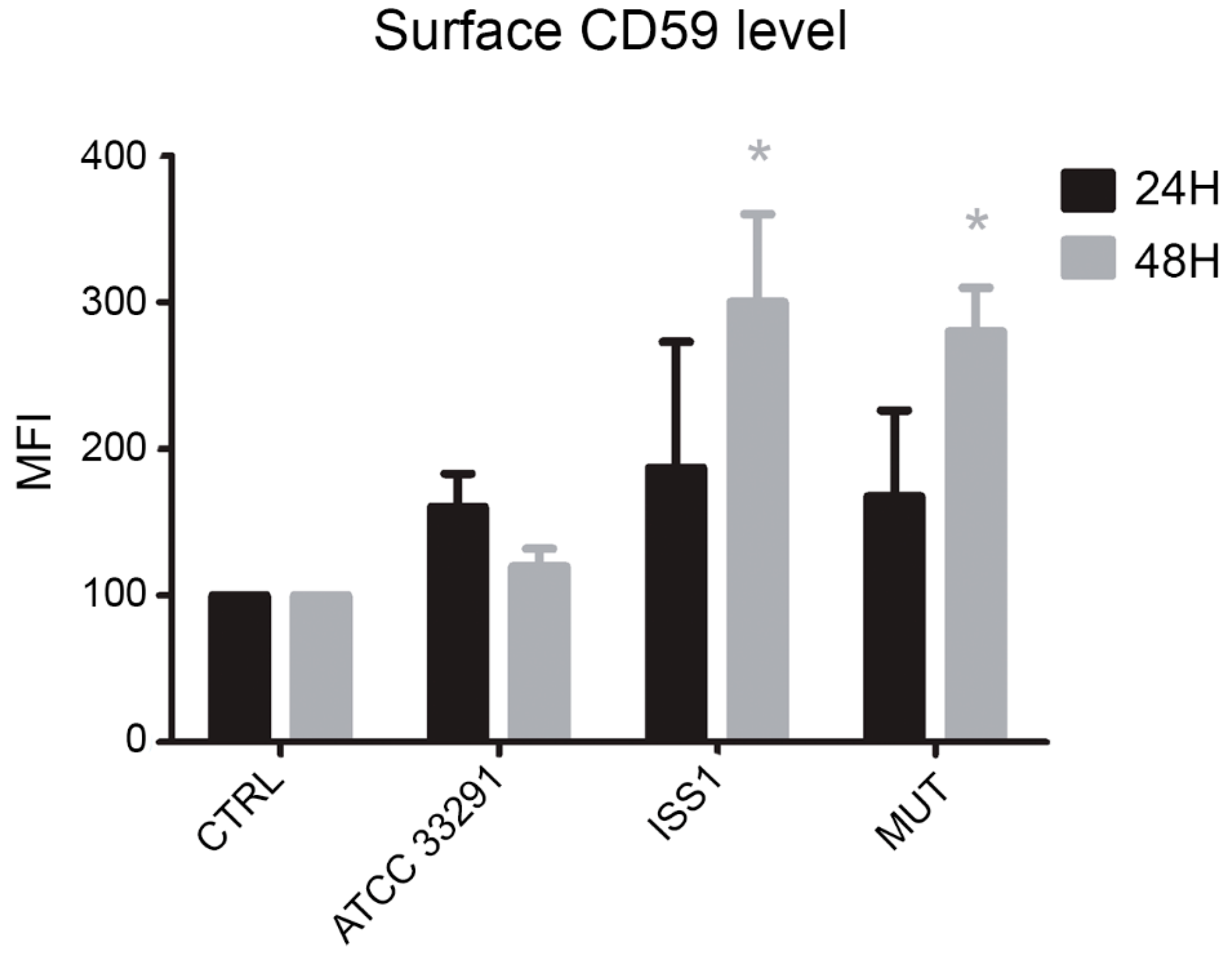
2.7. Surface CD59 Expression
3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. Growth Conditions
4.3. Cell Lines and Monocytes Isolation
4.4. C. jejuni Cell Lysate Preparation
4.5. Pretreatment of the Monocytes with C. jejuni Lysates
4.6. Morphological Feature Evaluation
4.7. Flow Cytometry and Confocal Microscopy Stainings
4.7.1. Flow Cytometric Detection of Cell Death and Flow Cytometric Absolute Count
4.7.2. Determination of Mitochondrial Potential (ΔΨm), Mitochondrial Mass, and Mitochondrial Reactive Oxygen Species (ROS)
4.7.3. Assessment of Lysosomal Involvement
4.7.4. ER Stress Evaluation
4.7.5. Intracellular Ca2+ Levels Detection
4.7.6. Surface Receptor Expressions Evaluation
4.7.7. Intracellular Detection of Bcl-2 and p53 Antigens
4.7.8. LAMP-1 Surface Expression (Lysosomal Exocytosis Assay)
4.7.9. Cytometric Investigations
4.7.10. Confocal Microscopy Analyses
4.8. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ketley, J.M. Pathogenesis of enteric infection by campylobacter. Microbiology 1997, 143, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Young, K.T.; Davis, L.M.; DiRita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Hoy, B.; Rohde, M.; Tegtmeyer, N.; Baek, K.T.; Oyarzabal, O.A.; Brondsted, L.; Wessler, S.; Backert, S. Rapid paracellular transmigration of campylobacter jejuni across polarized epithelial cells without affecting ter: Role of proteolytic-active htra cleaving e-cadherin but not fibronectin. Gut Pathog. 2012, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Ó Cróinín, T.; Backert, S. Host epithelial cell invasion by campylobacter jejuni: Trigger or zipper mechanism? Front. Cell. Infect. Microbiol. 2012, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Tian, F.F.; Liu, H.; Zhang, W.; Li, J.; Xiao, B.; Zhou, W.B. Macrophage migration inhibitory factor is necessary for the lipo-oligosaccharide-induced response by modulation of toll-like receptor 4 in monocytes from gbs patients. J. Neuroimmunol. 2013, 257, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, I.; Bleumink-Pluym, N.M.; Van Putten, J.P.; Van den Berg, L.H. Campylobacter DNA is present in circulating myelomonocytic cells of healthy persons and in persons with guillain-barre syndrome. J. Infect. Dis. 2002, 185, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Nyati, K.K.; Nyati, R. Role of campylobacter jejuni infection in the pathogenesis of guillain-barre syndrome: An update. BioMed Res. Int. 2013, 2013, 852195. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Nasher, F.; Jagatia, H.; Gundogdu, O.; Bajaj-Elliott, M.; Wren, B.; Dorrell, N. Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell e-cadherin and occludin. Cell. Microbiol. 2016, 18, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Yuki, N.; Hartung, H.P. Guillain-barre syndrome. N. Engl. J. Med. 2012, 366, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, S. Guillain-barre syndrome: Epidemiology, pathophysiology and management. Drugs 2004, 64, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The biology of the cytolethal distending toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Bayles, D.O. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit. Rev. Microbiol. 2006, 32, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.A.; Dreyfus, L.A. Dnase i homologous residues in cdtb are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 2000, 37, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Lara-Tejero, M.; Galan, J.E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease i-like protein. Science 2000, 290, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Dlakic, M. Is cdtb a nuclease or a phosphatase? Science 2001, 291, 547. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Tomiuk, S.; Wolff, G.; Stoffel, W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc. Natl. Acad. Sci. USA 2000, 97, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Canonico, B.; Campana, R.; Luchetti, F.; Arcangeletti, M.; Betti, M.; Cesarini, E.; Ciacci, C.; Vittoria, E.; Galli, L.; Papa, S.; et al. Campylobacter jejuni cell lysates differently target mitochondria and lysosomes on hela cells. Apoptosis 2014, 19, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Aaronson, S.A.; Abrams, J.; Alnemri, E.S.; Andrews, D.W.; Baehrecke, E.H.; Bazan, N.G.; Blagosklonny, M.V.; Blomgren, K.; Borner, C.; et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009, 16, 1093–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distelhorst, C.W.; McCormick, T.S. Bcl-2 acts subsequent to and independent of Ca2+ fluxes to inhibit apoptosis in thapsigargin- and glucocorticoid-treated mouse lymphoma cells. Cell Calcium 1996, 19, 473–483. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic bax and bak: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Cohen, I.; Zamzami, N.; Vieira, H.L.; Kroemer, G. Endoplasmic reticulum stress-induced cell death requires mitochondrial membrane permeabilization. Cell Death Differ. 2002, 9, 465–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacki, J.; Egger, L.; Monney, L.; Conus, S.; Rosse, T.; Fellay, I.; Borner, C. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by bcl-2. Oncogene 2000, 19, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Li, C.; Hatzivassiliou, G.; Lindsten, T.; Yu, Q.C.; Yuan, J.; Thompson, C.B. Bax and bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003, 162, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Menendez, D.; Inga, A.; Resnick, M.A. The expanding universe of p53 targets. Nat. Rev. Cancer 2009, 9, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Siegl, C.; Rudel, T. Modulation of p53 during bacterial infections. Nat. Rev. Microbiol. 2015, 13, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Fridman, J.S.; Yang, M.; Baranov, E.; Hoffman, R.M.; Lowe, S.W. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 2002, 1, 289–298. [Google Scholar] [CrossRef]
- Nalca, A.; Rangnekar, V.M. The g1-phase growth-arresting action of interleukin-1 is independent of p53 and p21/waf1 function. J. Biol. Chem. 1998, 273, 30517–30523. [Google Scholar] [CrossRef] [PubMed]
- Sampaziotis, F.; Kokotas, S.; Gorgoulis, V.G. P53 possibly upregulates the expression of cd58 (lfa-3) and cd59 (mirl). Med. Hypotheses 2002, 58, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Marudamuthu, A.S.; Tsukasaki, Y.; Ikebe, M.; Fu, J.; Shetty, S. P53- and pai-1-mediated induction of c-x-c chemokines and cxcr2: Importance in pulmonary inflammation due to cigarette smoke exposure. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L496–L506. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.G.; Zacharatos, P.; Kotsinas, A.; Kletsas, D.; Mariatos, G.; Zoumpourlis, V.; Ryan, K.M.; Kittas, C.; Papavassiliou, A.G. P53 activates icam-1 (cd54) expression in an nf-kappab-independent manner. EMBO J. 2003, 22, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Eckmann, L.; Savidge, T.C.; Kagnoff, M.F. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (icam)-1) expression and neutrophil adhesion. J. Clin. Investig. 1996, 98, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.C.; Appelqvist, H.; Nilsson, C.; Kagedal, K.; Roberg, K.; Ollinger, K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 2010, 15, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, P.L. Repairing a torn cell surface: Make way, lysosomes to the rescue. J. Cell Sci. 2002, 115, 873–879. [Google Scholar] [PubMed]
- Kannan, K.; Stewart, R.M.; Bounds, W.; Carlsson, S.R.; Fukuda, M.; Betzing, K.W.; Holcombe, R.F. Lysosome-associated membrane proteins h-lamp1 (cd107a) and h-lamp2 (cd107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells which mediate cell adhesion to vascular endothelium. Cell. Immunol. 1996, 171, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, B.J.; Lip, G.Y.; Shantsila, E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur. J. Heart Fail. 2011, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2007, 21, 335–376. [Google Scholar]
- Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Huber, M.; Kalis, C.; Keck, S.; Galanos, C.; et al. Cd14 is required for myd88-independent lps signaling. Nat. Immunol. 2005, 6, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, R.; Akashi, S.; Ogata, H.; Nagai, Y.; Fukudome, K.; Miyake, K.; Kimoto, M. Md-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J. Exp. Med. 1999, 189, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Murciano, J.C.; Muro, S.; Koniaris, L.; Christofidou-Solomidou, M.; Harshaw, D.W.; Albelda, S.M.; Granger, D.N.; Cines, D.B.; Muzykantov, V.R. Icam-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood 2003, 101, 3977–3984. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Serrano, D.; Bhowmick, T.; Kumar, K.; Shen, Y.; Kuo, Y.C.; Garnacho, C.; Muro, S. Enhanced endothelial delivery and biochemical effects of alpha-galactosidase by icam-1-targeted nanocarriers for fabry disease. J. Control Release 2011, 149, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Garnacho, C.; Dhami, R.; Simone, E.; Dziubla, T.; Leferovich, J.; Schuchman, E.H.; Muzykantov, V.; Muro, S. Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J. Pharmacol. Exp. Ther. 2008, 325, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Muro, S. Strategies for delivery of therapeutics into the central nervous system for treatment of lysosomal storage disorders. Drug Deliv. Transl. Res. 2012, 2, 169–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, A.J.; Huang, S.L.; Warnick, D.; Rabbat, M.; Kane, B.; Nagaraj, A.; Klegerman, M.; McPherson, D.D. Intravascular ultrasound molecular imaging of atheroma components in vivo. J. Am. Coll. Cardiol. 2004, 43, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, G.E.; Villanueva, F.S.; Tom, E.M.; Wagner, W.R. Targeted ultrasound contrast agents: In vitro assessment of endothelial dysfunction and multi-targeting to icam-1 and sialyl lewisx. Biotechnol. Bioeng. 2005, 92, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Kim, S.H.; Cai, Q.Y.; Kim, S.Y.; Kim, H.O.; Lee, H.J.; Kim, E.A.; Yoon, S.E.; Yun, K.J.; Yoon, K.H. Inflammation-specific t1 imaging using anti-intercellular adhesion molecule 1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid. Mol. Imaging 2007, 6, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chittasupho, C.; Duangrat, C.; Siahaan, T.J.; Berkland, C. Plga nanoparticle--peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug Chem. 2008, 19, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, R.C.; Auguste, D.T. Immunoliposomes that target endothelium in vitro are dependent on lipid raft formation. Mol. Pharm. 2010, 7, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Veach, A.J.; Vedvyas, Y.; Zarnegar, R.; Kim, J.Y.; Jin, M.M. Self-assembled nanoplatform for targeted delivery of chemotherapy agents via affinity-regulated molecular interactions. Biomaterials 2010, 31, 7766–7775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, T.; Zhou, L.; Maldonado, M.A.; Madaio, M.P.; Eisenberg, R.A.; Song, W.C. Absence of cd59 exacerbates systemic autoimmunity in mrl/lpr mice. J. Immunol. 2012, 189, 5434–5441. [Google Scholar] [CrossRef] [PubMed]
- Reeves, V.L.; Thomas, C.M.; Smart, E.J. Lipid rafts, caveolae and gpi-linked proteins. Adv. Exp. Med. Biol. 2012, 729, 3–13. [Google Scholar] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P.; Sansonetti, P.J. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science 2004, 304, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.D.; Huynh, M.M.; Tamilselvam, B.; Spiegelman, L.M.; Son, S.B.; Eshraghi, A.; Blanke, S.R.; Bradley, K.A. Distinct roles for cdta and cdtc during intoxication by cytolethal distending toxins. PLoS ONE 2015, 10, e0143977. [Google Scholar] [CrossRef] [PubMed]
- Marshansky, V.; Futai, M. The V-type H+-Atpase in vesicular trafficking: Targeting, regulation and function. Curr. Opin. Cell Biol. 2008, 20, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, S.; Schmidt, M.; August, C.; Cullen, P.; Rademaekers, A.; Pauels, H.G. Regulation of human monocyte apoptosis by the cd14 molecule. J. Immunol. 1997, 159, 3178–3188. [Google Scholar] [PubMed]
- Etienne-Manneville, S.; Manneville, J.-B.; Adamson, P.; Wilbourn, B.; Greenwood, J.; Couraud, P.-O. Icam-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J. Immunol. 2000, 165, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Tamai, R.; Asai, Y.; Ogawa, T. Requirement for intercellular adhesion molecule 1 and caveolae in invasion of human oral epithelial cells by porphyromonas gingivalis. Infect. Immun. 2005, 73, 6290–6298. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, P.; Welter-Stahl, L.; Ying, S.; Hansen, J.; Häcker, G.; Darville, T.; Ojcius, D.M. Recruitment of bad by the chlamydia trachomatis vacuole correlates with host-cell survival. PLoS Pathog. 2006, 2, e45. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.M. The unfolded protein response and cellular stress, part c. Preface. Methods Enzymol. 2011, 491, xix. [Google Scholar] [PubMed]
- Hüttemann, M.; Helling, S.; Sanderson, T.H.; Sinkler, C.; Samavati, L.; Mahapatra, G.; Varughese, A.; Lu, G.; Liu, J.; Ramzan, R.; et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 598–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierzynska-Mach, A.; Janowski, P.A.; Dobrucki, J.W. Evaluation of acridine orange, lysotracker red, and quinacrine as fluorescent probes for long-term tracking of acidic vesicles. Cytom. Part A 2014, 85, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Gruenberg, J. Ion flux and the function of endosomes and lysosomes: Ph is just the start: The flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal ph. Bioessays 2011, 33, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, A.M.; Trost, M.; Beyaert, R.; Hoffmann, E. Patterns, receptors, and signals: Regulation of phagosome maturation. Trends Immunol. 2017, 38, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.D.; Xavier, J.M.; Steer, C.J.; Rodrigues, C.M. The role of p53 in apoptosis. Discov. Med. 2010, 9, 145–152. [Google Scholar] [PubMed]
- Zaika, A.I.; Wei, J.; Noto, J.M.; Peek, R.M. Microbial regulation of p53 tumor suppressor. PLoS Pathog. 2015, 11, e1005099. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, U.; Fan, X.; Jack, R.S.; Workalemahu, G.; Kallies, A.; Stelter, F.; Schutt, C. Monocytes can phagocytose gram-negative bacteria by a cd14-dependent mechanism. J. Immunol. 1996, 157, 4119–4125. [Google Scholar] [PubMed]
- Comrie, W.A.; Li, S.; Boyle, S.; Burkhardt, J.K. The dendritic cell cytoskeleton promotes t cell adhesion and activation by constraining icam-1 mobility. J. Cell Biol. 2015, 208, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Behme, D.; Pantke, M.; Reiling, N.; Binder, C.; Pukrop, T.; Klemm, F. Isolation of human monocytes by double gradient centrifugation and their differentiation to macrophages in teflon-coated cell culture bags. J. Vis. Exp. 2014, 91, e51554. [Google Scholar] [CrossRef] [PubMed]
- Brando, B.; Barnett, D.; Janossy, G.; Mandy, F.; Autran, B.; Rothe, G.; Scarpati, B.; D’Avanzo, G.; D’Hautcourt, J.L.; Lenkei, R.; et al. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European working group on clinical cell analysis. Cytometry 2000, 42, 327–346. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Arcangeletti, M.; Guescini, M.; Cesarini, E.; Stocchi, V.; Degli Esposti, M.; Papa, S. Fas signalling promotes intercellular communication in t cells. PLoS ONE 2012, 7, e35766. [Google Scholar] [CrossRef] [PubMed]
- Canonico, B.; Cesarini, E.; Salucci, S.; Luchetti, F.; Falcieri, E.; Di Sario, G.; Palma, F.; Papa, S. Defective autophagy, mitochondrial clearance and lipophagy in niemann-pick type b lymphocytes. PLoS ONE 2016, 11, e0165780. [Google Scholar] [CrossRef] [PubMed]
- Chazotte, B. Labeling lysosomes in live cells with lysotracker. Cold Spring Harb. Protoc. 2011, 2011, pdb.prot5571. [Google Scholar] [CrossRef] [PubMed]
- Traganos, F.; Darzynkiewicz, Z. Lysosomal proton pump activity: Supravital cell staining with acridine orange differentiates leukocyte subpopulations. Methods Cell Biol. 1994, 41, 185–194. [Google Scholar] [PubMed]
- Hogg, R.C.; Adams, D.J. An atp-sensitive k(+) conductance in dissociated neurones from adult rat intracardiac ganglia. J. Physiol. 2001, 534, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, M.; Khodaei, N.; Salari, S.; Eliassi, A.; Saghiri, R. Gating behavior of endoplasmic reticulum potassium channels of rat hepatocytes in diabetes. Iran. Biomed. J. 2014, 18, 165–172. [Google Scholar] [PubMed]
- Li, H.; Mao, G.; Carlson, J.; Leng, S.X. A novel flow cytometry-based tool for determining the efficiency of human cytomegalovirus infection in thp-1 derived macrophages. J. Virol. Methods 2015, 221, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.W. Detection of lysosomal exocytosis by surface exposure of lamp1 luminal epitopes. In Lysosomes: Methods and Protocols; Öllinger, K., Appelqvist, H., Eds.; Springer: New York, NY, USA, 2017; pp. 205–211. [Google Scholar]
- Ivanov, A.I.; Nusrat, A.; Parkos, C.A. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 2004, 15, 176–188. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canonico, B.; Di Sario, G.; Cesarini, E.; Campana, R.; Luchetti, F.; Zamai, L.; Ortolani, C.; Nasoni, M.G.; Baffone, W.; Papa, S. Monocyte Response to Different Campylobacter jejuni Lysates Involves Endoplasmic Reticulum Stress and the Lysosomal–Mitochondrial Axis: When Cell Death Is Better Than Cell Survival. Toxins 2018, 10, 239. https://doi.org/10.3390/toxins10060239
Canonico B, Di Sario G, Cesarini E, Campana R, Luchetti F, Zamai L, Ortolani C, Nasoni MG, Baffone W, Papa S. Monocyte Response to Different Campylobacter jejuni Lysates Involves Endoplasmic Reticulum Stress and the Lysosomal–Mitochondrial Axis: When Cell Death Is Better Than Cell Survival. Toxins. 2018; 10(6):239. https://doi.org/10.3390/toxins10060239
Chicago/Turabian StyleCanonico, Barbara, Gianna Di Sario, Erica Cesarini, Raffaella Campana, Francesca Luchetti, Loris Zamai, Claudio Ortolani, Maria Gemma Nasoni, Wally Baffone, and Stefano Papa. 2018. "Monocyte Response to Different Campylobacter jejuni Lysates Involves Endoplasmic Reticulum Stress and the Lysosomal–Mitochondrial Axis: When Cell Death Is Better Than Cell Survival" Toxins 10, no. 6: 239. https://doi.org/10.3390/toxins10060239




