Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples
Abstract
:1. Introduction
2. Results and Discussion
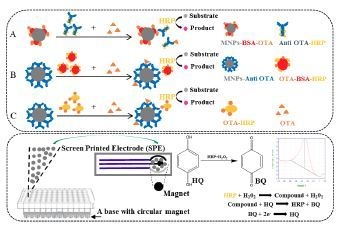
2.1. Optimizations and Comparisons of Three Variants of MNPs-ELISA
2.2. Specificity Study
2.3. Optimization of the Electrochemical Biosensor Immunoassay
2.4. Calibration Curve of the Electrochemical Biosensor Immunoassay
2.5. Recovery Studies
2.6. Commercial Samples Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Equipment
4.3. Synthesis of the OTA and Antibody Conjugates
4.4. Preparation of Magnetic Nanoparticles Conjugates
4.5. Development of MNP-ELISAs
4.6. Optimization of MNPs-ELISAs
4.7. Specificity Study
4.8. Development of the Electrochemical Biosensor Immunoassay
4.9. Recovery Study and Comparison of Detection in Commercial Samples by LC-MS/MS
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Vila-Donat, P.; Marin, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Ochratoxin, A. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occuring Substances: Food Items and Constitutions, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993. [Google Scholar]
- Liu, L.H.; Zhou, X.H.; Shi, H.C. Portable optical aptasensor for rapid detection of mycotoxin with a reversible ligand-grafted biosensing surface. Biosens. Bioelectron. 2015, 72, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.R.; Hoeltz, M.; Einloft, T.C.; Dottori, H.A.; Manfroi, V.; Noll, I.B. Determination of ochratoxin A in wine from the southern region of Brazil by thin layer chromatography with a charge-coupled detector. Food Addit. Contam. Part B Surveill. 2011, 4, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Cramer, B.; Iha, M.H.; Krska, R.; Lattanzio, V.M.T.; Macdonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M. Developments in mycotoxin analysis: An update for 2016–2017. World Mycotoxin J. 2018, 11, 5–32. [Google Scholar] [CrossRef]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of Mycotoxins in Food: A Review of Bioanalytical to Analytical Methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Meulenberg, E.P. Immunochemical methods for ochratoxin A detection: A review. Toxins 2012, 4, 244–266. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Isobe, S.; Zhu, Y.; Kiyama, R. Fluorescence-Based Bioassays for the Detection and Evaluation of Food Materials. Sensors 2015, 15, 25831–25867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazin, I.; Nabais, E.; Lopez-Ferber, M. Rapid visual tests: Fast and reliable detection of ochratoxin A. Toxins 2010, 2, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.Z.; Richard, J.L.; Binder, J. A review of rapid methods for the analysis of mycotoxins. Mycopathologia 2006, 161, 261–273. [Google Scholar] [PubMed]
- Li, Y.; Liu, X.; Lin, Z. Recent developments and applications of surface plasmon resonance biosensors for the detection of mycotoxins in foodstuffs. Food Chem. 2012, 132, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Badie Bostan, H.; Danesh, N.M.; Karimi, G.; Ramezani, M.; Mousavi Shaegh, S.A.; Youssefi, K.; Charbgoo, F.; Abnous, K.; Taghdisi, S.M. Ultrasensitive detection of ochratoxin A using aptasensors. Biosens. Bioelectron. 2017, 98, 168–179. [Google Scholar] [PubMed]
- Yu, J.C.; Lai, E.P. Molecularly imprinted polymers for ochratoxin a extraction and analysis. Toxins 2010, 2, 1536–1553. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.E.; Petrakova, A.V.; Vozniak, M.V.; Zherdev, A.V.; Dzantiev, B.B. Rapid immunoenzyme assay of aflatoxin B1 using magnetic nanoparticles. Sensors 2014, 14, 21843–21857. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, L.; Fang, C. The chemiluminescence immunoassay for aflatoxin B1 based on functionalized magnetic nanoparticles with two strategies of antigen probe immobilization. Lumin. J. Boil. Chem. Lumin. 2017, 32, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, J.; Wang, K.; Yang, X.; Liu, Q.; Hao, N.; Wang, C.; Dong, X.; Huang, X. Colorimetric aptasensing of ochratoxin A using Au@Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens. Bioelectron. 2016, 77, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Wang, Y.-C.; Wang, H.-A.; Ji, W.-H.; Sun, J.-H.; Yan, Y.-X. An immunomagnetic-bead-based enzyme-linked immunosorbent assay for sensitive quantification of fumonisin B1. Food Control 2014, 40, 41–45. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Sun, M.; Zhang, X.; Song, H.; Yan, Y.; Sun, J.; Li, X.; Fang, W. A Magnetic Nanoparticle Based Enzyme-Linked Immunosorbent Assay for Sensitive Quantification of Zearalenone in Cereal and Feed Samples. Toxins 2015, 7, 4216–4231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.K.; Yan, Y.X.; Mao, Z.W.; Wang, H.A.; Zou, Q.; Hao, Q.W.; Ji, W.H.; Sun, J.H. Highly sensitive electrochemical immunoassay for zearalenone in grain; and grain-based food. Microchim. Acta 2013, 180, 187–193. [Google Scholar] [CrossRef]
- Xing, R.; Zhao, X.; Liu, Y.; Liu, J.; Liu, B.; Ren, Y.; Liu, S.; Mao, L. Low Cost and Reliable Electrochemical Sensor for Rutin Detection Based on Au Nanoparticles-Loaded ZnS Nanocomposites. J. Nanosci. Nanotechnol. 2018, 18, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.R.S.; da Silva, J.S.; de Oliveira Farias, E.A.; Teixeira, P.R.S.; Eiras, C.; Nunes, L.C.C. Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosens. Bioelectron. 2018, 108, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jampasa, S.; Siangproh, W.; Laocharoensuk, R.; Vilaivan, T.; Chailapakul, O. Electrochemical detection of c-reactive protein based on anthraquinone-labeled antibody using a screen-printed graphene electrode. Talanta 2018, 183, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, S.H.; Palleschi, G.; Compagnone, D.; Pascale, M.; Visconti, A.; Barna-Vetro, I. Monoclonal antibody based electrochemical immunosensor for the determination of ochratoxin A in wheat. Talanta 2006, 69, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Hervas, M.; Lopez, M.A.; Escarpa, A. Simplified calibration and analysis on screen-printed disposable platforms for electrochemical magnetic bead-based immunosensing of zearalenone in baby food samples. Biosens. Bioelectron. 2010, 25, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.S.; Choi, E.H.; Chang, H.J.; Choi, S.W.; Eremin, S.A. A fluorescence polarization immunoassay for the detection of zearalenone in corn. Anal. Chim. Acta 2009, 639, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, Z.; Duan, Z.; He, Z.; Shu, M.; Wang, X.; Gee, S.J.; Hammock, B.D.; Xu, Y. Nanobody-based enzyme immunoassay for ochratoxin A in cereal with high resistance to matrix interference. Talanta 2017, 164, 154–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.K.; Yan, Y.X.; Ji, W.H.; Wang, H.A.; Zou, Q.; Sun, J.H. Novel chemiluminescence immunoassay for the determination of zearalenone in food samples using gold nanoparticles labeled with streptavidin-horseradish peroxidase. J. Agric. Food Chem. 2013, 61, 4250–4256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, M.; Kang, Y.; Xie, H.; Wang, X.; Song, H.; Li, X.; Fang, W. Identification of a high-affinity monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay. Toxicon Off. J. Int. Soc. Toxinol. 2015, 106, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Hong, W.; Zhang, X.; Lu, Y. Label-free and sensitive detection of Ochratoxin A based on dsDNA-templated copper nanoparticles and exonuclease-catalyzed target recycling amplification. Analyst 2018, 143, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Bueno, D.; Mishra, R.K.; Hayat, A.; Catanante, G.; Sharma, V.; Munoz, R.; Marty, J.L. Portable and low cost fluorescence set-up for in-situ screening of Ochratoxin A. Talanta 2016, 159, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, X.; Xiong, Y.; Pei, K.; Nie, L.; Xiong, Y. Silver Nanoparticle-Based Fluorescence-Quenching Lateral Flow Immunoassay for Sensitive Detection of Ochratoxin A in Grape Juice and Wine. Toxins 2017, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Samokhvalov, A.V.; Safenkova, I.V.; Eremin, S.A.; Zherdev, A.V.; Dzantiev, B.B. Use of anchor protein modules in fluorescence polarisation aptamer assay for ochratoxin A determination. Anal. Chim. Acta 2017, 962, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Dou, X.; Liang, R.; Li, M.; Kong, W.; Yang, X.; Luo, J.; Yang, M.; Zhao, M. A self-assembly aptasensor based on thick-shell quantum dots for sensing of ochratoxin A. Nanoscale 2016, 8, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ouyang, W.; Guo, L.; Lin, Z.; Jiang, X.; Qiu, B.; Chen, G. Facile synthesis of Fe3O4/g-C3N4/HKUST-1 composites as a novel biosensor platform for ochratoxin A. Biosens. Bioelectron. 2017, 92, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ding, A.; Zheng, J.; Chen, J.; Wang, B. A Label-Free Aptasensor for Ochratoxin a Detection Based on the Structure Switch of Aptamer. Sensors 2018, 18, 1769. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Matharu, Z.; Malhotra, B.D. Polyaniline Langmuir-Blodgett film based aptasensor for ochratoxin A detection. Biosens. Bioelectron. 2011, 26, 4006–4011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fang, Z.; Liu, J.; Zeng, L. A simple and rapid biosensor for ochratoxin A based on a structure-switching signaling aptamer. Food Control 2012, 25, 555–560. [Google Scholar] [CrossRef]
- Tian, J.; Wei, W.; Wang, J.; Ji, S.; Chen, G.; Lu, J. Fluorescence resonance energy transfer aptasensor between nanoceria and graphene quantum dots for the determination of ochratoxin A. Anal. Chim. Acta 2018, 1000, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mejri-Omrani, N.; Miodek, A.; Zribi, B.; Marrakchi, M.; Hamdi, M.; Marty, J.L.; Korri-Youssoufi, H. Direct detection of OTA by impedimetric aptasensor based on modified polypyrrole-dendrimers. Anal. Chim. Acta 2016, 920, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Liu, T.; Xu, N.; Zhang, Y.; Wang, S. Enzyme-linked immunosorbent assay and colloidal gold immunoassay for ochratoxin A: Investigation of analytical conditions and sample matrix on assay performance. Anal. Bioanal. Chem. 2007, 389, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Soleimany, F.; Jinapa, S. Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 2012, 130, 1055–1060. [Google Scholar] [CrossRef]





| Analytical Methods | Final Concentration (μg/mL) | Dilution Ratio | |||
|---|---|---|---|---|---|
| Anti OTA-HRP | OTA-BSA-HRP | OTA-HRP | MNPs-BSA-OTA | MNPs-Anti OTA | |
| Anti OTA-HRP MNPs-ELISA | 0.2 | NU a | NU | 1/50 | NU |
| OTA-BSA-HRP MNPs-ELISA | NU | 0.1 | NU | NU | 1/100 |
| OTA-HRP MNPs-ELISA | NU | NU | 0.5 | NU | 1/100 |
| Analytical Method | LOD (ng/mL) | IC50 (ng/mL) | Detection Range (IC20–IC80, ng/mL) | Regression Equation |
|---|---|---|---|---|
| Anti OTA-HRP MNPs-ELISA | 0.25 | 1.53 | 0.39–5.94 | y = 0.5083x + 0.4065 (R2 = 0.9908) |
| OTA-BSA-HRP MNPs-ELISA | 0.14 | 0.54 | 0.20–1.44 | y = 0.7032x + 0.6884 (R2 = 0.9927) |
| OTA-HRP MNPs-ELISA | 0.04 | 0.31 | 0.06–1.13 | y = 0.4836x + 0.7477 (R2 = 0.9906) |
| Methods | Matrix | LOD (ng/mL) | Recovery (%) | Detection Range (ng/mL) | Ref. |
|---|---|---|---|---|---|
| Nanobody-based ELISA | Cereal | 0.16 | 80–105 | 0.27–1.47 | [30] |
| dsDNA-base fluorescence method | Corn | 5 | 89.2–94.1 | 0.00–100 | [33] |
| Portable Flurescence | Cocoa | 1.25 | 79.05–83.25 | 1.25–10 | [34] |
| Silver nanoparticles-based LFIA | Juice and wine | 0.06 | 88.0–110.0 | 0.08–5.0 | [35] |
| Fluorescence polarisation aptamer | Wine | 1.1 | 83–113 | - | [36] |
| Quantum dots-based aptasensor | Foodstuff | 0.5 | - | 1–30 | [37] |
| Fluorescent biosensor | Corn | 2.57 | 96.5–101.4 | 5.0–160 | [38] |
| A Label-Free Aptasensor | Corn | 0.012 | 96–106 | 0.04–0.48 | [39] |
| A Polyaniline film-based aptasensor | - | 0.1 | - | 0.1–10 | [40] |
| Fluorescent aptamer-based sensor | Corn | 0.8 | 83–106 | 1–100 | [41] |
| FRET aptasensor | Peanut | 0.0025 | 90–110 | 0.01–20 | [42] |
| Impedimetric aptasensor | Wine | 0.002 | 102–107 | 0.002–6 | [43] |
| MNPs-SPEs sensor | Cereal and feed | 0.007 | 78.7–113.5 | 0.01–0.82 | This work |
| Samples | Concentrations (μg/kg) | Recovery Rate (%) (Mean ± SD a) | CV b (%) | |
|---|---|---|---|---|
| Spiked | Detected | |||
| 1 | 1.25 | 1.42 | 113.5 ± 3.6 | 3.2 |
| 2 | 2.5 | 1.97 | 78.7 ± 7.3 | 9.3 |
| 3 | 5 | 4.26 | 85.3 ± 9.8 | 11.5 |
| 4 | 10 | 8.12 | 81.2 ± 5.8 | 7.2 |
| 5 | 20 | 18.34 | 91.6 ± 7.4 | 8.1 |
| Samples | MNPs-SPEs Sensor (μg/kg), Mean ± SD a | LC-MS/MS (μg/kg), Mean ± SD |
|---|---|---|
| Corn 1 | 12.37 ± 1.86 | 14.87 ± 1.21 |
| Corn 2 | 19.75 ± 2.17 | 22.94 ± 1.32 |
| Corn 3 | 21.67 ± 1.69 | 25.57 ± 2.01 |
| Corn 4 | 2.94 ± 0.67 | 3.98 ± 0.73 |
| Corn 5 | 24.28 ± 2.41 | 27.71 ± 2.13 |
| Wheat 1 | 27.41 ± 1.61 | 20.99 ± 1.71 |
| Wheat 2 | 25.97 ± 2.03 | 20.01 ± 1.61 |
| Wheat 3 | 2.35 ± 0.97 | 1.36 ± 0.12 |
| Wheat 4 | 5.98 ± 1.03 | 4.18 ± 0.31 |
| Feedstuff 1 | 15.86 ± 1.15 | 18.39 ± 1.03 |
| Feedstuff 2 | 21.65 ± 1.24 | 15.97 ± 1.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, Z.; Xie, H.; Sun, R.; Cao, T.; Paudyal, N.; Fang, W.; Song, H. Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples. Toxins 2018, 10, 317. https://doi.org/10.3390/toxins10080317
Zhang X, Wang Z, Xie H, Sun R, Cao T, Paudyal N, Fang W, Song H. Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples. Toxins. 2018; 10(8):317. https://doi.org/10.3390/toxins10080317
Chicago/Turabian StyleZhang, Xian, Zuohuan Wang, Hui Xie, Renjie Sun, Tong Cao, Narayan Paudyal, Weihuan Fang, and Houhui Song. 2018. "Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples" Toxins 10, no. 8: 317. https://doi.org/10.3390/toxins10080317