Comparative Study of Various Immune Parameters in Three Bivalve Species during a Natural Bloom of Dinophysis acuminata in Santa Catarina Island, Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and experimental design
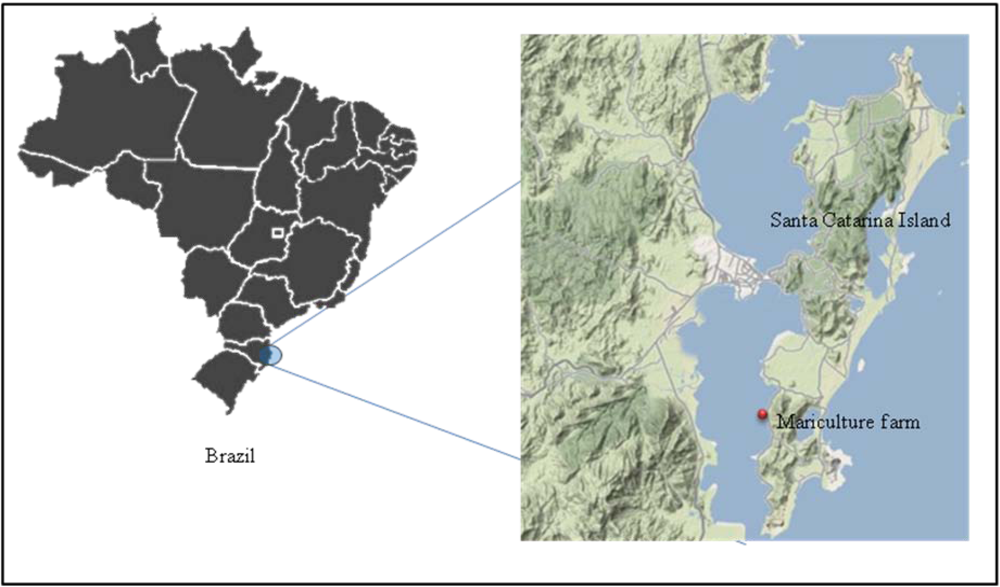
2.2. Algal cell counts and determination of okadaic acid (OA) concentration in bivalve tissues
2.3. Hemolymph preparation
2.4. Hemograms-Total (THC) and differential (DHC) hemocyte counts
2.5. Quantification of apoptotic hemocytes (AH)
2.6. Hemagglutinating activity of total hemolymph (HA)
2.7. Determination of phenoloxidase (PO) activity
2.8. Total protein concentration (PC)
2.9. Statistical analysis
3. Results
3.1. Algal cell counts and okadaic acid (OA) concentration in bivalve tissues
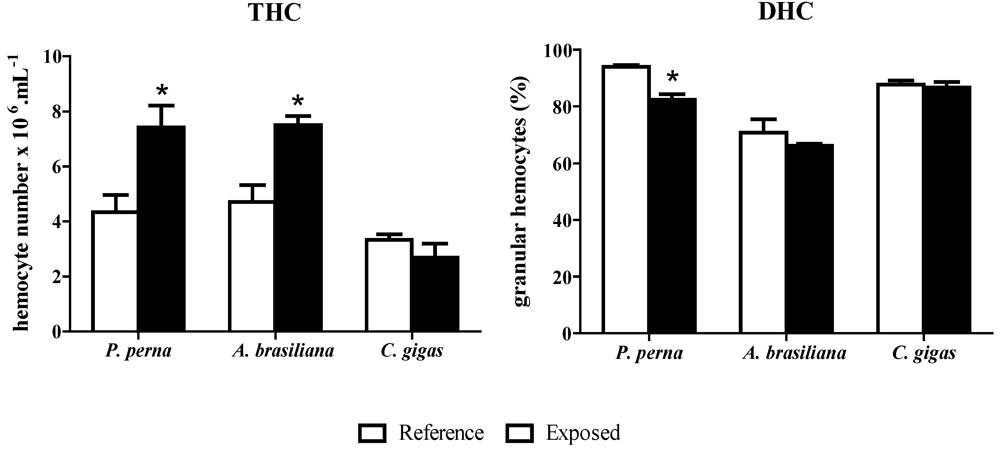
3.2. Hemograms: THC and DHC
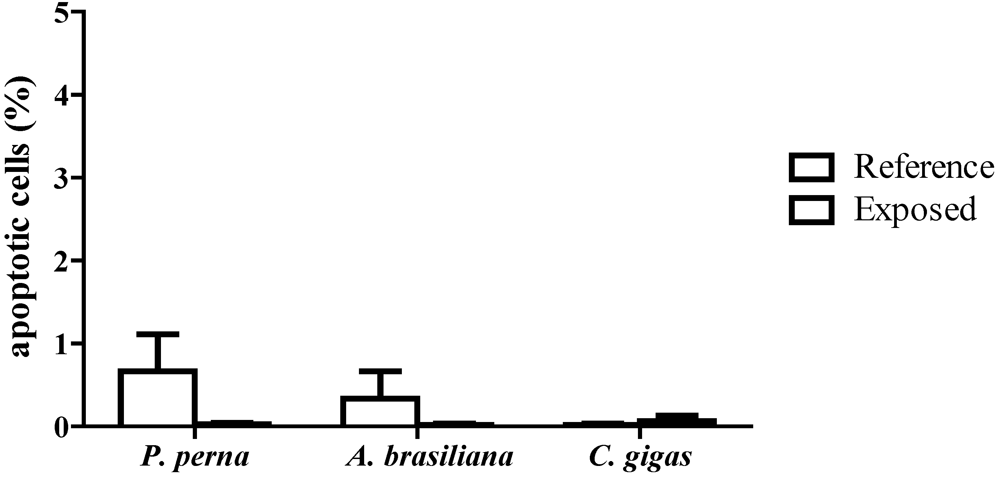
3.3. Percentage of apoptotic hemocytes (AH)
3.4. Hemagglutinating activity (HA)

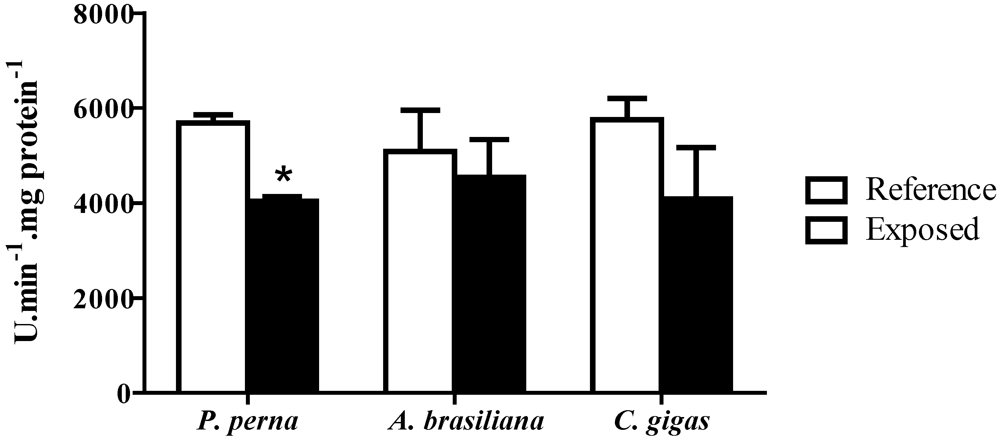
3.5. Phenoloxidase (PO) activity
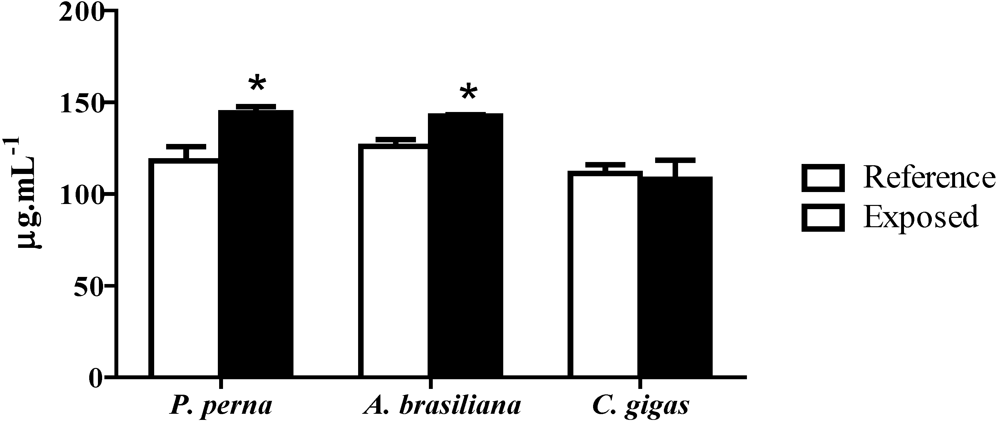
3.6. Total protein concentration in hemolymph (PC)
4. Discussion
Acknowledgements
References
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Harmful algal blooms: A global overview. In Manual on Harmful Marine Algae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; United Nations Educacional, Scientific, and Cultural Organization: Paris, France, 2003; pp. 25–49. [Google Scholar]
- Proenca, L.A.O.; Schramm, M.A.; Tamanaha, M.S.; Alves, T.P. Diarrhetic shellfish poisoning (DSP) outbreak in Subtropical Southwest Atlantic. Harmful Algal News 2007, 33, 19–20. [Google Scholar]
- FAO, Marine Biotoxins. In FAO Food and Nutrition Paper 80; Food and Agriculture Organization: Rome, Italy, 2004; p. 295.
- Hegaret, H.; Wikfors, G.H. Time-dependent changes in hemocytes of eastern oysters, Crassostrea virginica, and northern bay scallops, Argopecten irradians irradians, exposed to a cultured strain of Prorocentrum minimum. Harmful Algae 2005, 4, 187–199. [Google Scholar] [CrossRef]
- Hegaret, H.; Wikfors, G.H. Effects of natural and field-simulated blooms of the dinoflagellate Prorocentrum minimum upon hemocytes of eastern oysters, Crassostrea virginica, from two different populations. Harmful Algae 2005, 4, 201–209. [Google Scholar] [CrossRef]
- Hegaret, H.; da Silva, P.M.; Wikfors, G.H.; Lambert, C.; De Bettignies, T.; Shumway, S.E.; Soudant, P. Hemocyte responses of Manila clams, Ruditapes philippinarum, with varying parasite, Perkinsus olseni, severity to toxic-algal exposures. Aquat. Toxicol. 2007, 84, 469–479. [Google Scholar] [CrossRef]
- Hegaret, H.; Shumway, S.E.; Wikfors, G.H. Effects of harmful algae on physiology and hemocyte parameters of the Northern Bay scallop, Argopecten irradians irradians. J. Shellfish Res. 2007, 26, 1316–1316. [Google Scholar]
- da Silva, P.M.; Hegaret, H.; Lambert, C.; Wikfors, G.H.; Le Goic, N.; Shumway, S.E.; Soudant, P. Immunological responses of the Manila clam (Ruditapes philippinarum) with varying parasite (Perkinsus olseni) burden, during a long-term exposure to the harmful alga, Karenia selliformis, and possible interactions. Toxicon 2008, 51, 563–573. [Google Scholar]
- Galimany, E.; Sunila, I.; Hegaret, H.; Ramon, M.; Wikfors, G.H. Pathology and immune response of the blue mussel (Mytilus edulisi L.) after an exposure to the harmful dinoflagellate Prorocentrum minimum. Harmful Algae 2008, 7, 630–638. [Google Scholar] [CrossRef]
- Galimany, E.; Sunila, I.; Hegaret, H.; Ramon, M.; Wikfors, G.H. Experimental exposure of the blue mussel (Mytilus edulisi, L.) to the toxic dinoflagellate Alexandrium fundyense: Histopathology, immune responses, and recovery. Harmful Algae 2008, 7, 702–711. [Google Scholar] [CrossRef]
- Hegaret, H.; da Silva, P.M.; Sunila, I.; Shumway, S.E.; Dixon, M.S.; Alix, J.; Wikfors, G.H.; Soudant, P. Perkinsosis in the Manila clam Ruditapes philippinarum affects responses to the harmful-alga, Prorocentrum minimum. J. Exp. Mar. Biol. Ecol. 2009, 371, 112–120. [Google Scholar] [CrossRef]
- Galimany, E.; Place, A.R.; Ramon, M.; Jutson, M.; Pipe, R.K. he effects of feeding Mytilus galloprovincialis (PLY # 103; Gymnodinium veneficum Ballantine) to the blue mussel Mytilus edulisi. Harmful Algae 2008, 7, 91–98. [Google Scholar] [CrossRef]
- Malagoli, D.; Ottaviani, E. Yessotoxin affects fMLP-induced cell shape changes in Mytilus galloprovincialis immunocytes. Cell Biol. Int. 2004, 28, 57–61. [Google Scholar] [CrossRef]
- Malagoli, D.; Casarini, L.; Ottaviani, E. Algal toxin yessotoxin signalling pathways involve immunocyte mussel calcium channels. Cell Biol. Int. 2006, 30, 721–726. [Google Scholar] [CrossRef]
- Malagoli, D.; Casarini, L.; Ottaviani, E. Effects of the marine toxins okadaic acid and palytoxin on mussel phagocytosis. Fish Shellfish Immunol. 2008, 24, 180–186. [Google Scholar] [CrossRef]
- Haberkorn, H.; Lambert, C.; Le Goïc, N.; Guéguen, M.; Moal, J.; Palacios, E.; Lassus, P.; Soudant, P. Effects of Alexandrium minutum exposure upon physiological and hematological variables of diploid and triploid oysters, Crassostrea gigas. Aquat. Toxicol. 2009, 97, 96–108. [Google Scholar]
- Vargas-Albores, F.; Barracco, M.A. Mecanismos de defensa de los moluscos bivalvos con énfasis en pectinídeos. In Los Moluscos Pectínidos de Iberoamérica; Maeda-Martínez, A.N., Ed.; Ciencia y Acuicultura: Limusa, La Paz, Bolivia, 2001; pp. 127–140. [Google Scholar]
- Proenca, L.A.O. Algal blooms in coastal zones: Examples of harmful impacts from the Brazilian coast. J. Coast. Res. 2006, 1, 76–78. [Google Scholar]
- Utermöhl, H. Zur vervollkommung der quantitativen phytoplankton-methodic. Mitt. Int. Ver. Theor. Angew. Limnol. Oceanogr. 1958, 9, 1–38. [Google Scholar]
- Söderhäll, K.; Häll, L. Lipopolysaccharide-induced activation of prophenoloxidase activating system in crayfish haemocyte lysate. Biochim. Biophys. Acta 1984, 797, 99–104. [Google Scholar]
- Suzuki, T.; Mitsuya, T. Comparison of dinophysistoxin-1 and esterified dinophysistoxin-1 (dinophysistoxin-3) contents in the scallop Patinopecten yessoensis and the mussel Mytilus galloprovincialis. Toxicon 2001, 39, 905–908. [Google Scholar]
- Sidari, L.; Nichetto, P.; Cok, S.; Sosa, S.; Tubaro, A.; Honsell, G.; Della Loggia, R. Phytoplankton selection by mussels, and diarrhetic shellfish poisoning. Mar. Biol. 1998, 131, 103–111. [Google Scholar] [CrossRef]
- Lindegarth, S.; Torgersen, T.; Lundve, B.; Sandvik, M. Differential Retention of Okadaic Acid (Oa) Group Toxins and Pectenotoxins (Ptx) in the Blue Mussel, Mytilus edulisi (L.), and European Flat Oyster, Ostrea Edulis (L.). J. Shellfish Res. 2009, 28, 313–323. [Google Scholar] [CrossRef]
- Mydlarz, L.D.; Jones, L.E.; Harvell, C.D. Innate immunity environmental drivers and disease ecology of marine and freshwater invertebrates. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 251–288. [Google Scholar] [CrossRef]
- Hegaret, H.; Wikfors, G.H.; Soudant, P.; Lambert, C.; Shumway, S.E.; Berard, J.B.; Lassus, P. Toxic dinoflagellates (Alexandrium fundyense and A-catenella) have minimal apparent effects on oyster hemocytes. Mar. Biol. 2007, 152, 441–447. [Google Scholar]
- da Silva, P.M.; Magalhaes, A.R.M.; Barracco, M.A. Effects of Bucephalus sp (Trematoda: bucephalidae) on Perna perna mussels from a culture station in ratones Grande Island, Brazil. J. Inver. Pathol. 2002, 79, 154–162. [Google Scholar] [CrossRef]
- Auffret, M.; Rousseau, S.; Boutet, I.; Tanguy, A.; Baron, J.; Moraga, D.; Duchemin, M. A multiparametric approach for monitoring immunotoxic responses in mussels from contaminated sites in Western Mediterranea. Ecotoxicol. Environ. Saf. 2006, 63, 393–405. [Google Scholar] [CrossRef]
- Ordas, M.C.; Albaiges, J.; Bayona, J.M.; Ordas, A.; Figueras, A. Assessment of in vivo effects of the prestige fuel oil spill on the Mediterranean mussel immune system. Arch. Environ. Contam. Toxicol. 2007, 52, 200–206. [Google Scholar]
- Schleder, D.D.; Kayser, M.; Suhnel, S.; Ferreira, J.F.; Rupp, G.S.; Barracco, M.A. Evaluation of hemato-immunological parameters during the reproductive cycle of the scallop Nodipecten nodosus in association with a carotenoid-enriched diet. Aquaculture 2008, 280, 256–263. [Google Scholar] [CrossRef]
- Canesi, L.; Gallo, G.; Gavioli, M.; Pruzzo, C. Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microsc. Res. Tech. 2002, 57, 469–476. [Google Scholar] [CrossRef]
- Baier-Anderson, C.; Anderson, R.S. Immunotoxicity of Environmental Pollutants in Marine Bucephalus. In Recent Advances in Marine Biotechnology; Fingerman, M., Nagabhushamam, R., Eds.; Science Publishers Inc.: New Hampshire, NH, USA, 2000. [Google Scholar]
- Coles, J.A.; Pipe, R.K. Phenoloxidase activity in the hemolymph and hemocytes of the marine mussel Mytilus edulis. Fish Shellfish Immunol. 1994, 4, 337–352. [Google Scholar] [CrossRef]
- Gagnaire, B.; Soletchnik, P.; Madec, P.; Gealron, P.; Le Moine, O.; Renault, T. Diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg), reared at two heights above sediment in Marennes-Oleron Basin, France: Difference in mortality, sexual maturation and hemocyte parameters. Aquaculture 2006, 254, 606–616. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mello, D.F.; Proença, L.A.d.O.; Barracco, M.A. Comparative Study of Various Immune Parameters in Three Bivalve Species during a Natural Bloom of Dinophysis acuminata in Santa Catarina Island, Brazil. Toxins 2010, 2, 1166-1178. https://doi.org/10.3390/toxins2051166
Mello DF, Proença LAdO, Barracco MA. Comparative Study of Various Immune Parameters in Three Bivalve Species during a Natural Bloom of Dinophysis acuminata in Santa Catarina Island, Brazil. Toxins. 2010; 2(5):1166-1178. https://doi.org/10.3390/toxins2051166
Chicago/Turabian StyleMello, Danielle Ferraz, Luis Antonio de Oliveira Proença, and Margherita Anna Barracco. 2010. "Comparative Study of Various Immune Parameters in Three Bivalve Species during a Natural Bloom of Dinophysis acuminata in Santa Catarina Island, Brazil" Toxins 2, no. 5: 1166-1178. https://doi.org/10.3390/toxins2051166



