1. Introduction
Up to now, an antidote for tetrodotoxin (TTX) poisoning has not been developed. It is necessary to find an effective method to minimize the risks associated with clinical trials of TTX.
Many researchers proved that TTX was produced by symbiotic bacteria, and then accumulated in different organs of puffer fishes [
1,
2,
3,
4]. TTX was also found commonly in various types of animal including the California newt [
5], the goby [
6], the gastropod mollusks [
7], the xanthid crab [
8], and the blue-ringed octopus [
9]. TTX inhibits the initiation and conduction of potential action by selectively blocking sodium channels on the nerve and muscle membrane at extremely low concentrations [
10]. Furthermore, TTX-containing puffer fish meat was traditionally fermented before use in Japan [
11].
Lactic acid bacteria (LAB) are used as probiotics. Lactic acid bacteria can be divided into homopolysaccharides or heteropolysaccharides to protect themselves from unfavorable culture conditions such as desiccation, osmotic stress, antibiotics or toxic compounds, predation by protozoans, phagocytosis and phage attack [
12,
13]. There are varieties of heteropolysaccharide categorized according to their structure, composition, molecular mass, and functionalities. Exopolysaccharide (EPS) production from LAB is strongly influenced by culture conditions, resulting in different structures and functions [
14]. The EPS from
Streptococcus thermophilus CRL 1190 was found to be effective for preventing chronic gastritis [
15]. The EPS of
Lactobacillus paracasei subsp.
paracasei NTU 101 and
Lactobacillus plantarum NTU 102 demonstrated potential antioxidant properties [
16]. EPS can also interact with copper forming a complex structure and conferring specific activities in EPS [
17].
As the above stated above, the study investigated TTX detoxification using Lactobacilli exopolysaccharide.
2. Results
2.1. Identification of Leuconostoc mesenteroides N3
After checking the bacterium showing short chain of coccal shape under the microscope, bacterium was identified by biochemical tests and 16S rRNA sequencing analysis. As a result, the bacterium was found to be a facultative anaerobe. It can utilize and produce acid by fermenting d-glucose, mannitol, rhamnose, d-sorbitol, mannose, d-galactose, d-fructose, and 10% lactose. In addition, this bacterium appeared positive for indole, urease, amylolysis, phenylalanine deaminase, utilization of malonate, and methyl red tests, but negative for sucrose, maltose and l-arabinose, Voges–Proskauer reaction, gelatin liquefaction, tryptophan deaminase, ornithine decarboxylase, phenylalanine deaminase, and arginine decarboxylase. The partial 16S rRNA sequence was 425 bp deposited in DDBJ (accession number: LC066674). The partial 16S rRNA gene sequence was analyzed using NCBI BLAST, showing 99% of the homology to Leuconostoc mesenteroides. The isolated strain was identified as Leuconostoc mesenteroides N3.
2.2. EPS Isolation
The EPS yield of
Leuconostoc mesenteroides,
Lactobacillus plantarum, and
Lactobacillus rhamnosus was 140 mg, 150 mg, and 140 mg per 10
6 cfu/mL, respectively. However, the EPS solubility in water of isolated
Leuconostoc mesenteroides,
Lactobacillus plantarum, and
Lactobacillus rhamnosus was 9.88%, 67.73%, and 9.26%, respectively (
Table 1).
2.3. Monomer Detection
By thin layer chromatography (TLC) analysis, the EPS of
Leuconostoc mesenteroides,
Lactobacillus plantarum, and
Lactobacillus rhamnosus was constituted mostly by glucose. The R
f value of standard
d-glucose was 0.73, similarly to the spots of monomer from the EPS of
Leuconostoc mesenteroides,
Lactobacillus plantarum, and
Lactobacillus rhamnosus (
Figure 1).
The monomer detection was also confirmed by high-performance liquid chromatography (HPLC). The results of the HPLC showed that EPS from
Leuconostoc mesenteroides,
Lactobacillus plantarum, and
Lactobacillus rhamnosus consisted of
d-glucose and one unknown monomer. All hydrolyzed peaks appear at the same retention time of standard
d-glucose (
Table 2). Moreover, the ratio between
d-glucose and the unknown monomer was highest in the EPS of
Lactobacillus plantarum, then the EPS of
Leuconostoc mesenteroides, and finally lowest in the EPS of
Lactobacillus rhamnosus. These differences could result in a different TTX detoxification capacity.
2.4. Functional Group Detection
FTIR was performed for more clarification on the structure of EPS and summarized in
Table 3. The polymers had group frequencies including OH, CH
2, CH, C–C, C=O, C–O at 3425 cm
−1, 2934 cm
−1, 1457 cm
−1, 1224 cm
−1, 1645 cm
−1, and 1056 cm
−1, respectively. Significantly, the EPS of
Lactobacillus plantarum contained a different frequency at 1380.7 cm
−1 while 1456.3 cm
−1 and 1457.4 cm
−1 in the EPS of
Lactobacillus rhamnosus and
Leuconostoc mesenteroides, respectively. It was revealed that the EPS of
Lactobacillus plantarum contained a dimethyl group in its monomer. This difference could make the EPS of
Lactobacillus plantarum have a different activity in comparison with that of the EPS of
Lactobacillus rhamnosus and
Leuconostoc mesenteroides.
2.5. Mice Assay for TTX Detoxification Capacity of EPS
TTX was incubated with the EPS of
Leuconostoc mesenteroides,
Lactobacillus plantarum, and
Lactobacillus rhamnosus together with cuprous oxide at room temperature for 20 to 60 min. On the basis of the lethal dose (0.22 µg TTX/mouse unit) that caused 100% of mice to die within 30 min, the TTX dose applied to the mice depending on their weight is presented in
Table 4.
2.6. TTX Detoxification Capacity of EPS of Lactobacillus rhamnosus
Experimentally, the optimal combination of EPS of
Lactobacillus rhamnosus (147.946 µg) with cuprous oxide (55.325 µg) incubated for 40.415 min showed the highest TTX detoxification capacity, resulting in all mice recovering after injection (
Figure 2). EPS or cuprous oxide alone did not help mice recover from TTX. There was no significant difference in the incubation time from 20 to 60 min in the TTX detoxification capacity.
Table 5 showed the reduction percentage of TTX incubated with EPS (28.99%) or both EPS and cuprous oxide (37.81%). The interaction between the EPS of
Lactobacillus rhamnosus and cuprous oxide in TTX detoxification may occur in a shorter time, less than 20 min.
2.7. TTX Detoxification Capacity of EPS of Lactobacillus plantarum
In the
Figure 3, the mixture of the EPS of
Lactobacillus plantarum and cuprous oxide did not show any TTX detoxification in mice. However, the EPS of
Lactobacillus plantarum showed TTX detoxification capacity in mice. Actually, EPS showed the higher reduction percentage of TTX than EPS incubated with cuprous oxide when analyzed by HPLC (
Table 5).
2.8. TTX Detoxification Capacity of EPS of Leuconostoc mesenteroides
The combination of the EPS of
Leuconostoc mesenteroides and cuprous oxide significantly affected the survival in mice (
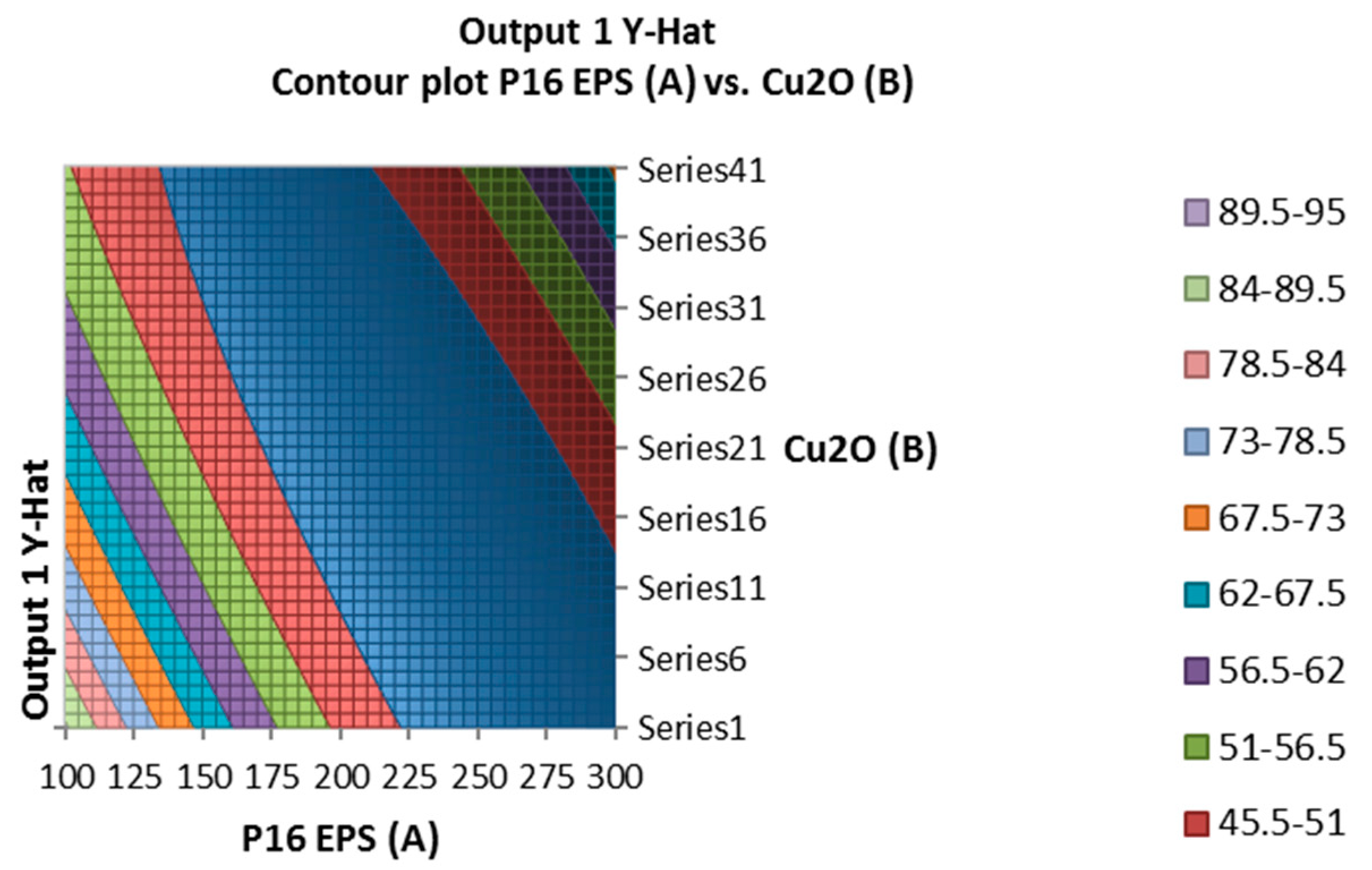
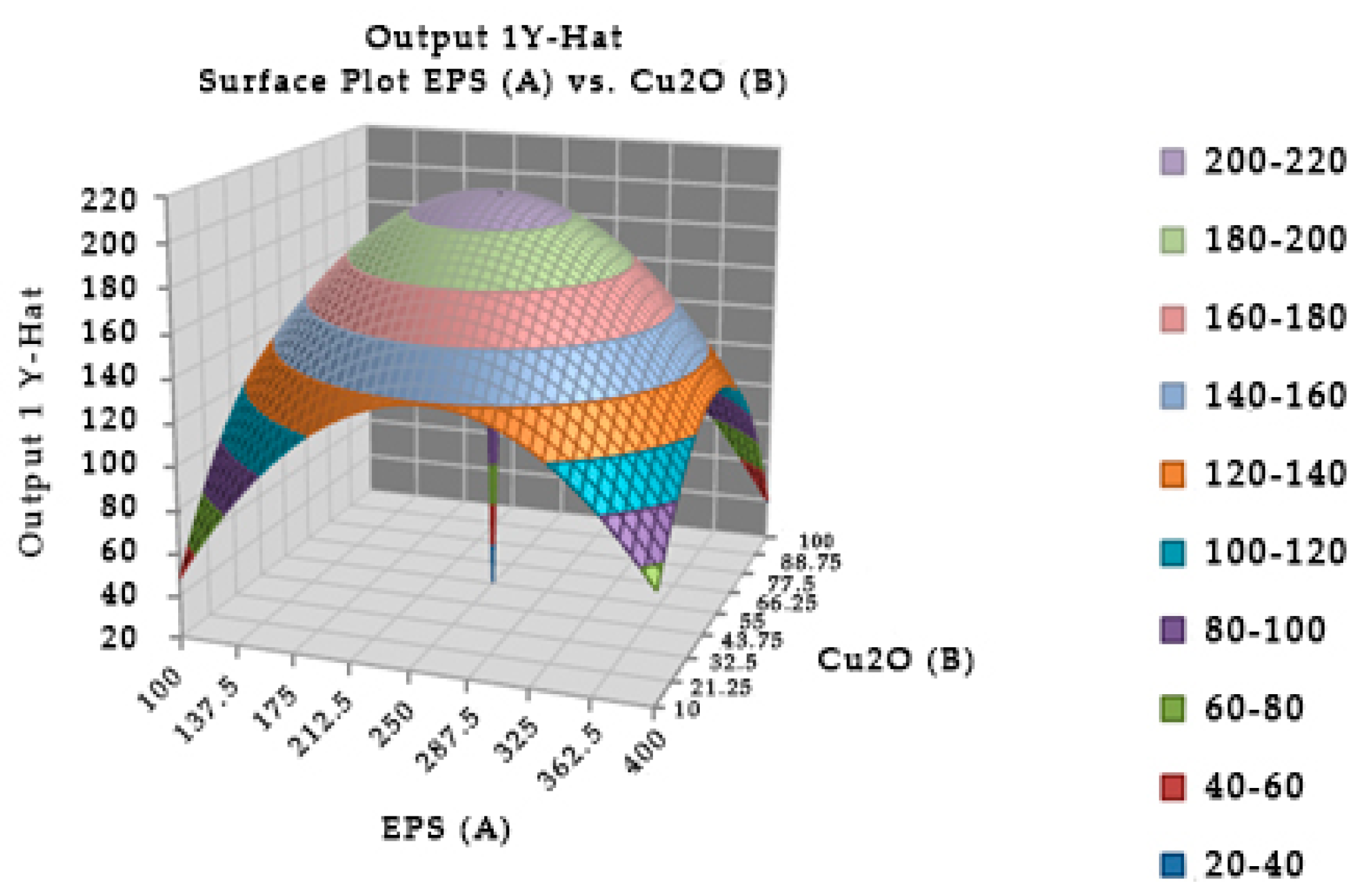
Figure 4). In experiment, the optimal incubation time was 40.04 min, the optimal EPS dose was 248.449 µg, and cuprous oxide dose was 54.204 µg for mice survival after injection. In the study, the incubation times of EPS and cuprous oxide were changed from 20 to 60 min which insignificantly affected the survival in mice.
Table 5 showed that the reduction percentage of TTX incubated with EPS (5.96%) was lower than the EPS incubated with cuprous oxide (37.54%). The interaction between the EPS of
Leuconostoc mesenteroides and cuprous oxide in TTX detoxification may occur in a shorter time, less than 20 min.
3. Discussion
Lactobacillus plantarum PN05,
Leuconostoc mesenteroides N3, and
Lactobacillus rhamnosus PN04 were chosen to clarify TTX detoxification. EPS is a countering compound produced by lactic acid bacteria in response to disadvantageous conditions, hypothesized as a TTX detoxification compound in this study. Different bacteria may produce EPS differently in response to hard conditions, resulting in different activities [
18]. EPS may directly bind and reduce the toxicity of antibiotic or prevent antibiotic binding to active sites. Other studies revealed that TTX can be removed from puffer fish meat by traditional fermentation in which lactic acid bacteria play the main roles [
11]. According to researched results, EPS was chosen and scanned for its TTX detoxification capacity.
Lactobacillus plantarum, Leuconostoc mesenteroides, and Lactobacillus rhamnosus had an equal productivity of EPS, but varied their solubility in water largely because of the difference of functional groups detected by FTIR, leading to the different interaction with water and other compounds.
From the Box–Behnken design, the experimentally optimal results indicated that the EPS of Leuconostoc mesenteroides and Lactobacillus rhamnosus can detoxify TTX and help mice survive when combined with cuprous oxide, while the EPS of Lactobacillus plantarum alone showed TTX detoxification capacity. This variation may be due to the different structure of EPS of the three strains. The study suggested that the EPS of Lactobacillus plantarum alone could be used in prevent TTX contamination.
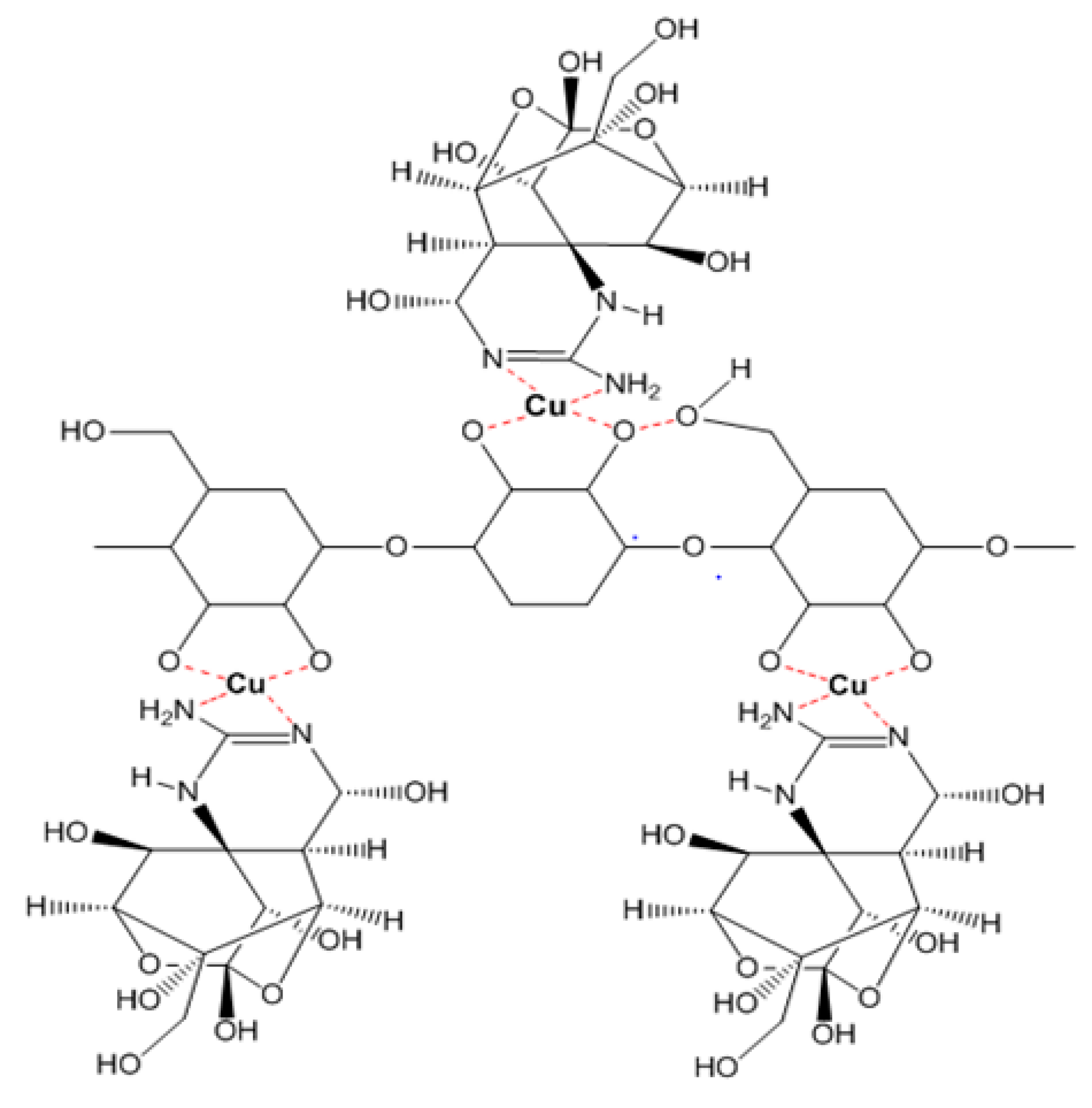
In the cases of the EPS of
Leuconostoc mesenteroides and
Lactobacillus rhamnosus, the EPS showed their TTX detoxification capacity when combined with copper ion. EPS is a long chain polymer while tetrodotoxin contains many strong functional groups in a compact structure. In the presence of copper, both EPS structures of
Leuconostoc mesenteroides and
Lactobacillus rhamnosus contain functional groups (O–H, N–H) that interact with copper forming a complexly compact structure that can bind to TTX through many hydrogen bonds (
Figure 5), then TTX was not enough amount to cause TTX toxicity in mice. According to the FTIR result, the EPS of
Lactobacillus plantarum was methylated. This group makes the electric density in oxygen become more negative in charge allowing them to interact with hydrogen of the hydroxyl groups of TTX (
Figure 6). These interactions might happen faster than the interaction of copper with nitrogen in nitro moiety of TTX leading to TTX detoxification without the presence of copper. Further investigations must be conducted to optimize the way EPS originated in many sources in TTX detoxification.
4. Conclusions
EPS from Leuconostoc mesenteroides N3 and Lactobacillus rhamnosus PN04 combined with cuprous oxide can detoxify TTX in mouse. However, because cuprous oxide is toxic, these combinations should be considered well. Fortunately, EPS from Lactobacillus plantarum PN05 alone showed TTX detoxification capacity in mice. Therefore, the study may bring out the potential way in TTX prevention.
5. Materials and Methods
5.1. Isolation and Identification of Leuconostoc mesenteroides N3
The seawater samples were collected at different places in the Vung Tau sea in Vietnam. These samples were handled in sterile condition. The isolation for lactic acid bacteria was done according to Schillinger [
19] and Rodriguez et al. [
15]. The media including the de Man–Rogosa–Sharpe (MRS) agar or broth (Merck, Darmstadt, Germany) were used. Briefly, a 10 g of sample was mixed with 90 mL saline solution (0.9%) to get a ratio of 1/10. The solutions were shaken for 10 min to homogenize. A volume of 1 mL was plated on MRS agar and incubated in CO
2 (5%) conditions at 45 °C for 48 h. About ten colonies were grown at the incubated condition. All colonies were taken for identification. The isolated colonies were tested by microscopic examination with gram stain and catalase production. The pure colonies were also characterized using an uronic acid test. Then, the strains were identified by biochemical characterization based on the ability of the isolates to utilize different carbon sources, which were determined by API CHL 50 (bioMerieux, Lyon, France) and 16S rRNA sequencing analysis. The forward primer was f1 (5′-GCAAGTCGAACGCACAGCGA-3′) and the reverse primer was f2 (5′-CACGTATTTAGCCGTCCCTTTC-3′). The PCR reaction was performed as follows: 95 °C for 5 min; 30 cycles of 95 °C for 20 s, 50 °C for 20 s, and 72 °C for 3 min; and a final extension at 72 °C for 10 min. The PCR product was stored at 4 °C. The purified PCR product was then sequenced. The homology comparison of 16S rRNA gene sequence with the others was performed using BLAST (NCBI).
5.2. Cultivation of Lactic Acid Bacteria for EPS Isolation
Lactic acid bacteria strains used in this study including
Leuconostoc mesenteroides N3,
Lactobacillus plantarum PN05 isolated from
Coriandrum sativum [
20], and
Lactobacillus rhamnosus PN04 isolated from
Hottuynia cordata Thunb [
21]. All these strains were cultured in MRS broth at room temperature for 48 h.
5.3. Mice
Mice (20 ± 1 g) selected for this experiment were male, provided by the cell reprograming laboratory, Hochiminh City International University (HCM-IU), Vietnam National University—Hochiminh city. All the procedures were done according to the rules of HCM-IU (Ethical approval code: 302/QD-DHQT-QLKH; Renewed date of approval: 2 July 2018).
5.4. Isolation of EPS
All supernatant was harvested after centrifugation at 10,000 rpm, 4 °C for 30 min. The procedure was done according to Wei’s group [
22]. The dried EPS was used for quantification, further purification for structure determination, and testing for TTX neutralization.
5.5. EPS Determination
The EPS concentration was determined by the phenol-sulphuric acid method [
23]. The absorbance was measured at 490 nm. The concentration of EPS was determined in triplicate. Distilled water was used as blank. The EPS content of each sample was calculated based on the standard curve.
5.6. Molecular Weight Determination
The extracted EPS was purified according to the method [
24]. The precipitated EPS was dissolved in water, dialyzed in water, and lyophilized. The UV spectrum of the purified EPS was investigated for the presence of protein or nucleic acid. The molecular weight of EPS was determined by performing gel permeation chromatography (GPC). The EPS was eluted with 0.1 NaNO
3 at a flow rate of 0.6 mL/min. Dextran was used as standard to estimate the molecular weight of EPS [
25].
5.7. Monomer Detection
Monomers of EPS were preliminary detected by thin layer chromatography [
26]. After that, high performance liquid chromatography was performed to thoroughly detect for monomers. The hydrolyzed EPS was dissolved in Milli-Q water and applied into the monosaccharide Ca
2+ column. Milli-Q water was used as mobile phase. The flow rate was 0.6 mL/min. The monosaccharides such as
d-glucose,
d-galactose, fructose,
d-mannose,
d-xylose, and
l-rhamnose were used as standards. Refractive Index Detector (RID) was used in the study [
26].
5.8. FT-IR
FTIR was performed to detect functional groups or chemical bonds in the compound according to the previous method [
27]. To detect functional group present in the EPS, FTIR was carried out by micro-KBr pellet technique. Lyophilized EPS powder was ground with potassium bromide powder finely. A spectrum was recorded using FTIR spectrophotometer (Nicolet-5700 Thermo Electron Corporation, Madison, WI, USA) in the frequency range of 400–4000 cm
−1. Potassium bromide was used as a background reference.
5.9. Test for TTX Neutralization Capacity of EPS
TTX in a range of 0.1–0.5 µg was screened on mice via intraperitoneal injection to determine minimal dose at which 100% mice died within 30 min. After determination of exact lethal dose, TTX was combined with EPS of Leuconostoc mesenteroides, Lactobacillus plantarum, and Lactobacillus rhamnosus with and without cuprous oxide. The mixtures were incubated for 20–60 min, then intraperitoneally injected in mice. The symptoms and time of death of injected mice were recorded carefully. In parallel, we intraperitoneally injected mice with TTX, and then injected EPS or EPS combined with cuprous oxide. All the symptoms and time of death were then recorded. Box–Behnken design was used to set up the doses for TTX detoxification in mice.
5.10. Statistical Analysis and Box–Behnken Design
In order to maximize the TTX detoxification capacity of EPS, the different concentrations of EPS and Cu
2O as well as incubated time were screened. A set of factors was studied at three levels (−1, 0, +1) those are standing for the lower extreme, central point and the higher extreme of each factor in the design respectively (
Table 6,
Table 7 and
Table 8). These factors were symbolized as A, B, and C, respectively.
The experimental matrix was designed by using the Design-Expert@ version 8.06 (Stat-Ease Inc, East Hennepin Avenue, Minneapolis, MN, USA) design was arranged in
Table 9.
On the basis of Box–Behnken design, the experiments for determination of optimal combination of EPS and cuprous oxide in a suitable incubation time were done. All the results were statistically analyzed.
Author Contributions
Data curation, N.H.K.T., N.V.D. and L.V.C.; Formal analysis, N.H.K.T., N.V.D., L.V.C. and D.T.T.V.; Investigation, N.H.K.T.; Methodology, N.H.K.T. and N.V.D.; Supervision, N.H.K.T.; Validation, N.H.K.T. and N.V.D.; Writing—original draft, N.V.D., L.V.C. and D.T.T.V.; Writing—review & editing, N.H.K.T.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Campbell, S.; Harada, R.M.; DeFelice, S.V.; Bienfang, P.K.; Li, Q.X. Bacterial production of tetrodotoxin in the pufferfish Arothron hispidus. Nat. Prod. Res. 2009, 23, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.C.; Yu, P.H.; Ho, K.C.; Lee, F.W. Isolation and identification of a new tetrodotoxin-producing bacterial species, Raoultella terrigena, from Hong Kong marine puffer fish Takifugu niphobles. Mar. Drugs 2011, 9, 2384–2396. [Google Scholar] [CrossRef] [PubMed]
- Tu, N.; Tu, Q.; Tung, H.; Hieu, D.; Romero-Jovel, S. Detection of tetrodotoxin-producing Providencia rettgeri T892 in Lagocephalus pufferfish. World J. Microbiol. Biotechnol. 2014, 30, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Nguyen, H.N.; Nghe, D.V.; Nguyen, K.H. Biological activities of tetrodotoxin-producing Enterococcus faecium AD1 isolated from puffer fishes. BioMed Res. Int. 2015, 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, G.M.; Li, A.; Zimmer, R.K.; Kats, L.B.; Green, D.B. Quantifying tetrodotoxin levels in the California newt using a non-destructive sampling method. Toxicon 2014, 80, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Hashimoto, Y. Isolation of tetrodotoxin from a goby Gobius criniger. Toxicon 1973, 11, 305–307. [Google Scholar] [CrossRef]
- Yang, C.C.; Han, K.C.; Lin, T.J.; Tsai, W.J.; Deng, J.F. An outbreak of tetrodotoxin poisoning following gastropod mollusc consumption. Hum. Exp. Toxicol. 1995, 14, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O.; Daigo, K.; Hashimoto, K. Local differences in toxin composition of a xanthid crab Atergatis floridus inhabiting Ishigaki Island, Okinawa. Toxicon 1986, 24, 705–711. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D.; Flachsenberger, W. Distribution of tetrodotoxin in the body of the blue-ringed octopus (Hapalochlaena maculosa). Toxicon 2007, 49, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T. Pharmacology of tetrodotoxin. J. Toxicol. 2001, 20, 67–84. [Google Scholar] [CrossRef]
- Anraku, K.; Nonaka, K.; Yamaga, T.; Yamamoto, T.; Shin, M.C.; Wakita, M.; Hamamoto, A.; Akaike, N. Removal of toxin (Tetrodotoxin) from puffer ovary by traditional fermentation. Toxins 2013, 5, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Prajapat, J.B. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv. Dairy Res. 2013, 1, 1–8. [Google Scholar]
- Rodriguez, J.M.; Martinez, M.I.; Horn, N.; Dodd, H.M. Heterologous production of bacteriocins by lactic acid bacteria. Int. J. Food Microbiol. 2003, 80, 101–116. [Google Scholar] [CrossRef]
- Iliev, I.; Ivanova, I.; Ignatova, C. Glucansucrases from lactic acid bacteria (Lab). Biotechnol. Biotechnol. Equip. 2006, 20, 15–20. [Google Scholar] [CrossRef]
- Rodriguez, C.; Medici, M.; Rodriguez, A.V.; Mozzi, F.; Font de Valdez, G. Prevention of chronic gastritis by fermented milks made with exopolysaccharide-producing Streptococcus thermophilus strains. J. Dairy Sci. 2009, 92, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and antioxidant potential of lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, Z.; Hossien, F.; Zahra, A.T. Glucose interaction with Au, Ag, and Cu clusters: Theoretical investigation. Int. J. Quantum Chem. 2013, 113, 1062–1070. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U. Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. Int. J. Food Microbiol. 1999, 47, 79–87. [Google Scholar] [CrossRef]
- Nguyen, T.; Le, C.; Tran, N. The adjunct therapy of study on antibiotic and Lactobacillus plantarum PN05 isolated in Coriandrum sativum. Wulfenia J. 2015, 22, 195–221. [Google Scholar]
- Nguyen, T.H.K.; Doan, V.T.T.; Ha, L.D.; Nguyen, H.N. Molecular cloning, expression of minD gene from Lactobacillus acidophilus VTCC-B-871 and analyses to identify Lactobacillus rhamnosus PN04 from Vietnam Hottuynia cordata thumb. Indian J. Microbiol. 2013, 53, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fang, L.; Cai, P.; Huang, Q.; Chen, H.; Liang, W.; Rong, X. Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ. Pollut. 2011, 15, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Li, J.-Q.; Guo, H.-T.; Wang, J.; Xu, R.-H. Selection of exopolysaccharide-producing lactic acid bacteria isolates from Inner Mongolian traditional yoghurt. Mljekarstvo 2014, 64, 254–260. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Grosu-Tudor, S.S.; Zamfir, M. Exopolysaccharide production by selected lactic acid bacteria isolated from fermented vegetables. Sci. Bull. Ser. F Biotechnol. 2014, 18, 107–114. [Google Scholar]
- Ying, C.; Liping, S.; Yong, Z.; Lei, W.; Liguo, A. The production-influencing factors of extracellular polysaccharide (EPS) from a strain of lactic acid bacteria and EPS extraction. Front. Biol. China 2006, 1, 236–240. [Google Scholar] [CrossRef]
- Yadav, V.; Prappulla, S.G.; Jha, A.; Poonia, A. A novel exopolysaccharide from probiotic Lactobacillus fermentum CFR 2195: Production, purification and characterization. Biotechnol. Bioinf. Bioeng. 2011, 1, 415–421. [Google Scholar]
Figure 1.
The hydrolyzed exopolysaccharide (EPS) of Leuconostoc mesenteroides (1), Lactobacillus plantarum (2), and Lactobacillus rhamnosus (3) were detected by thin layer chromatography (TLC). Standard glucose (4).
Figure 1.
The hydrolyzed exopolysaccharide (EPS) of Leuconostoc mesenteroides (1), Lactobacillus plantarum (2), and Lactobacillus rhamnosus (3) were detected by thin layer chromatography (TLC). Standard glucose (4).
Figure 2.
Surface and contour plot of combination of EPS of Lactobacillus rhamnosus, cuprous oxide, and time treatment for TTX detoxification capacity on mice.
Figure 2.
Surface and contour plot of combination of EPS of Lactobacillus rhamnosus, cuprous oxide, and time treatment for TTX detoxification capacity on mice.
Figure 3.
Surface and contour plot of combination of EPS of Lactobacillus plantarum, Cu2O, and time treatment for TTX detoxification capacity on mice.
Figure 3.
Surface and contour plot of combination of EPS of Lactobacillus plantarum, Cu2O, and time treatment for TTX detoxification capacity on mice.
Figure 4.
Surface and contour plot of combination of EPS of Leuconostoc mesenteroides, cuprous oxide, and time treatment for TTX detoxification capacity on mice.
Figure 4.
Surface and contour plot of combination of EPS of Leuconostoc mesenteroides, cuprous oxide, and time treatment for TTX detoxification capacity on mice.
Figure 5.
The interaction model of TTX and EPS from Leuconostoc mesenteroides N3 and Lactobacillus rhamnosus PN04 through Cu bridges.
Figure 5.
The interaction model of TTX and EPS from Leuconostoc mesenteroides N3 and Lactobacillus rhamnosus PN04 through Cu bridges.
Figure 6.
The interaction model of TTX and EPS isolated from Lactobacillus plantarum PN05.
Figure 6.
The interaction model of TTX and EPS isolated from Lactobacillus plantarum PN05.
Table 1.
Characteristics of exopolysaccharide (EPS) obtained from lactic acid bacteria.
Table 1.
Characteristics of exopolysaccharide (EPS) obtained from lactic acid bacteria.
| Selected LAB | Crude EPS (mg/0.5 L Culture) | Water Solubilizing EPS (mg/0.5 L Culture) | Percentage (%) | Molecular Weight (Da) |
|---|
| Leuconostoc mesenteroides | 140 | 13.83 | 9.88 | 3.8 × 104 |
| Lactobacillus plantarum | 150 | 101.6 | 67.73 | 4 × 104 |
| Lactobacillus rhamnosus | 140 | 12.96 | 9.26 | 4.4 × 104 |
Table 2.
The retention time and peak area of monomer revealed by high-performance liquid chromatography (HPLC).
Table 2.
The retention time and peak area of monomer revealed by high-performance liquid chromatography (HPLC).
| Hydrolyzed EPS from Selected Lactic Acid Bacteria (LAB) | Peak Area | Retention Time (min) |
|---|
| Leuconostoc mesenteroides | 143,843 | 13.133 |
| 87,661 | 15.599 |
| Lactobacillus plantarum | 82,310 | 13.125 |
| 53,584 | 15.575 |
| Lactobacillus rhamnosus | 120,313 | 13.152 |
| 34,456 | 15.620 |
| Standard d-glucose | 579,056 | 15.512 |
Table 3.
The Wavenumber (cm−1) corresponding to chemical bonds from FTIR spectrum.
Table 3.
The Wavenumber (cm−1) corresponding to chemical bonds from FTIR spectrum.
| EPS from Selected LAB | Wavenumber (cm−1) |
|---|
| OH | C–H | C=O | N–H | C–H | C–C | C–O | C–H |
|---|
| Leuconostoc mesenteroides | 3427.5 | 2934.4 | 1645.1 | 1542.5 | 1457.4 | 1224.6 | 1056.2 | 813.4 |
| Lactobacillus plantarum | 3438.2 | 2936.8 | 1654.2 | 1550.9 | 1380.7 | 1223.4 | 1052.4 | 814.8 |
| Lactobacillus rhamnosus | 3421.9 | 2934.1 | 1652.1 | 1539.6 | 1456.3 | 1221.6 | 1057.2 | 813.6 |
Table 4.
The dead time of mouse treated with different dose of TTX.
Table 4.
The dead time of mouse treated with different dose of TTX.
| Mice | Weight of Mouse (g) | Dose of Tetrodotoxin (TTX) (µg/Mouse Unit) | Time Until Death (min) |
|---|
| 1 | 19.53 | 0.20 | 57 |
| 2 | 20.52 | 0.20 | 65 |
| 3 | 21.02 | 0.20 | 49 |
| 4 | 19 | 0.22 | 28 |
| 5 | 20.81 | 0.22 | 30 |
| 6 | 21 | 0.22 | 26 |
| 7 | 19.95 | 0.24 | 18 |
| 8 | 20.30 | 0.24 | 22 |
| 9 | 20.86 | 0.24 | 15 |
Table 5.
The reduction percentage of TTX by EPS in combination with Cu2O reveal by HPLC.
Table 5.
The reduction percentage of TTX by EPS in combination with Cu2O reveal by HPLC.
| EPS from Selected LAB | TTX Dose (μ/mu) | EPS Dose (μ/mu) | Cuprous Oxide (μ/mu) | Incubation Time (min) | Peak Area | Retention Time (min) | % Reduction |
|---|
| Leuconostoc mesenteroides | 0.5 | 250 | 55 | 40 | 30,026 | 15.038 | 37.54 |
| 0.5 | 250 | 0 | 40 | 45,212 | 15.046 | 5.96 |
| Lactobacillus rhamnosus | 0.5 | 150 | 55 | 40 | 29,899 | 15.068 | 37.81 |
| 0.5 | 150 | 0 | 40 | 34,139 | 15.064 | 28.99 |
| Lactobacillus plantarum | 0.5 | 100 | 55 | 40 | 44,618 | 15.063 | 7.19 |
| 0.5 | 100 | 0 | 40 | 40,968 | 15.063 | 14.78 |
| Cuprous oxide | 0.5 | 0 | 55 | 40 | 31,547 | 15.083 | 34.38 |
| Standard TTX | 0.5 | 0 | 0 | 40 | 48,077 | 15.121 | 0 |
Table 6.
Code level of Plackett–Burman design for Lactobacillus plantarum.
Table 6.
Code level of Plackett–Burman design for Lactobacillus plantarum.
| Income Variables | Code Levels |
|---|
| −1 | 0 | 1 |
|---|
| EPS (µg/mouse unit) (A) | 100 | 200 | 300 |
| Cuprous oxide (µg/mouse unit) (B) | 10 | 55 | 100 |
| Incubated time (min) (C) | 20 | 40 | 60 |
Table 7.
Code level of Plackett–Burman design for Lactobacillus rhamnosus.
Table 7.
Code level of Plackett–Burman design for Lactobacillus rhamnosus.
| Income Variables | Code Levels |
|---|
| −1 | 0 | 1 |
|---|
| EPS (µg/mouse unit) (A) | 100 | 150 | 200 |
| Cuprous oxide (µg/mouse unit) (B) | 10 | 55 | 100 |
| Incubated time (min) (C) | 20 | 40 | 60 |
Table 8.
Code level of Plackett–Burman design for Leuconostoc mesenteroides.
Table 8.
Code level of Plackett–Burman design for Leuconostoc mesenteroides.
| Income Variables | Code Levels |
|---|
| −1 | 0 | 1 |
|---|
| EPS (µg/mouse unit) (A) | 100 | 250 | 400 |
| Cuprous oxide (µg/mouse unit) (B) | 10 | 55 | 100 |
| Incubated time (min) (C) | 20 | 40 | 60 |
Table 9.
Plackett–Burman experimental design matrix.
Table 9.
Plackett–Burman experimental design matrix.
| Experiment | Factors |
|---|
| A | B | C |
|---|
| 1 | −1 | −1 | 0 |
| 2 | −1 | 1 | 0 |
| 3 | 1 | −1 | 0 |
| 4 | 1 | 1 | 0 |
| 5 | −1 | 0 | −1 |
| 6 | −1 | 0 | 1 |
| 7 | 1 | 0 | −1 |
| 8 | 1 | 0 | 1 |
| 9 | 0 | −1 | −1 |
| 10 | 0 | −1 | 1 |
| 11 | 0 | 1 | −1 |
| 12 | 0 | 1 | 1 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).