Ribosome-Inactivating Proteins: From Plant Defense to Tumor Attack
Abstract
:1. Ribosome Inactivating Proteins and Plant Defense Mechanism(s)
2. Biochemical Characteristics
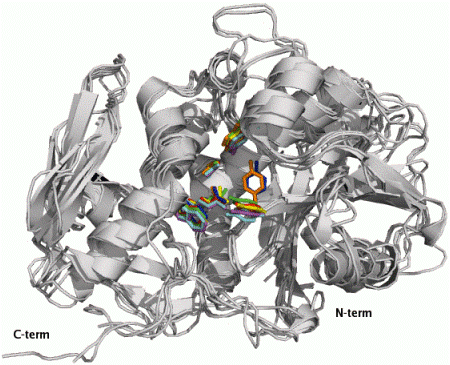
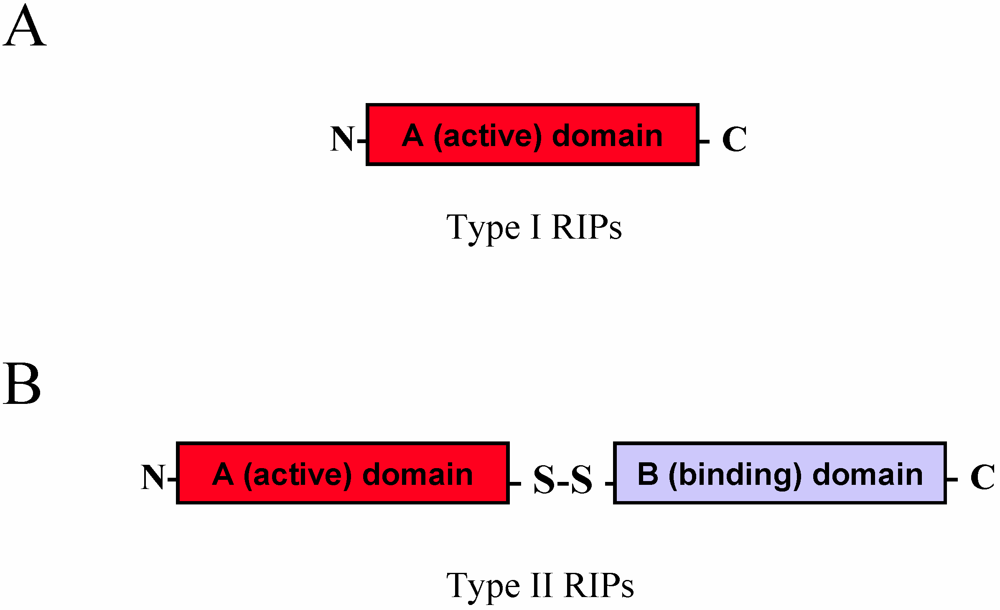
2.1. RIP Toxic Domains: Saporin Isoforms, RTA and 3-D Structures of Type I RIPs
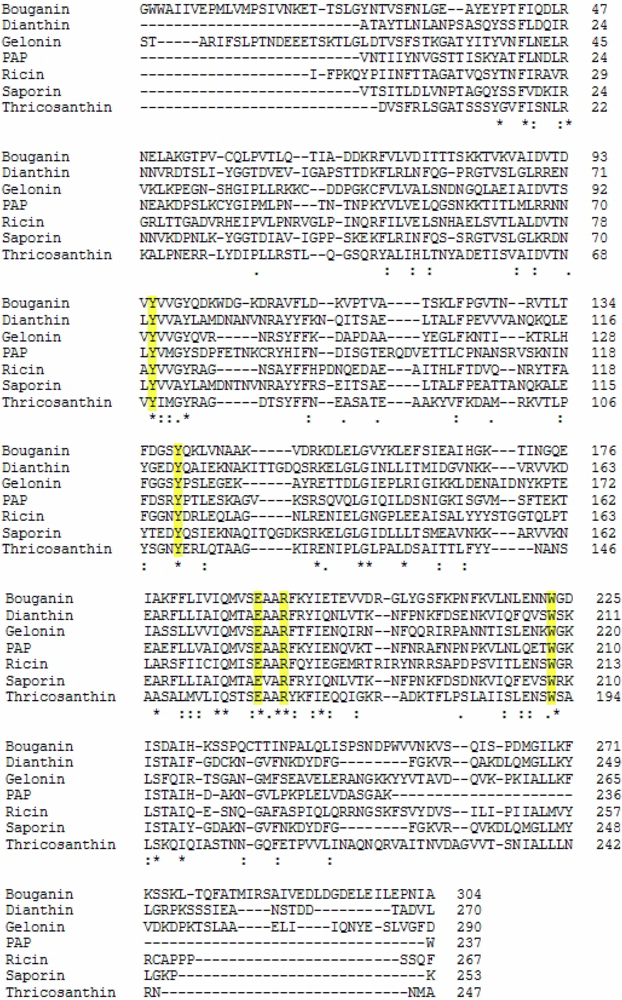
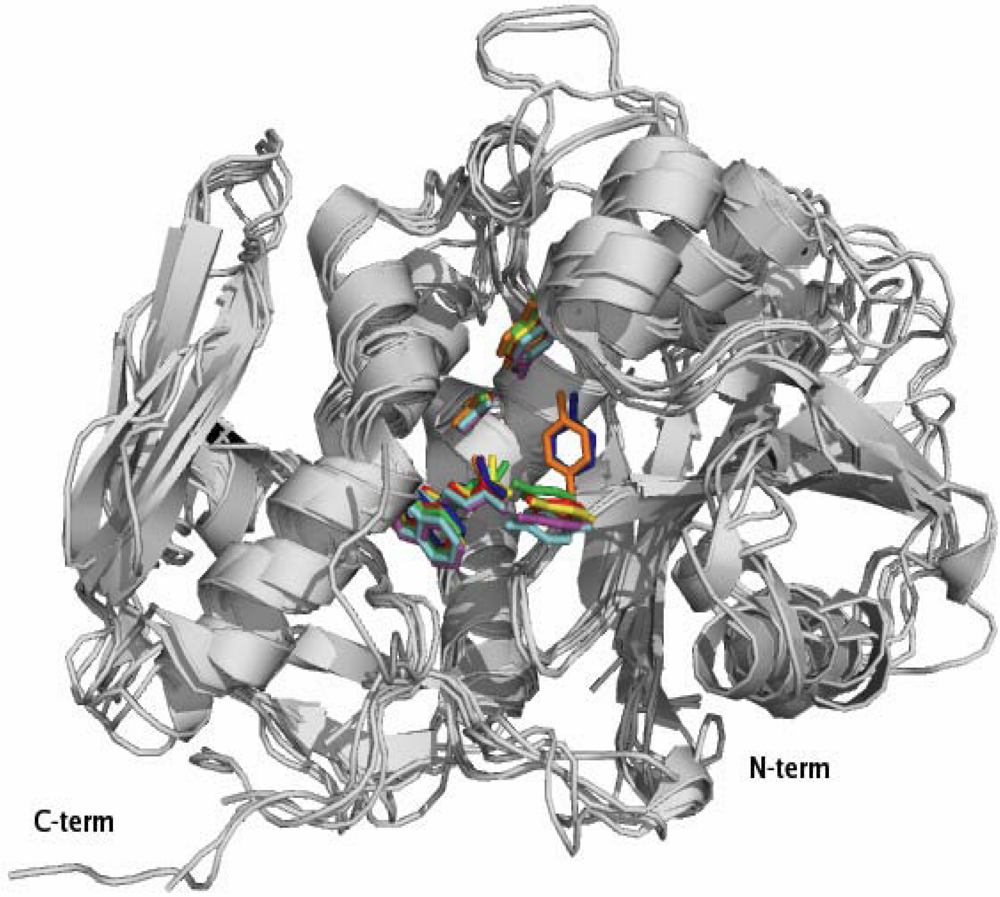
2.2. Interaction with Substrates and Catalytic Activity
2.3. Extra Enzymatic Activities
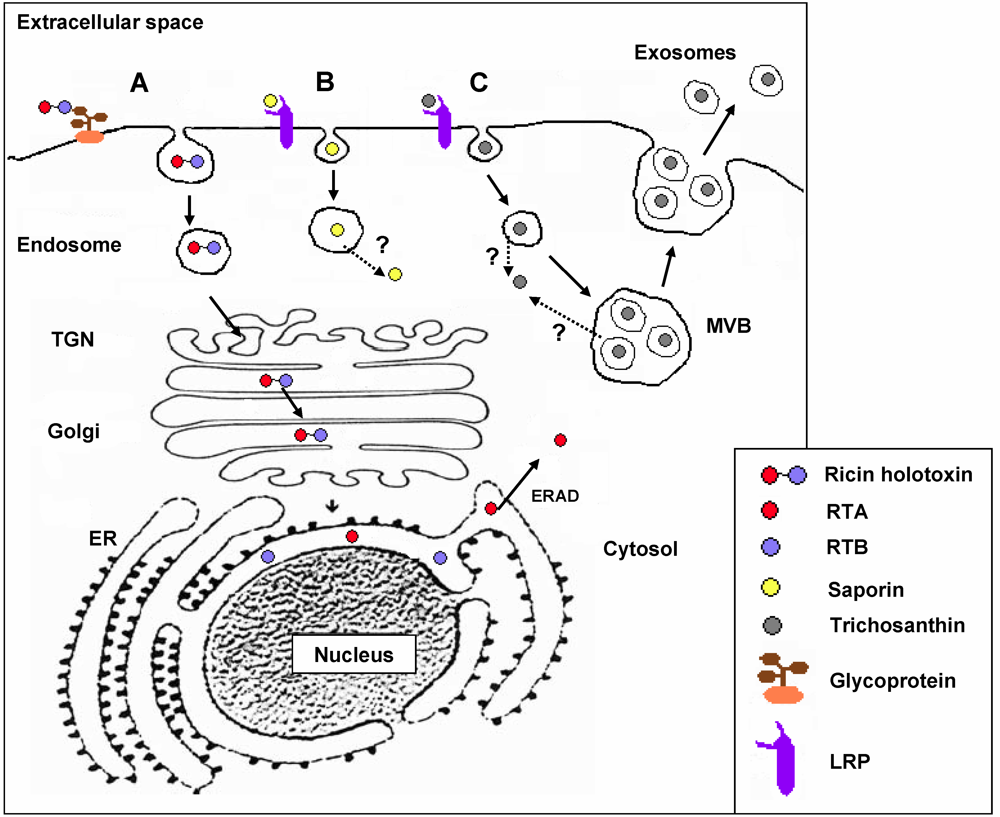
3. RIP Intoxication Routes in Mammalian Cells
4. Historical Uses of Plant RIPs
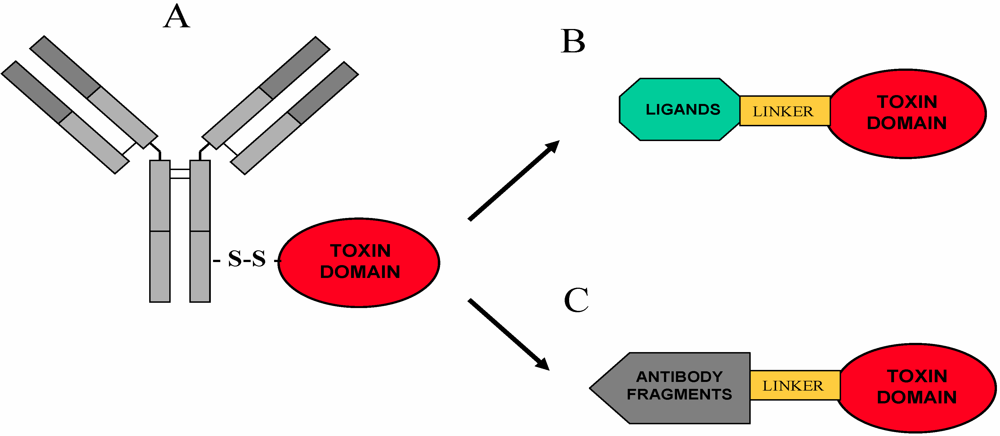
5. Immunotoxins and Targeted Chimeric Toxins
5.1. Overview
5.2. RIP-Based Immunotoxins
5.3. RIPs and Recombinant Fusions Expressed in Bacteria: Targets and Molecular Design
6. Yeast/Eukaryotic Expression Systems
7. Immunogenicity and Non Specific Toxicity Issues
8. Complementary Approaches
8.1. Proof-of-Concept IT Potential Evaluation with Streptavidin-Saporin
8.2. IT Potentiation Approaches
8.3. Non Viral Gene Delivery Approaches
9. Conclusions and Perspectives
Acknowledgements
References
- Stirpe, F. Ribosome-inactivating proteins. Toxicon 2004, 44, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Battelli, M.G. Ribosome-inactivating proteins: Progress and problems. Cell. Mol. Life Sci. 2006, 44, 1850–1866. [Google Scholar]
- Wang, P.; Tumer, N.E. Virus resistance mediated by ribosome inactivating proteins. Adv. Virus Res. 2000, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S.; Pihl, A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry 1973, 12, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M.; Roberts, L.M.; Robertus, J.D. Ricin: Structure, mode of action, and some current applications. FASEB J. 1994, 8, 201–208. [Google Scholar] [PubMed]
- Peumans, W.J.; Hao, Q.; Van Damme, E.J. Ribosome-inactivating proteins from plants: More than N-glycosidases? FASEB J. 2001, 15, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Barbieri, L. Ribosome-inactivating proteins up to date. FEBS Lett. 1986, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; An, C.S.; Liu, J.R.; Kwak, S-S.; Lee, H.S.; Lim, J.K.; Paek, K.H. Molecular cloning of a cDNA encoding ribosome-inactivating protein from Amaranthus viridis and its expression in E. coli. Mol. Cells 2000, 10, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.K.; Kaniewski, W.K.; Tumer, N.E. Broad-spectrum virus resistance in transgenic plants expressing pokeweed antiviral protein. Proc. Natl. Acad. Sci. USA 1993, 90, 7089–7093. [Google Scholar]
- Lam, Y.H.; Wong, Y.S.; Wang, B.; Wong, R.N.S.; Yeung, H.M.; Shaw, P.C. Use of trichosanthin to reduce infection by turnip mosaic virus. Plant Sci. 1996, 114, 111–117. [Google Scholar] [CrossRef]
- Moon, Y.H.; Song, S.K.; Choi, K.W.; Lee, J.S. Expression of a cDNA encoding Phytolacca insularis antiviral protein confers virus resistance of transgenic potato plants. Mol. Cells 1997, 7, 807–815. [Google Scholar] [PubMed]
- Taylor, S.; Massiah, A.; Lomonossoff, G.; Roberts, L.M.; Lord, J.M.; Hartley, M. Correlation between the activities of five ribosome-inactivating proteins in depurination of tobacco ribo-somes and inhibition of tobacco mosaic virus infection. Plant J. 1994, 5, 827–835. [Google Scholar] [PubMed]
- Tarantini, A.; Pittaluga, E.; Marcozzi, G.; Testone, G.; Rodrigues-Pousada, R.A.; Giannino, D.; Spanò, L. Differential expression of saporin genes upon wounding, ABA treatment and leaf development. Physiol. Plant. 2010, 140, 141–152. [Google Scholar] [PubMed]
- Carzaniga, R.; Sinclair, L.; Fordham-Skeleton, A.P.; Harris, N.; Croy, R.R.D. Cellular and subcellular distribution of saporins, type I ribosome-inactivating proteins, in soapwort (Saponaria officinalis L.). Planta 1994, 194, 461–470. [Google Scholar] [CrossRef]
- Ready, M.; Brown, D.T.; Robertus, J.D. Extracellular localization of pokeweed antiviral protein. Proc. Natl. Acad. Sci. USA 1986, 83, 5053–5056. [Google Scholar]
- Roberts, L.M.; Lord, J.M. The synthesis of Ricinus communis agglutinin-cotranslational and posttranslational modification of agglutinin polypeptides. J. Eur. Biochem. 1981, 119, 31–41. [Google Scholar] [CrossRef]
- Nielsen, K.; Boston, R.S. Ribosome-inactivating proteins: A plant perspective. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 785–816. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Crabtree, J.; Zhao, Q.; Lorenzi, H.; Orvis, J.; Puiu, D.; Melake-Berhan, A.; Jones, K.M.; Redman, J.; Chen, G.; Cahoon, E.B.; Gedil, M.; Stanke, M.; Haas, B.J.; Wortman, J.R.; Fraser-Liggett, C.M.; Ravel, J.; Rabinowicz, P.D. Draft genome sequence of the oilseed species Ricinus communis. Nat. Biotechnol. 2010, 28, 951–956. [Google Scholar] [PubMed]
- Weeks, A.; Leshin, J.A.; Dretchen, K.L.; Skowronski, E.W.; O'Connel, K.P. Population-level variation of the preproricin gene contradicts expectation of neutral equilibrium for generalist plant defense toxins. Toxicon 2010, 55, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.R.; Lord, J.M. Cytotoxic ribosome-inactivating lectins from plants. Biochim. Biophys. Acta. 2004, 1701, 1–14. [Google Scholar]
- Frigerio, L.; Jolliffe, N.A.; Di Cola, A.; Felipe, D.H.; Paris, N.; Neuhaus, J.M.; Lord, J.M.; Ceriotti, A.; Roberts, L.M. The internal propeptide of the ricin precursor carries a sequence-specific determinant for vacuolar sorting. Plant. Physiol. 2001, 126, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, L.; Vitale, A.; Lord, J.M.; Cerotti, A.; Roberts, L.M. Free Ricin A chain, proricin, native toxin have different cellular fates when expressed in tobacco protoplasts. J. Biol. Chem. 1998, 273, 14194–14199. [Google Scholar] [PubMed]
- Di Cola, A.; Frigerio, L.; Lord, J.M.; Ceriotti, A.; Roberts, L.M. Ricin A chain without its partner B chain is degraded after retrotranslocation from the endoplasmic reticulum to the cytosol in the plant cells. Proc. Natl. Acad. Sci. USA 2001, 98, 14726–14731. [Google Scholar]
- Vitale, A.; Boston, R.S. Endoplasmic reticulum quality control and the unfolded protein response: Insights from plants. Traffic 2008, 9, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Jolliffe, N.A.; Ceriotti, A.; Snowden, C.J.; Lord, J.M.; Frigerio, L.; Roberts, L.M. The role of CDC48 in the retro-translocation of non-ubiquitinated toxin substrates in plant cells. J. Biol. Chem. 2008, 283, 15869–15877. [Google Scholar] [PubMed]
- Prestle, J.; Schönfelder, M.; Adam, G.; Mundry, K.-W. Type 1 ribosome-inactivating proteins depurinate plant 25S rRNA without species specificity. Nucl. Acid. Res. 1992, 20, 3179–3182. [Google Scholar] [CrossRef]
- Bonness, M.S.; Ready, M.P.; Irvin, J.D.; Mabri, T.J. Pokeweed antiviral protein inactivates pokeweed ribosomes; implications for the antiviral mechanism. Plant J. 1994, 5, 173–183. [Google Scholar] [PubMed]
- Kataoka, J.; Habuka, N.; Miyano, M.; Masuta, C.; Koiwai, A. Adenine depurination and inactivation of plant ribosomes by an antiviral protein of Mirabilis jalapa (MAP). Plant Mol. Biol. 1992, 20, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Peumans, W.J.; Desmyter, S.; Proost, P.; Ciani, M.; Van Damme, E.J. The type 1 and type 2 ribosome-inactivating proteins from Iris confer transgenic tobacco plants local but not systemic protection against viruses. Planta 2004, 220, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Desvoyes, B.; Proyet, J.L.; Schlik, J.L.; Adami, P.; Jouvenot, M.; Dulieu, P. Identification of a biological inactive complex from a pokeweed antiviral protein. FEBS Lett. 1997, 410, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; D’Avila, F.; Di Cola, A.; Traini, R.; Spanò, L.; Fabbrini, M.S.; Ceriotti, A. Signal peptide-regulated toxicity of a plant ribosome inactivating protein during cell stress. Plant J. 2010, in press.. [Google Scholar]
- Kang, S.W.; Rane, N.S.; Kim, S.J.; Garrison, J.L.; Taunton, J.; Hegde, R.S. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 2006, 127, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Marshall, R.S.; Savino, C.; Fabbrini, M.S.; Ceriotti, A. Toxic Plant Proteins, Plant Cell Monographs; Lord, J.M., Hartley, M.R., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2010; Volume 18, p. 55. Chapter 4. [Google Scholar]
- Fabbrini, M.S.; Rappocciolo, E.; Carpani, D.; Solinas, M.; Valsasina, B.; Breme, U.; Cavallaro, U.; Nikjaer, A.; Rovida, E.; Legname, G.; Soria, M.R. Characterization of a saporin isoform with lower ribosome-inhibiting activity. Biochem. J. 1997, 322, 719–727. [Google Scholar] [PubMed]
- Rosenblum, M.G.; Kohor, W.A.; Beattie, K.L.; Beattie, W.G.; Marks, J.W.; Toman, P.D.; Cheung, L.H. Amino acid sequence analysis, gene construction, cloning, and expression of gelonin, a toxin derived from Gelonium multiflorum. J. Interf. Cytok. Res. 1995, 15, 547–555. [Google Scholar] [CrossRef]
- Montfort, W.; Villafranca, J.E.; Monzingo, A.F.; Ernst, S.R.; Katzin, B.; Rutenber, E.; Xuong, N.H.; Hamlin, R.; Robertus, J.D. The three-dimensional structure of ricin at 2.8 Å. J. Biol. Chem. 1987; 262, 5398–5403. [Google Scholar]
- Monzingo, A.F.; Collins, E.J.; Ernst, S.R.; Irwin, J.D.; Robertus, J.D. The 2.5 Å structure of pokeweed antiviral protein. J. Mol. Biol. 1993, 233, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Fu, Z.; Chen, M.; Lin, Y.; Pan, K. Structure of trichosanthin at 1.88 Å resolution. Proteins 1994, 19, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Hosur, M.V.; Nair, B.; Satyamurthy, P.; Misquith, S.; Surolia, A.; Kannan, K.K. X-ray structure of gelonin at 1.8 Å resolution. J. Mol. Biol. 1995, 250, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Savino, C.; Federici, L.; Ippoliti, R.; Lendaro, E.; Tsernoglou, D. The crystal structure of saporin SO6 from Saponaria officinalis and its interaction with the ribosome. FEBS Lett. 2000, 470, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Fermani, S.; Falini, G.; Ripamonti, A.; Polito, L.; Stirpe, F.; Bolognesi, A. The 1.4 Å structure of dianthin 30 indicates a role of surface potential at the active site of type 1 ribosome-inactivating proteins. J. Struct. Biol. 2005, 149, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Weston, S.A.; Tucker, A.D.; Thatcher, D.R.; Derbyshire, D.J.; Pauptit, R.A. X-ray structure of recombinant ricin A-chain at 1.8 Å resolution. J. Mol. Biol. 1994, 244, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.A.; Bartle, L.M.; Leszyk, J.D.; Lambert, J.M.; Goldmacher, V.S. Ricin A chain can be chemically crosslinked to the mammalian ribosomal proteins L9 and L10e. J. Biol. Chem. 1995, 270, 12933–12940. [Google Scholar] [PubMed]
- Hudak, K.A.; Dinman, J.D.; Tumer, N.E. Pokeweed antiviral protein accesses ribosomes by binding to L3. J. Biol. Chem. 1999, 274, 3859–3864. [Google Scholar] [PubMed]
- Ippoliti, R.; Lendaro, E.; Bellelli, A.; Brunori, M. A ribosomal protein is specifically recognized by saporin, a plant toxin which inhibits protein synthesis. FEBS Lett. 1992, 298, 145–148. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, A.J.; Poon, G.M.K.; Bolewska-Pedyczak, E.; Srikumar, T.; Jeram, S.M.; Raught, B.; Gariepy, J. The catalytic subunit of Shiga-like toxin 1 interacts with ribosomal stalk proteins and is inhibited by their conserved C-terminal domain. J. Mol. Biol. 2008, 378, 375–386. [Google Scholar] [PubMed]
- Korennykh, A.V.; Correll, C.C.; Piccirilli, A.J. Evidence for the importance of electrostatics in the function of two distinct families of ribosome inactivating toxins. RNA 2007, 13, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y. Mechanism of action of ricin and related toxins on the inactivation of eukaryotic ribosomes. Canc. Treat. Res. 1988, 37, 75–89. [Google Scholar]
- Endo, Y.; Tsurugi, K.; Lambert, J.M. The site of action of six different ribosome-inactivating proteins from plants on eukaryotic ribosomes. The RNA N-glycosidase activity of the proteins. Biochem. Biophys. Res. Commun. 1988, 150, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Gorini, P.; Valbonesi, P.; Castiglioni, P.; Stirpe, F. Unexpected activity of saporins. Nature 1994, 372, 624. [Google Scholar] [PubMed]
- Barbieri, L.; Valbonesi, P.; Bonora, E.; Gorini, P.; Bolognesi, A.; Stirpe, F. Polynucleotide: adenosine glycosidase activity of ribosome-inactivating proteins: Effect on DNA, RNA and poly(A). Nucl. Acid. Res. 1997, 25, 518–522. [Google Scholar]
- Bagga, S.; Seth, D.; Batra, J.K. The cytotoxic activity of ribosome-inactivating protein saporin-6 is attributed to its rRNA N-glycosidase and internucleosomal DNA fragmentation activities. J. Biol. Chem. 2003, 278, 4813–4820. [Google Scholar] [PubMed]
- Zarovni, N.; Vago, R.; Soldà, T.; Monaco, L.; Fabbrini, M.S. Saporin as a novel suicide gene in anticancer gene therapy. Canc. Gene Ther. 2007, 14, 165–173. [Google Scholar] [CrossRef]
- Lombardi, A.; Bursomanno, S.; Lopardo, T.; Traini, R.; Colombatti, M.; Ippoliti, R.; Flavell, D.J.; Flavell, S.U.; Cerotti, A.; Fabbrini, M.S. Pichia pastoris as a host for secretion of toxic saporin chimeras. FASEB J. 2010, 24, 253–265. [Google Scholar] [PubMed]
- Rajamohan, F.; Pugmire, M.J.; Kurinov, I.V.; Uckun, F.M. Modeling and alanine scanning mutagenesis studies of recombinant pokeweed antiviral protein. J. Biol. Chem. 2000, 275, 3382–3390. [Google Scholar] [PubMed]
- Hudak, K.A.; Wang, P.; Tumer, N.E. A novel mechanism for inhibition of translation by pokeweed antiviral protein: Depurination of the capped RNA template. RNA 2000, 6, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Roncuzzi, L.; Gasperi-Campani, A. DNA-nuclease activity of single-chain ribosome-inactivating proteins dianthin 30, saporin 6 and gelonin. FEBS Lett. 1996, 392, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, E.; Beggs, J.M.; Haltiwanger, B.M.; Taraschi, T.F. A new class of DNA glycosylase/apurinic/apyrimidinic lyases that act on specific adenines in single-stranded DNA. J. Biol. Chem. 1998, 273, 17216–1720. [Google Scholar] [PubMed]
- Fermani, S.; Tosi, G.; Farini, V.; Polito, L.; Falini, G.; Ripamonti, A.; Barbieri, L.; Chambery, A.; Bolognesi, A. Structure/function studies on two type 1 ribosome inactivating proteins: Bouganin and Lychinin. J. Struct. Biol. 2009, 168, 278–287. [Google Scholar] [PubMed]
- Li, X.-D.; Chen, W.-F.; Liu, W.-Y.; Wang, G.-H. Large-scale preparation of two new ribosome-inactivating proteins, Cinnamomin and Camphorin, from the seeds of Cinnamomum camphora. Protein Expr. Purif. 1997, 10, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.; Lombard, S.; Pieroni, G. Ricin RCA60: Evidence of its phospholipase activity. Biochem. Biophys. Res. Commun. 1999, 258, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Park, S-W.; Vepachedu, R.; Barbieri, L.; Ciani, M.; Stirpe, F.; Savary, B.J.; Vivanco, J.M. Isolation and characterization of a RIP-like protein from Nicotiana tabacum with dual enzymatic activity. Plant Physiol. 2004, 134, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.B.; Tyler, P.C.; Evans, G.B.; Schramm, V.L. Transition state analogues rescue ribosomes from saporin L1 ribosome inactivating protein. Biochemistry 2009, 48, 9941–9948. [Google Scholar] [PubMed]
- Vago, R.; Marsden, C.J.; Lord, J.M.; Ippoliti, R.; Flavell, D.J.; Flavell, S.U.; Ceriotti, A.; Fabbrini, M.S. Saporin and ricin A chain follow different intracellular routes to enter the cytosol of intoxicated cells. FEBS J. 2005, 272, 4983–4995. [Google Scholar] [CrossRef] [PubMed]
- Day, P.J.; Lord, J.M.; Roberts, L.M. The deoxyribonuclease activity attributed to ribosome inactivating proteins is due to contamination. Eur. J. Biochem. 1998, 258, 540–545. [Google Scholar] [PubMed]
- Barbieri, L.; Valbonesi, P.; Righi, F.; Zucceri, G.; Monti, F.; Gorini, P.; Samori, B.; Stirpe, F. Polynucleotide: Adenosine glycosidase is the sole activity of ribosome-inactivating proteins DNA. J. Biochem. 2000, 128, 883–889. [Google Scholar] [PubMed]
- Sandvig, K.; van Deurs, B. Delivery into cells: Lessons learned from plant and bacterial toxins. Gene Ther. 2005, 12, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.A; Watson, P.D; Marsden, C.J.; Smith, D.C.; Moore, K.A.H.; Cook, J.P.; Lord, J.M.; Roberts, L.M. Protein disulphide isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem. J. 2004, 383, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D.; Cook, J.P.; Day, P.J.; Smith, D.C.; Roberts, L.M.; Lord, J.M. The low lisine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochemistry. 2002, 41, 3405–3413. [Google Scholar] [PubMed]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL Receptor Related Protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008, 88, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Kreitman, J.K. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the a2-macroglobulin receptor. J. Biol. Chem. 2000, 275, 7566–7573. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, M.S.; Carpani, D.; Bello-Rivero, I.; Soria, M.R. The amino-terminal fragment of human urokinase directs a recombinant chimeric toxin to target cells: Internalization is toxin-mediated. FASEB J. 1997, 11, 1169–1176. [Google Scholar] [PubMed]
- Ippoliti, R.; Lendaro, E.; Benedetti, P.A.; Torrisi, M.R.; Belleudi, F.; Carpani, D.; Soria, M.R.; Fabbrini, M.S. Endocytosis of a chimera between human pro-urokinase and the plant toxin saporin: An unusual internalization mechanism. FASEB J. 2000, 14, 1335–1344. [Google Scholar] [PubMed]
- Wales, R.; Roberts, L.M.; Lord, J.M. Addition of an endoplasmic reticulum retrieval sequence to ricin A chain significantly increases its cytotoxicity to mammalian cells. J. Biol. Chem. 1993, 268, 23986–23990. [Google Scholar] [PubMed]
- Geden, S.; Gardner, R.; Fabbrini, M.S.; Ohashi, M.; Phanstiel, I.O.; Teter, K. Lipopolyamine treatment increases the efficacy of intoxication with saporin and an anticancer saporin conjugate. FEBS J. 2007, 274, 4825–4836. [Google Scholar] [PubMed]
- Fabbrini, M.S.; Carpani, D.; Soria, M.R.; Ceriotti, A. Cytosolic immunization allows the expression of preATF-saporin chimeric toxin in eukaryotic cells. FASEB J. 2000, 14, 391–398. [Google Scholar] [PubMed]
- Zhang, F; Sun, S; Feng, D; Zhao, W.L.; Sui, S.F. A novel strategy for the invasive toxin: Hijacking exosome-mediated intercellular trafficking. Traffic 2009, 10, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.; Shaw, P.C.; Tam, S.C.; Jacobsen, C.; Gliemann, J.; Nielsen, M.S. Trichosanthin interacts with and enters cells via LDL receptor family members. Biochem. Biophys. Res. Commun. 2000, 270, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.G.; Cheung, L.H.; Liu, Y.; Marks, J.W. Design, expression, Purification and characterization, in vitro and in vivo, of an antimelanoma single-chain Fv antibody fused to the toxin gelonin. Canc. Res. 2003, 63, 3995–4002. [Google Scholar]
- Vallera, D.A.; Oh, S.; Chen, H.; Shu, Y.; Frankel, A.E. Bioengineering a unique deimmunized bispecific targeted toxin that simultaneously recognizes human CD22 and CD19 receptors in a mouse model of B-cell metastases. Mol. Canc. Ther. 2010, 9, 1872–1883. [Google Scholar] [CrossRef]
- Amessou, M.; Fradagrada, A.; Falguières, T.; Lord, J.M.; Smith, D.C.; Roberts, L.M.; Lamaze, C.; Johannes, L. Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J. Cell Sci. 2007, 120, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Wesche, J.; Rapak, A.; Olsnes, S. Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 1999, 274, 34443–34449. [Google Scholar]
- Mayerhofer, P.U.; Cook, J.P.; Wahlman, J.; Pinheiro, T.T.; Moore, K.A.; Lord, J.M.; Johnson, A.E.; Roberts, L.M. Ricin A chain insertion into endoplasmic reticulum membranes is triggered by a temperature increase to 37 ºC. J. Biol. Chem. 2009, 284, 10232–10242. [Google Scholar] [PubMed]
- Li, S.; Spooner, R.A.; Allen, S.C.; Guise, C.P.; Ladds, G.; Schnöder, T.; Schmitt, M.J.; Lord, J.M.; Roberts, L.M. Folding-competent and folding-defective forms of ricin A chain may have different fates. Mol. Biol. Cell. 2010, 21, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.A.; Hart, P.J.; Cook, J.P.; Pietroni, P.; Rogon, C.; Höhfeld, J.; Roberts, L.M.; Lord, J.M. Cytosolic chaperones influence the fate of a toxin dislocated from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2008, 105, 17408–17413. [Google Scholar]
- Austin, C.D.; Wen, X.; Gazzard, L.; Nelson, C.; Scheller, R.H.; Scales, S.J. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc. Natl. Acad. Sci. USA 2005, 102, 17987–17992. [Google Scholar]
- Bellisola, G.; Fracasso, G.; Ippoliti, R.; Menestrina, G.; Rosén, A.; Soldà, S.; Udali, S.; Tomazzolli, R.; Tridente, G.; Colombatti, M. Reductive activation of ricin and ricin A-chain immunotoxins by protein disulfide isomerase and thioredoxin reductase. Biochem. Pharmacol. 2004, 67, 1721–1731. [Google Scholar]
- Zarling, J.M.; Moran, P.A.; Haffar, O.; Sias, J.; Richman, D.D.; Spina, C.A.; Myers, D.E.; Kuebelbeck, V.; Ledbetter, J.A.; Uckun, F.M. Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4+ cells by monoclonal antibodies. Nature 1990, 347, 92–95. [Google Scholar]
- McGrath, M.S.; Hwang, K.M.; Caldwell, S.E.; Gaston, I.; Luk, K.C.; Wu, P.; Ng, W.L.; Crowe, S.; Daniels, J.; Marsh, J. GLQ223: An inhibitor of human immunodeficiency virus replication in acutely and chronically infected cells of lymphocyte and mononuclear phagocyte lineage. Proc. Natl. Acad. Sci. USA. 1989, 86, 2844–2848. [Google Scholar]
- Byers, V.S.; Levin, A.S.; Malvino, A.; Waites, L.A.; Robins, R.A.; Baldwin, R.W. A phase II study of effect of addition of trichosanthin to zidovudine in patients with HIV disease and failing antiretroviral agents. AIDS Res. Hum. Retrovir. 1994, 10, 413–420. [Google Scholar] [CrossRef]
- Yeung, H.W.; Li, W.W.; Feng, Z.; Barbieri, L.; Stirpe, F. Trichosanthin, alpha-momorcharin and beta-momorcharin: Identity of abortifacient and ribosome-inactivating proteins. Int. J. Pept. Protein Res. 1988, 31, 265–268. [Google Scholar]
- Battelli, M.G.; Mantacuti, V.; Stirpe, F. High sensitivity of cultured human trophoblasts to ribosome-inactivating proteins. Exp. Cell Res. 1992, 201, 109–112. [Google Scholar]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar]
- Fredriksson, S.A.; Hulst, A.G.; Artursson, E.; de Jong, A.L.; Nilsson, C.; van Baar, B.L. Forensic identification of neat ricin and of ricin from crude castor bean extracts by mass spectrometry. Anal. Chem. 2005, 77, 1545–1555. [Google Scholar]
- Rubina, A.Y.; Dyukova, V.I.; Dementieva, E.I.; Stomakhin, A.A.; Nesmeyanov, V.A.; Grishin, E.V.; Zasedatelev, A.S. Quantitative immunoassay of biotoxins on hydrogelbased protein microchips. Anal. Biochem. 2005, 340, 317–329. [Google Scholar]
- Guo, J.W.; Shen, B.F.; Feng, J.N.; Sun, Y.X.; Yu, M.; Hu, M.R. A novel neutralizing monoclonal antibody against cell-binding polypeptide of ricin. Hybridoma (Larchmt) 2005, 24, 263–266. [Google Scholar]
- Vitetta, E.S.; Smallshaw, J.E.; Coleman, E.; Jafri, H.; Foste, C.; Munford, R.; Schindler, J. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc. Natl. Acad. Sci. USA 2006, 103, 2268–2273. [Google Scholar]
- Marconescu, P.S.; Smallshaw, J.E.; Pop, L.M.; Ruback, S.L.; Vitetta, E.S. Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine 2010, 28, 5315–5322. [Google Scholar]
- Campoli, M.; Ferris, R.; Ferrone, S.; Wang, X. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin. Canc. Res. 2010, 16, 11–20. [Google Scholar]
- Fracasso, G.; Stirpe, F.; Colombatti, M. Toxic Plant Proteins, Plant Cell Monographs; Lord, J.M., Hartley, M.R., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2010; Volume 18, p. 255. Chapter 12. [Google Scholar]
- Huston, J.S.; Mudgett-Hunter, M.; Tai, M.S.; McCartney, J.; Warren, F.; Haber, E.; Oppermann, H. Protein engineering of single-chain Fv analogs and fusion proteins. Meth. Enzymol. 1991, 203, 46–88. [Google Scholar]
- Huston, J.S.; Tai, M.S.; McCartney, J.; Keck, P.; Oppermann, H. Antigen recognition and targeted delivery by the single-chain Fv. Cell Biophys. 1993, 22, 189–224. [Google Scholar]
- Kreitman, R.J. Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies. BioDrugs 2009, 23, 1–13. [Google Scholar]
- Kreitman, R.J. Toxin-labeled monoclonal antibodies. Curr. Pharm. Biotechnol. 2001, 2, 313–325. [Google Scholar]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin treatment of cancer. Annu. Rev. Med. 2007, 58, 221–237. [Google Scholar]
- Savage, P; Rowlinson-Busza, G.; Verhoeyen, M.; Spooner, R.A.; So, A.; Windust, J.; Davis, P.J.; Epenetos, A.A. Construction, characterisation and kinetics of a single chain antibody recognising the tumour associated antigen placental alkaline phosphatase. Br. J. Canc. 1993, 68, 738–742. [Google Scholar] [CrossRef]
- Batra, S.K.; Jain, M.; Wittel, U.A.; Chauhan, S.C.; Colcher, D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr. Opin. Biotechnol. 2002, 13, 603–608. [Google Scholar]
- Jain, M.; Chauhan, S.C.; Singh, A.P.; Venkatraman, G.; Colcher, D.; Batra, S.K. Penetratin improves tumor retention of single-chain antibodies: A novel step toward optimization of radioimmunotherapy of solid tumors. Canc. Res. 2005, 65, 7840–7846. [Google Scholar]
- Fabbrini, M.S.; Flavell, D.J.; Ippoliti, R. Bacterial Plant and Animal Toxins; Ascenzi, P., Polticelli, F., Visca, P., Eds.; Research Signpost: Kerala, India, 2003; p. 69. Chapter 4. [Google Scholar]
- Amlot, P.L.; Stone, M.J.; Cunningham, D.; Fay, J.; Newman, J.; Collins, R.; May, R.; McCarthy, M.; Richardson, J.; Ghetie, V.; et al. A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapy. Blood 1993, 82, 2624–2633. [Google Scholar] [PubMed]
- Falini, B.; Bolognesi, A.; Flenghi, L.; Tazzari, P.L.; Broe, M.K.; Stein, H.; Dürkop, H.; Aversa, F.; Corneli, P.; Pizzolo, G.; et al. Response of refractory Hodgkin's disease to monoclonal anti-CD30 immunotoxin. Lancet 1992, 339, 1195–1196. [Google Scholar] [PubMed]
- Flavell, D.J.; Noss, A.; Pulford, K.; Flavell, S. Systemic therapy with 3BIT, a triple combination cocktail of anti-CD19, -CD22, and -CD38-saporin immunotoxins, is curative of human B-cell lymphoma in severe combined immunodeficient mice. Canc. Res. 1997, 57, 4824–4829. [Google Scholar]
- Laske, D.W.; Muraszko, K.M.; Oldfield, E.H.; DeVroom, H.L.; Sung, C.; Dedrik, R.L.; Simon, T.R.; Colandrea, J.; Copeland, C.; Katz, D.; Greenfield, L.; Grooves, E.S.; Houston, L.L.; Youle, R.J. Intraventricular immunotoxin therapy for leptomeningeal neoplasia. Neurosurgery 1997, 41, 1039–1049. [Google Scholar]
- Pagliaro, L.C.; Liu, B.; Bunker, R.; Andreef, M.; Freireich, E.J.; Scheinberg, D.A.; Rosenblum, M.G. Humanized M195 monoclonal antibody conjugated to recombinant gelonin: An anti-CD33 immunotoxin with antileukemic activity. Clin. Canc. Res. 1998, 4, 1971–1976. [Google Scholar]
- Pennell, C.A.; Pauza, M.E. CD7-specific single chain Fv immunotoxins. Design and expression. Meth. Mol. Biol. 2001, 166, 17–29. [Google Scholar]
- Chignola, R.; Anselmi, C.; Dalla Serra, M.; Franceschi, A.; Fracasso, G.; Pasti, M.; Chiesa, E.; Lord, J.M.; Tridente, G.; Colombatti, M. Self-potentiation of ligand-toxin conjugates containing ricin A chain fused with viral structures. J. Biol. Chem. 1995, 270, 23345–23351. [Google Scholar]
- Tazzari, P.L.; Polito, L.; Bolognesi, A.; Pistillo, M.P.; Capanni, P.; Palmisano, G.L.; Lemoli, R.M.; Curti, A.; Biancone, L.; Camussi, G.; Conte, R.; Ferrara, G.B.; Stirpe, F. Immunotoxins containing recombinant anti-CTLA-4 single-chain fragment variable antibodies and saporin: in vitro results and in vivo effects in an acute rejection model. J. Immunol. 2001, 167, 4222–4229. [Google Scholar] [PubMed]
- Piazza, T.; Cha, E.; Bongarzone, I.; Canevari, S.; Bolognesi, A.; Polito, L.; Bargellesi, A.; Sassi, F.; Ferrini, S.; Fabbi, M. Internalization and recycling of ALCAM/CD166 detected by a fully human single-chain recombinant antibody. J. Cell Sci. 2005, 118, 1515–1525. [Google Scholar]
- Fuchs, H.; Bachran, C.; Li, T.; Heisler, I.; Durkop, H.; Sutherland, M. A cleavable molecular adapter reduces side effects and concomitantly enhances efficacy in tumor treatment by targeted toxins in mice. J. Control. Release 2007, 117, 342–350. [Google Scholar]
- O’Hare, M.; Brown, A.N.; Hussain, K.; Gebhardt, A.; Watson, G.; Roberts, L.M. Cytotoxicity of a recombinant ricin-A-chain fusion protein contain a proteolytically-cleavable spacer sequence. FEBS Lett. 1990, 273, 200–204. [Google Scholar]
- Hartley, M.R.; Legname, G.; Osborn, R.; Chen, Z.; Lord, J.M.S. Single chain ribosome inactivating proteins from plants depurinate Escherichia coli 23S ribosomal RNA. FEBS Lett. 1991, 290, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Chaddock, J.A; Lord, J.M; Hartley, M.R; Roberts, L.M. Pokeweed antiviral protein (PAP) mutations which permit E. coli growth do not eliminate catalytic activity towards prokaryotic ribosomes. Nucl. Acid. Res. 1994, 22, 1536–1540. [Google Scholar] [CrossRef]
- Kataoka, J.; Ago, H.; Habuka, N.; Furano, M.; Masuta, C.; Miyano, M.; Koiwai, A. Expression of a pokeweed antiviral protein in Escherichia coli and its characterization. FEBS Lett. 1990, 320, 31–34. [Google Scholar]
- Liu, L.; Wang, R.; He, W.; He, F.; Huang, G. Cloning and soluble expression of mature α-luffin from Luffa cylindrica and its antitumor activities in vitro. Acta Biochim. Biophys. Sin. (Shanghai) 2010, 42, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Villemain, J; Padilla, R; Sousa, R. Mechanisms by which T7 lysozyme specifically regulates T7 RNA polymerase during different phases of transcription. J. Mol. Biol. 1999, 293, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Di Leandro, L.; Koutris, I.; Pitari, G.; Fabbrini, M.S.; Lombardi, A.; Flavell, D.J.; Flavell, S.U.; Gianni, S.; Ippoliti, R. Engineering a switchable toxin: The potential use of PDZ domains in the expression, targeting and activation of modified Saporin variants. Protein Eng. Des. Sel. 2010, 23, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, C.; Roberts, L.; Fawell, S.; Zdanovsky, A.G.; FitzGerald, D.J.; Lord, J.M. Generation of a potent chimeric toxin by replacement of domain III of Pseudomonas exotoxin with ricin A chain KDEL. Bioconjug. Chem. 1995, 6, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Dore, J.M.; Gras, E.; Wijdenes, J. Expression and activity of a recombinant chimeric protein composed of pokeweed antiviral protein and of human interleukin-2. FEBS Lett. 1997, 402, 50–52. [Google Scholar]
- Qi, L.; Nett, T.M.; Allen, M.C.; Sha, X.; Harrison, G.S.; Federick, B.A.; Crawford, E.D.; Glode, L.M. Binding and cytotoxicity of conjugated and recombinant fusion proteins targeted to the Gonadotropin-releasing hormone receptor. Canc. Res. 2004, 64, 2090–2095. [Google Scholar]
- Lappi, D.A.; Ying, W.; Barthelemy, I.; Martineau, D.; Prieto, I.; Benatti, L.; Soria, M.R.; Baird, A. Expression and activities of a recombinant basic fibroblast growth factor-saporin fusion protein. J. Biol. Chem. 1994, 269, 12552–12558. [Google Scholar]
- Davol, P.A.; Beitz, J.G.; Mohler, M.; Ying, W.; Cook, J.; Lappi, D.A.; Frackelton, A.R., Jr. Saporin toxins directed to basic fibroblast growth factor receptors effectively target human ovarian teratocarcinoma in an animal model. Cancer 1995, 76, 79–85. [Google Scholar]
- Davol, P.A.; Garza, S.; Frackelton, A.R., Jr. Combining suramin and a chimeric toxin directed to basic fibroblast growth factor receptors increases therapeutic efficacy against human melanoma in an animal model. Cancer 1999, 86, 1733–1741. [Google Scholar]
- Chandler, L.A.; Sosnowski, B.A.; McDonald, J.R.; Price, J.E.; Aukerman, S.L.; Baird, A.; Pierce, G.F.; Houston, L.L. Targeting tumor cells via EGF receptors: Selective toxicity of an HBEGF-toxin fusion protein. Int. J. Canc. 1998, 78, 106–111. [Google Scholar]
- Ellis, V.; Scully, M.F.; Kakkar, V.V. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator.Potentiation by U937 monocytes. J. Biol. Chem. 1989, 264, 2185–2188. [Google Scholar] [PubMed]
- Ragno, P. The urokinase receptor ligand: A ligand or a receptor? Story of a sociable molecule. Cell. Mol. Life Sci. 2006, 63, 1028–1037. [Google Scholar]
- Stephens, R.W.; Nielsen, H.J.; Christensen, I.J.; Thorlacius-Ussing, O.; SØrensen, S.; DanØ, K.; Brünner, N. Plasma urokinase receptor levels in patients with colorectal cancer: Relationship to prognosis. J. Natl. Canc. Inst. 1999, 91, 869–874. [Google Scholar]
- Mustijoki, S.; Alitalo, R.; Stephens, R.W.; Vaheri, A. Blast cell-surface and plasma soluble urokinase receptor in acute leukaemia patients: relationship to classification and response to therapy. Thromb. Haemost. 1999, 81, 705–710. [Google Scholar]
- Sier, C.F.; Sidenius, N.; Mariani, A.; Aletti, G.; Agape, V.; Ferrai, A.; Casetta, G.; Stephens, R.W.; Brünner, N.; Blasi, F. Presence of urokinase-type plasminogen activator receptor in urine of cancer patients and its possibile clinical relevance. Lab. Invest. 1999, 79, 717–722. [Google Scholar]
- Rasch, M.G.; Lund, I.K.; Almasi, C.E.; HØyer-Hansen, G. Intact and cleaved uPAR forms: Diagnostic and prognostic value in cancer. Front. Biosc. 2008, 13, 6752–6762. [Google Scholar]
- Degryse, B.; Fernandez-Recio, J.; Citro, V.; Blasi, F.; Cubellis, M.V. In silico docking of urokinase plasminogen activator and integrins. BMC Bioinform. 2008, 9, S8. [Google Scholar]
- Potala, S.; Sahoo, S.K.; Verma, R.S. Target therapy of cancer using diphtheria toxin-derived immunotoxins. Drug. Discov. Today 2008, 13, 809–815. [Google Scholar]
- Vallera, D.A.; Li, C.; Jin, N.; Panoskaltis-Mortari, A.; Hall, W.A. Targeting urokinase-type plasminogen activator receptor on human glioblastoma tumors with diphtheria toxin fusion protein DTAT. J. Nat Canc. Inst. 2002, 94, 597–606. [Google Scholar]
- Ramage, J.G.; Vallera, D.A.; Black, J.H.; Aplan, P.D.; Kees, U.R.; Frankel, A.E. The diphtheria toxin/urokinase fusion protein (DTAT) is selectively toxic to CD87 expressing leukemic cells. Leuk. Res. 2003, 27, 79–84. [Google Scholar]
- Rustamzadeh, E.; Hall, W.A.; Todhunter, D.A.; Vallera, V.D.; Low, W.C.; Liu, H.; Panoskaltis-Mortari, A.; Vallera, D.A. Intracranial therapy of glioblastoma with the fusion protein DTAT in immunodeficient mice. Int. J. Canc. 2007, 120, 411–419. [Google Scholar]
- Todhunter, D.A.; Mhall, W.A; Rustamzedeh, E.; Shu, Y; Doumbia, S.O; Vallera, D.A. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng. Des. Sel. 2004, 17, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rustamzadeh, E.; Vallera, D.A.; Todhunter, D.A.; Low, W.C.; Panoskaltis-Mortari, A.; Hall, W.A. Immunotoxin pharmacokinetics: a comparison of the anti-glioblastoma bi-specific fusion protein (DTAT13) to DTAT and DTL13. J. Neurooncol. 2006, 77, 257–266. [Google Scholar]
- Frankel, A.E. Reducing the Immune Response to Immunotoxin. Clinic. Canc. Res. 2004, 10, 16–18. [Google Scholar]
- Hall, P.D.; Virella, G.; Willoughby, T.; Atchley, D.H.; Kreitman, R.J.; Frankel, A.E. Antibody Response to DT-GM, a novel fusion toxin consisting of truncated diphtheria toxin (DT) linked to human granulocyte-macrophage colony stimulating factor (GM), during a phase I trial of patient with relapsed or refractory acute myeloid leukaemia. Clin. Immunol. 2001, 100, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Deckert, P.M. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr. Drugs Targets 2009, 10, 158–175. [Google Scholar]
- Carter, P.J.; Senter, P.D. Antibody-drug conjugates for cancer therapy. Canc. J. 2008, 14, 154–169. [Google Scholar]
- Whitlow, M.; Bell, B.A.; Feng, S.L.; Filpula, D.; Hardman, K.D.; Hubert, S.L.; Rollence, M.L.; Wood, J.F.; Schott, M.E.; Milenic, D.E.; Yokota, T.; Schlom, J. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993, 6, 989–995. [Google Scholar]
- Kim, J.H.; Weaver, R.F. Construction of a recombinant expression plasmid encoding a staphylococcal protein A-ricin A fusion protein. Gene 1988, 68, 315–321. [Google Scholar]
- Cao, Y.; Marks, J.D.; Marks, J.W.; Cheung, L.H.; Sehoon, K.; Rosenblum, M.G. Construction and characterization of novel, recombinant immunotoxins targeting the Her2/neu oncogene product: in vitro and in vivo studies. Canc. Res. 2009, 69, 8987–8995. [Google Scholar]
- Nimmanapalli, R.; Lyu, M.-A.; Du, M.; Keating, M.J.; Rosenblum, M.G.; Gandhi, V. The growth factor fusion construct containing B-lymphocyte stimulator (BLyS) and the toxin rGel induces apoptosis specifically in BAFF-R-positive CLL cells. Blood 2007, 109, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.-A.; Cheung, L.H.; Hittelman, W.N.; Marks, J.W.; Aguiar, R.C.; Rosenblum, M.G. The rGel/BLyS fusion toxin specifically targets malignant B cells expressing the BLyS receptors BAFF-R, TACI, and BCMA. Mol. Canc. Ther. 2007, 6, 460–470. [Google Scholar] [CrossRef]
- Wen, X.; Lyu, M.-A.; Rui, Z.; Lu, W.; Huang, Q.; Liang, D.; Rosenblum, M.G.; Li, C. Biodistribution, pharmacokinetics, and nuclear imaging studies of 111In-labeled rGel/BLyS fusion toxin in SCID mice bearing B cell lymphoma. Mol. Imag. Biol. 2010, in press.. [Google Scholar]
- Lyu, M.-A.; Rai, D.; Ahn, K.S.; Sung, B.; Cheung, L.H.; Marks, J.W.; Aggarwall, B.B.; Aguiar, R.C.T.; Gandhi, V.; Rosenblum, M.G. The rGel/BLyS fusion toxin inhibits diffuse large B-cell lymphoma growth in vitro and in vivo. Neoplasia 2010, 12, 366–375. [Google Scholar] [PubMed]
- Thorpe, P.E. Vascular targeting agents as cancer therapeutics. Clin. Canc. Res. 2004, 10, 415–427. [Google Scholar] [CrossRef]
- Kim, S.; Mohamedali, K.A.; Cheung, L.H.; Rosenblum, M.G. Overexpression of biologically active VEGF121 fusion proteins in Escherichia coli. J. Biotechnol. 2007, 128, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Smagur, A.; Boyko, M.M.; Biront, N.V.; Cichoń, T.; Szala, S. Chimeric protein ABRaA-VEGF121 is cytotoxic towards VEGFR-2-expressing PAE cells and inhibits B16-F10 melanoma growth. Acta Biochim. Pol. 2009, 56, 115–124. [Google Scholar]
- Cook, J.P.; Savage, P.M.V.; Lord, J.M.; Roberts, L.M. Biologically active interleukin 2-ricin A chain fusion proteins may require intracellular proteolytic cleavage to exhibit a cytotoxic effect. Bioconjug. Chem. 1993, 4, 440–447. [Google Scholar]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, F.; Doumbia, S.O.; Engstrom, C.R.; Pendergras, S.L.; Maher, D.L.; Uckun, F.M. Expression of biologically active recombinant pokeweed antiviral protein in methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 2000, 18, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Liu, Y.; Mathias, A.; Stavrou, S.; Wang, Z.; Thompson, J.; Neville, D.M., Jr. Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris. Protein Expr. Purif 2002, 25, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Della Cristina, P.; Lombardi, A.; Ippoliti, R.; Flavell, D.J.; Flavell, S.U.; Cerotti, A.; Colombatti, M.; Fabbrini, M.S. Systematic comparison between single-chain fusion toxin contructs containing saporin or PEA produced in different expression systems. 2010. in preparation.. [Google Scholar]
- Holmes, M.A.; Foote, J. Structural consequences of humanizing an antibody. J. Immunol. 1997, 158, 2192–2201. [Google Scholar]
- Presta, L.G. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv. Drug Deliv. Rev. 2006, 58, 640–656. [Google Scholar]
- Sharkey, R.M.; Goldenberg, D.M. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv. Drug Deliv. Rev. 2008, 60, 1407–1420. [Google Scholar]
- Kreitman, R.J.; Wilson, W.H.; White, J.D.; Stetler-Stevenson, M.; Jaffe, E.S.; Giardina, S.; Waldmann, T.A.; Pastan, I. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J. Clin. Oncol. 2000, 18, 1622–1636. [Google Scholar]
- Hassan, R.; Bullock, S.; Premkumar, A.; Kreitman, R.J.; Kindler, H.; Willingham, M.C.; Pastan, I. Phase I study of SS1P a recombinant anti-mesothelin immunotoxin given as a bolus IV infusion to patients with mesothelin-expressing mesothelioma, ovarian and pancreatic cancers. Clin. Canc. Res. 2007, 13, 5144–5149. [Google Scholar]
- Molineux, G. Pegylation:engineering improved biopharmaceuticals for oncology. Pharmacotherapy 2003, 23, S3–S8. [Google Scholar]
- Onda, M.; Nagata, S.; FitzGerald, D.J.; Beers, R.; Fisher, R.J.; Vincent, J.J.; Lee, B.; Nakamura, M.; Hwang, J.; Kreitman, R.J.; Hassan, R.; Pastan, I. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J. Immunol. 2006, 177, 8822–8834. [Google Scholar] [PubMed]
- Onda, M.; Beers, R.; Xiang, L.; Nagata, S.; Wang, Q.; Pastan, I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc. Natl. Acad. Sci. USA 2008, 105, 11311–11316. [Google Scholar]
- Chan, S.H.; Shaw, P.C.; Mulot, S.F.; Xu, L.H.; Chan, W.L.; Tam, S.C.; Wong, K.B. Engineering of a mini-trichosanthin that has lower antigenicity by deleting its C-terminal amino acid residues. Biochem. Biophys. Res. Commun. 2000, 270, 279–285. [Google Scholar]
- Bolognesi, A.; Polito, L.; Olivieri, F.; Valbonesi, P.; Barbieri, L.; Battelli, M.G.; Carusi, M.V.; Benvenuto, E.; Del Vecchio Bianco, F.; Di Maro, A.; Parente, A.; Di Loreto, M.; Stirpe, F. New ribosome-inactivating proteins with polynucleotide: Adenosine glycosidase and antiviral activities from Basella rubra L.and Bougainvillea spectabilis Willd. Planta 1997, 203, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Cizeau, J.; Grenkow, D.M.; Brown, J.G.; Entwistle, J.; McDonald, G.C. Engineering and biological characterization of VB6-845, an anti-EpCAM immunotoxin containing a T-cell epitope -depleted variant of the plant toxin bouganin. J. Immunother. 2009, 32, 574–584. [Google Scholar]
- MacDonald, G.C.; Rasamoelisolo, M.; Entwistle, J.; Cizeau, J.; Bosc, D.; Cuthbert, W.; Kowalski, M.; Spearman, M.; Glover, N. A phase I clinical study of VB4-845: Weekly intratumoral administration of an anti-EpCAM recombinant fusion protein in patients with squamous cell carcinoma of the head and neck. Drug. Des. Devel. Ther. 2009, 6, 105–114. [Google Scholar]
- MacDonald, G.C.; Rasamoelisolo, M.; Entwistle, J.; Cuthbert, W.; Kowalski, M.; Spearman, M.; Glover, N. A phase I clinical study of intratumorally administered VB4-845, an anti-epithelial cell adhesion molecule recombinant fusion protein, in patients with squamous cell carcinoma of the head and neck. Med. Oncol. 2009, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Baluna, R.; Rizo, J.; Gordon, B.E.; Ghetie, V.; Vitetta, E.S. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc. Natl. Acad. Sci. USA. 1999, 96, 3957–3962. [Google Scholar]
- Baluna, R.; Coleman, E.; Jones, C.; Ghetie, V.; Vitetta, E.S. The effect of a monoclonal antibody coupled to ricin A chain-derived peptides on endothelial cells in vitro: Insights into toxin mediated vascular damage. Exp. Cell Res. 2000, 258, 417–424. [Google Scholar]
- Vitetta, E.S. Immunotoxins and vascular leak syndrome. Canc. J. 2000, 6, 218–224. [Google Scholar]
- Coulson, B.S.; Londrigan, S.L.; Lee, D.J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA 1997, 94, 5389–5394. [Google Scholar]
- Smallshaw, J.E.; Ghetie, V.; Rizo, J.; Fulmer, J.R.; Trahan, L.L.; Ghetie, M.A.; Vitetta, E.S. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat. Biotechnol. 2003, 21, 387–391. [Google Scholar]
- Messmann, R.A.; Vitetta, E.S.; Headlee, D.; Senderowicz, A.M.; Figg, W.D.; Schindler, J.; Michiel, D.F.; Creekmore, S.; Steinberg, S.M.; Kohler, D.; Jaffe, E.S.; Stetler-Stevenson, M.; Chen, H.; Ghetie, V.; Sausville, E.A. A phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin. Canc. Res. 2000, 6, 1302–1313. [Google Scholar]
- Kuroda, K.; Liu, H.; Kim, S.; Guo, M.; Navarro, V.; Bander, N.H. Saporin toxin-conjugated monoclonal antibody targeting prostate-specific membrane antigen has potent anticancer activity. Prostate 2010, 70, 1286–1294. [Google Scholar]
- Yip, W.L.; Weyergang, A.; Berg, K.; Tønnesen, H.H.; Selbo, P.K. Targeted delivery and enhanced cytotoxicity of cetuximab-saporin by photochemical internalization in EGFR-positive cancer cells. Mol Pharm. 2007, 4, 241–245. [Google Scholar]
- Fuchs, H.; Bachran, D.; Panjideh, H.; Schellmann, N.; Weng, A.; Melzig, M.F.; Sutherland, M.; Bachran, C. Saponins as tools for improved targeted tumor therapies. Curr. Drug Targets 2009, 10, 140–151. [Google Scholar]
- Weng, A.; Bachran, C.; Fuchs, H.; Krause, E.; Stephanowitz, H.; Melzig, M.F. Enhancement of saporin cytotoxicity by Gypsophila saponins more than stimulation of endocytosis. Chem. Biol. Interact. 2009, 176, 204–211. [Google Scholar]
- Bachran, D.; Schneider, S.; Urban, .C; Weng, A.; Melzig, M.F.; Hoffman, C.; Kaufman, A.M.; Fuchs, H. Epidermal growth factor receptor expression affects the efficacy of the combined application of saponin and a targeted toxin on human cervical carcinoma cells. Int. J. Canc. 2010, 127, 1453–1461. [Google Scholar]
- Bachran, C.; Dürkop, H.; Sutherland, M.; Bachran, D.; Müller, C.; Weng, A.; Melzig, M.F.; Fuchs, H. Inhibition of tumor growth by targeted toxins in mice is dramatically improved by saponinum album in a synergistic way. J. Immunother. 2009, 32, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010, 28, 181–188. [Google Scholar]
- Hoganson, D.K.; Chandler, L.A.; Fleurbaaij, G.A.; Ying, W.; Black, M.E.; Doukas, J.; Pierce, G.F.; Baird, A.; Sosnowski, B.A. Targeted delivery of DNA encoding cytotoxic proteins through high-affinity fibroblast growth factor receptors. Hum. Gene Ther. 1998, 9, 2565–2575. [Google Scholar]
- Baird, J.A.; Chandler, L.A.; Sosnowski, B.A. Compositions containing nucleic acids and ligands for therapeutic treatment. US Patent 6,503,886, 13 January 2003. [Google Scholar]
- Sajja, H.K.; East, M.P.; Mao, H.; Wang, Y.A.; Nie, S.; Yang, L.; Jankun, J.; Hart, R. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr. Drug. Discov. Technol. 2009, 6, 43–51. [Google Scholar]
- Bar, H.; Yacoby, I.; Benhar, I. Killing cancer cells by targeted drug-carrying phage nanomedicines. BMC Biotechnol. 2008, 8, 37. [Google Scholar]
- Zarovni, N.; Vago, R.; Fabbrini, M.S. Saporin suicide gene therapy. Meth. Mol. Biol. 2009, 542, 261–283. [Google Scholar]
- Jankun, J.; Hart, R. Method of delivery of a medicament to a cancer cell using a pathway of plasminogen activator material. US Patent 5,679,350, 21 October 1997. [Google Scholar]
- Weber, R.; Feng, X.; Foord, O.; Green, L.; Gudas, J.M.; Keyt, B.; Liu, Y.; Rathanaswami, P. Antibodies directed to the deletion mutants of epidermal growth factor receptor and uses thereof. US Patent 7,628,986, 8 December 2009. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Virgilio, M.d.; Lombardi, A.; Caliandro, R.; Fabbrini, M.S. Ribosome-Inactivating Proteins: From Plant Defense to Tumor Attack. Toxins 2010, 2, 2699-2737. https://doi.org/10.3390/toxins2112699
Virgilio Md, Lombardi A, Caliandro R, Fabbrini MS. Ribosome-Inactivating Proteins: From Plant Defense to Tumor Attack. Toxins. 2010; 2(11):2699-2737. https://doi.org/10.3390/toxins2112699
Chicago/Turabian StyleVirgilio, Maddalena de, Alessio Lombardi, Rocco Caliandro, and Maria Serena Fabbrini. 2010. "Ribosome-Inactivating Proteins: From Plant Defense to Tumor Attack" Toxins 2, no. 11: 2699-2737. https://doi.org/10.3390/toxins2112699