Monoclonal Antibodies and Toxins—A Perspective on Function and Isotype
Abstract
:1. Introduction
2. Selective Characterization of mAbs
| Toxin | Authors | Reference | Binding sites/ domains/subunits | Protective | Indifferent | Enhancing | Uncharacterized | In vivo model |
|---|---|---|---|---|---|---|---|---|
| Shiga Toxin / Shiga-like Toxin | Griffin et al. (1983) | [26] | Stx | 25 (IgG1) | 63 | - | - | - |
| Donohue-Rolfe et al. (1984) | [27] | Stx | 3 (IgG1) | - | - | - | - | |
| Strockbine et al. (1985) | [28] | SLT | 3 (IgG1) | - | - | - | mouse (CD-1) | |
| Downes et al. (1988) | [29] | SLT-II | 5 (IgG1) | - | - | - | - | |
| Perera et al. (1988) | [30] | SLT-II | 5 (80% IgM and 20% IgG1) | - | - | - | - | |
| Donohue-Rolfe et al. (1989) | [31] | Stx, SLT-II | 3 (66.6% IgG1 and 33.3% IgG2b) | 2 (IgM) | - | - | - | |
| Padhye et al. (1989) | [32] | SLT-I, SLT-II | 3 (66.6% IgG1 and 33.3% IgG2b) | 11 (90.9% IgM and 9.1% IgG2b) | - | - | mouse | |
| Islam and Stimson (1990) | [33] | Stx | 4 (IgG1) | - | - | - | mouse | |
| Qadri et al. (1993) | [34] | Stx | - | 1 (IgM) | - | - | - | |
| Nakao et al. (1999) | [35] | SLT-II | 1 (IgG1) | - | - | - | - | |
| Mukherjee et al. (2002) | [36] | SLT-I | 5 (40% IgM and 60% IgG1) | 5 (IgM) | - | - | mouse (Swiss Webster) | |
| Mukherjee et al. (2002) | [37] | SLT-II | 8 (IgG1) | 28 (96.4% IgG1 and 3.6% IgG3) | - | - | mouse (Swiss Webster) and gnotobiotic piglet | |
| Nakao et al. (2002) | [38] | SLT-I | 1 | - | - | - | - | |
| Tanikawa et al. (2008) | [39] | SLT-I | 1 (IgG1) | 1 (IgA) | - | - | - | |
| Pertussis Toxin | Sato et al. (1984) | [40] | S1, S4 | 1 (IgG2a) | 2 (IgG1) | - | - | mouse (Slc:ddY) |
| Frank and Parker (1984) | [41] | S2 | - | 2 (IgG1) | - | - | mouse (CFW) | |
| Sato et al. (1987) | [42] | S2, S3 | 2 (IgG1) | - | - | - | - | |
| Kenimer et al. (1989) | [43] | S1, S4 | 3 (IgG1) | 3 (IgG1) | - | - | - | |
| Lang et al. (1989) | [44] | S2, S3, S4 | 5 (40% IgG1 and 60% IgG2a) | 8 (25% IgG1, 62.5% IgG2a and 12.5% IgG2b) | - | - | - | |
| Sato and Sato (1990) | [45] | S1, S2, S3, S2–3, S4 | 5 (IgG1) | 15 (IgG1) | - | - | mouse | |
| Halperin et al. (1991) | [46] | S1, S3 | 3 | - | - | - | mouse (CFW) | |
| Kenimer et al. (1991) | [47] | S1 | - | 2 (IgG1 and IgG3) | - | - | - | |
| Sato et al. (1991) | [48] | S2, S3, S2–3, S4, S5 | 2 | 10 | - | - | mouse | |
| Walker et al. (1991) | [49] | S1, S2–3, S4 | 3 (IgG1) | - | - | - | - | |
| Zaccolo et al. (1992) | [50] | S3 | 1 (IgG1) | - | - | - | mouse (BALB/c) | |
| Lee et al. (1999) | [51] | Adenylate cyclase toxin | 4 (75% IgG1 and 25% IgG2a) | 8 (62.5% IgG1, 25% IgG2a, and 12.5% IgG2b) | - | - | - | |
| Pootong et al. (2007) | [52] | S1 | 1 (IgG1) | - | - | 6 | - | |
| Anthrax Toxin | Little et al. (1988) | [53] | PA | 2 (IgG1) | 35 (17.1% IgM, 62.9% IgG1, 11.4% IgG2a, 8.6% IgG2b) | - | - | Fisher 344 rat |
| Little et al. (1990) | [54] | LF | 4 (IgG1) | 59 (30.5% IgM, 40.7% IgG1, 22% IgG2a, 3.4% IgG3, 3.4% IgA) | - | - | Fisher 344 rat | |
| Little et al. (1994) | [55] | EF | 1 (IgG1) | 9 (88.9% IgG1, 11.1% IgG2a) | - | - | - | |
| Little et al. (1996) | [56] | PA63 | 2 (IgG1 and IgG2b) | - | - | - | Fisher 344 rat | |
| Zhao et al. (2003) | [57] | LF | 1 | - | - | - | mouse (nude) | |
| Belova et al. (2004) | [17] | PA | 2 | - | 1 | - | - | |
| Brossier et al. (2004) | [58] | PA | 9 | 87 | - | - | mouse (OF1) | |
| Kozel et al. (2004) | [59] | poly γ-D-glutamic acid | 1 (IgG1) | - | - | 4 (IgG3) | mouse (BALB/c) | |
| Mohamed et al. (2004) | [18] | PA | - | 22 | 21 (IgG2a) | - | - | |
| Sawada-Hirai et al. (2004) | [60] | PA | 3 (IgG1) | 1 (IgG1) | - | - | Fisher 344 rat | |
| Lim et al. (2005) | [61] | LF | 2 (IgG1) | - | - | - | Fisher 344 rat | |
| Chen et al. (2006) | [62] | PA, LF | 2 | 4 | - | - | Fisher 344 rat | |
| Gubbins et al. (2006) | [63] | PA | 3 (IgG1) | 8 (IgG1) | - | - | - | |
| Rivera et al. (2006) | [64] | PA | 2 (IgG1 and IgG2b) | - | - | - | mouse (BALB/c) | |
| Vitale et al. (2006) | [65] | PA | 1 (IgG1) | - | - | - | rabbit | |
| Albrecht et al. (2007) | [66] | PA, LF | 2 (IgG1) | - | - | - | mouse (A/J) | |
| Kozel et al. (2007) | [67] | poly γ-D-glutamic acid | 5 (IgG3) | 1 (IgG1) | - | - | mouse (BALB/c) | |
| Staats et al. (2007) | [68] | PA, LF | 2 | 2 | - | - | mouse (BALB/c) | |
| Abboud et al. (2009) | [69] | PA | 1 (IgG1) | 3 (33.3% IgM, 66.7% IgG1) | - | - | mouse (BALB/c) | |
| Chen et al. (2009) | [70] | LF | 2 (IgG1) | 1 | - | 89 | Fisher 344 rat | |
| Kelly-Cirino and Mantis (2009) | [71] | PA | 1 (IgG1) | 2 (IgG1 and IgG2a) | - | 2 | mouse (BALB/c) | |
| Rosenfeld et al. (2009) | [72] | PA | 101 | 499 | - | - | Fisher 344 rat, Hartley guinea pig | |
| Winterroth et al. (2010) | [73] | EF | 1 (IgM) | 5 (20% IgM, 80% IgG1) | - | - | mouse (A/JCr) | |
| Chen et al. (2011) | [74] | poly γ-D-glutamic acid | 2 (IgG1 and IgG3) | - | - | 3 | mouse (BALB/c) | |
| Kulshreshtha and Bhatnagar (2011) | [75] | LF and EF | 1 (IgG2b) | - | - | - | mouse (BALB/c) | |
| Leysath et al. (2011) | [76] | EF | 3 (IgG1) | 1 (IgG1) | - | 78 | mouse (BALB/cJ, C57BL/6J) | |
| Little et al. (2011) | [19] | PA | - | 56 | 17 | - | Fisher 344 rat | |
| vor dem Esche et al. (2011) | [77] | LF | 1 (IgG1) | 17 | - | - | mouse (A/J) | |
| Chow et al. (manuscript in prep.) | - | PA | 2 (IgG2a) | 16 (IgG1) | 6 (83.3% IgG1, 16.7% IgG2a) | - | mouse (BALB/c) | |
| Ricin Toxin | Colombatti et al. (1986) | [20] | RT, RTA | 3 | 4 | 1 | 13 | - |
| Colombatti et al. (1987) | [78] | RTB | 1 (IgG2a) | - | - | - | - | |
| Chanh et al. (1993) | [79] | RT | 1 (IgG1) | 19 | - | - | mouse (BALB/c) | |
| Lemley et al. (1994) | [80] | RTA | 2 (IgG1) | - | - | - | mouse (BALB/c) | |
| Maddaloni et al. (2004) | [21] | RT, RTA, RTB | 18 | 19 | 1 (IgG1) | - | mouse (CD-1) | |
| Dertzbaugh et al. (2005) | [81] | RT, RTA, RTB | 6 (IgG1) | 23 | - | - | - | |
| Mantis et al. (2006) | [82] | RTA, RTB | 4 (IgA) | - | - | 20 (IgA) | - | |
| McGuinness and Mantis (2006) | [83] | RTB | 1 (IgG1) | - | - | - | - | |
| Pelat et al. (2009) | [84] | RTA | 1 | 18 | - | - | - | |
| Neal et al. (2010) | [85] | RTA | 1 (IgG1) | - | - | - | mouse (BALB/c) | |
| O'Hara et al. (2010) | [86] | RTA | 24 | 394 | - | - | mouse (BALB/c) | |
| Dai et al. (2011) | [87] | RTA | 3 (IgG1) | 14 | - | - | mouse | |
| Prigent et al. (2011) | [88] | RTA, RTB | 7 | 24 | - | - | - | |
| Yermakova and Mantis (2011) | [89] | RTB | 2 | ~100 | - | - | mouse (BALB/c) | |
| Botulinum Toxin | Oguma et al. (1982) | [90] | Type C1 | 2 (IgG1) | 2 (IgG1) | - | - | mouse (ddY) |
| Oguma et al. (1984) | [91] | Type C1, D | 17 | 11 | - | - | - | |
| Kozaki et al. (1986) | [92] | Type E | 3 (IgG1) | 3 (66.7% IgG1, 33.3% IgG2b) | - | - | - | |
| Ferreira et al. (1987) | [93] | Type A | - | 1 (IgG1) | - | 60 | mouse (Swiss Webster) | |
| Simpson et al. (1990) | [94] | Type E | 3 | 1 | - | - | mouse | |
| Toratani et al. (1993) | [95] | ADP-ribosyltransferase C3 | 4 (IgG2b and IgG3) | - | - | - | - | |
| Cenci Di Bello et al. (1994) | [96] | Type A | - | 7 (85.7% IgG1, 14.3% IgG2b) | - | - | mouse | |
| Noah et al. (1995) | [97] | Type B | - | 4 | - | - | mouse | |
| Amersdorfer et al. (1997) | [98] | Type A | 2 | 3 | - | - | - | |
| Brown et al. (1997) | [99] | Type F | 3 | 23 | - | - | mouse | |
| Pless et al. (2001) | [100] | Type A | 33 | 455 | - | - | mouse | |
| Wu et al. (2001) | [101] | Type A | 2 (IgG1) | 14 | - | - | mouse (ICR) | |
| Kamata et al. (2002) | [102] | ADP-ribosyltransferase C3 | 1 (IgG1) | - | - | - | - | |
| Yang et al. (2004) | [103] | Type B | 1 (IgG1) | - | - | - | mouse (ICR) | |
| Adekar et al. (2008) | [104] | Type A | 1 (IgG1) | 19 | - | - | mouse (Swiss Webster) | |
| Adekar et al. (2008) | [105] | Type A | 1 (IgG1) | - | - | - | mouse | |
| Adekar et al. (2008) | [106] | Type A | 1 (IgM) | 1 (IgM) | - | - | mouse (Swiss Webster) | |
| Zhou et al. (2009) | [107] | Type B | 1 | - | - | - | - | |
| Mazuet et al. (2010) | [108] | Type A | 12 (66.7% IgG1, 33.3% IgG2a) | 2 (IgG1) | - | - | mouse (Swiss Webster) | |
| Corbett et al. (2011) | [109] | Type A | 1 (IgG1) | - | - | 7 | mouse (Swiss Webster) | |
| Montgomery et al. (2011) | [110] | Type C | - | 1 | - | - | mouse (CD-1) | |
| Enterotoxin B (SEB) | Lin et al. (1988) | [111] | SEB | 4 (25% IgM, 75% IgG1) | 1 | - | - | - |
| Hamad et al. (1994) | [112] | SEB | 2 (IgG1) | 2 (IgG1 and IgG2b) | - | - | - | |
| Pang et al. (2000) | [113] | SEB | 1 (IgG1) | - | - | - | - | |
| Tilahun et al. (2010) | [114] | SEB | 21 (IgG1) | - | - | - | - | |
| Larkin et al. (2010) | [115] | SEB | 4 | 6 | - | - | mouse (BALB/c) | |
| Drozdowski et al. (2010) | [116] | SEB | 3 (IgG1) | - | - | - | mouse (BALB/c) | |
| Varshnev et al. (2011) | [117] | SEB | 3 (IgM, IgG1, IgA) | 8 (62.5% IgG1, 37.5% IgG2a) | - | - | mouse (BALB/c) |
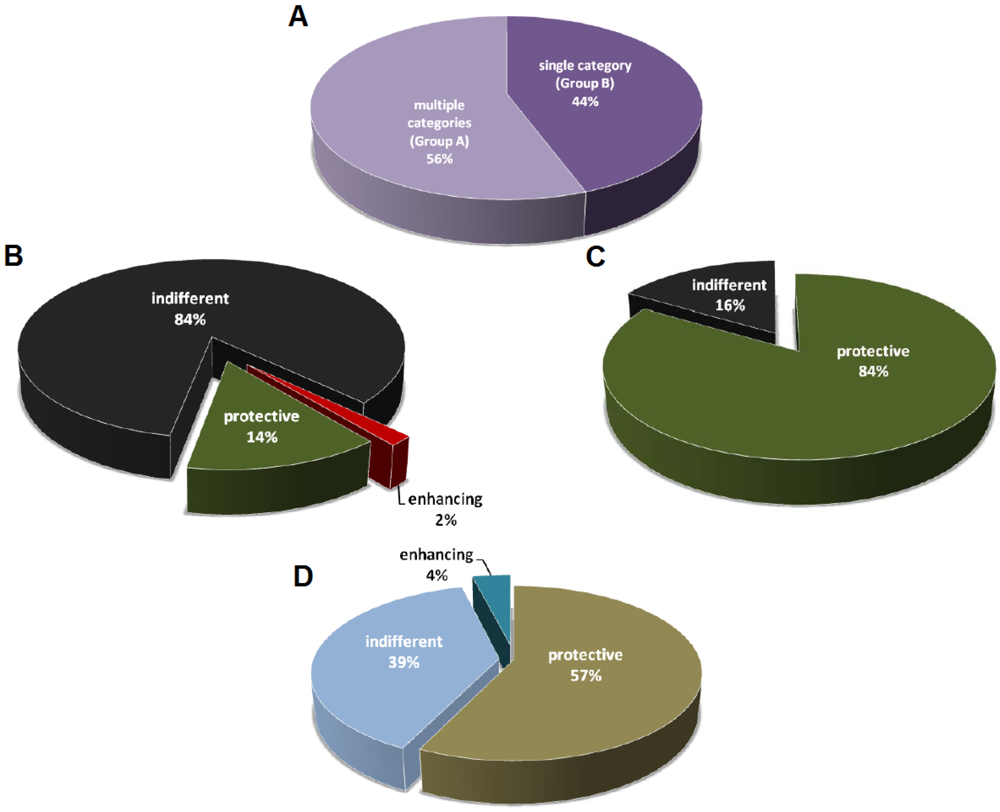
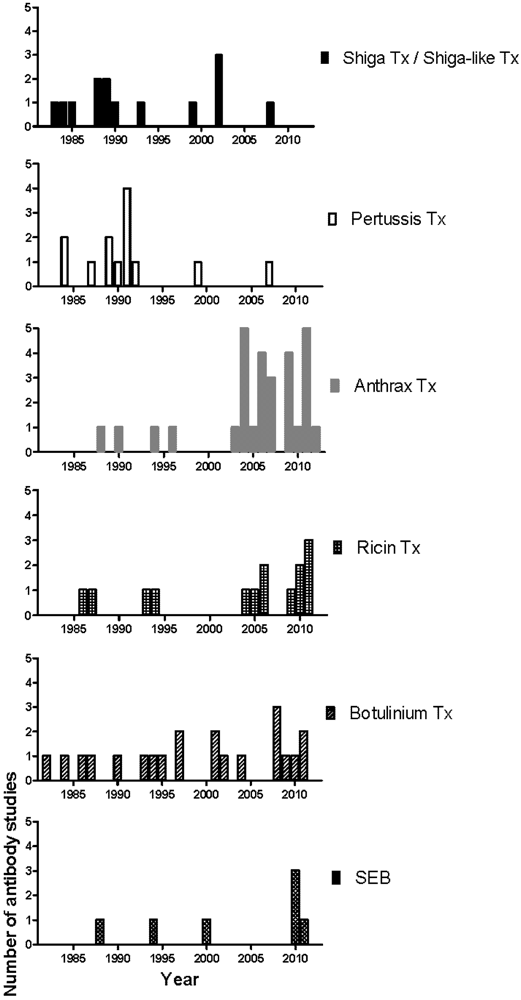

3. Caveats of Protection Studies
3.1. Definition of Protection
3.2. Toxin and Bacterium Inoculum
3.3. Screening Methods
3.4. Interactions of Multiple Monoclonal Antibodies
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Brock, T.D. Milestones in Microbiology: 1556 to 1940; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Casadevall, A. The third age of antimicrobial therapy. Clin. Infect. Dis. 2006, 42, 1414–1416. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Casadevall, A.; Scharff, M.D. Serum therapy revisited: Animal models of infection and development of passive antibody therapy. Antimicrob. Agents Chemother. 1994, 38, 1695–1702. [Google Scholar]
- Khazaeli, M.B.; Conry, R.M.; LoBuglio, A.F. Human immune response to monoclonal antibodies. J. Immunother. Emphasis. Tumor. Immunol. 1994, 15, 42–52. [Google Scholar] [CrossRef]
- Lang, A.B.; Cryz, S.J., Jr.; Schurch, U.; Ganss, M.T.; Bruderer, U. Immunotherapy with human monoclonal antibodies. Fragment a specificity of polyclonal and monoclonal antibodies is crucial for full protection against tetanus toxin. J. Immunol. 1993, 151, 466–472. [Google Scholar]
- Saylor, C.; Dadachova, E.; Casadevall, A. Monoclonal antibody-based therapies for microbial diseases. Vaccine 2009, 27, S38–S46. [Google Scholar]
- Berry, J.D.; Gaudet, R.G. Antibodies in infectious diseases: Polyclonals, monoclonals and niche biotechnology. N. Biotechnol. 2011, 28, 489–501. [Google Scholar]
- Laffly, E.; Danjou, L.; Condemine, F.; Vidal, D.; Drouet, E.; Lefranc, M.P.; Bottex, C.; Thullier, P. Selection of a macaque fab with framework regions like those in humans, high affinity, and ability to neutralize the protective antigen (pa) of bacillus anthracis by binding to the segment of pa between residues 686 and 694. Antimicrob. Agents Chemother. 2005, 49, 3414–3420. [Google Scholar]
- Pelat, T.; Hust, M.; Laffly, E.; Condemine, F.; Bottex, C.; Vidal, D.; Lefranc, M.P.; Dubel, S.; Thullier, P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (lf) of bacillus anthracis by inhibiting protective antigen-lf complex formation. Antimicrob. Agents Chemother. 2007, 51, 2758–2764. [Google Scholar] [CrossRef]
- Chahboun, S.; Hust, M.; Liu, Y.; Pelat, T.; Miethe, S.; Helmsing, S.; Jones, R.G.; Sesardic, D.; Thullier, P. Isolation of a nanomolar scfv inhibiting the endopeptidase activity of botulinum toxin a, by single-round panning of an immune phage-displayed library of macaque origin. BMC Biotechnol. 2011, 11, 113. [Google Scholar] [CrossRef]
- Peiris, J.S.; Porterfield, J.S. Antibody-mediated enhancement of flavivirus replication in macrophage-like cell lines. Nature 1979, 282, 509–511. [Google Scholar] [CrossRef]
- Takeda, A.; Tuazon, C.U.; Ennis, F.A. Antibody-enhanced infection by hiv-1 via fc receptor-mediated entry. Science 1988, 242, 580–583. [Google Scholar]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar]
- Takada, A.; Watanabe, S.; Okazaki, K.; Kida, H.; Kawaoka, Y. Infectivity-enhancing antibodies to ebola virus glycoprotein. J. Virol. 2001, 75, 2324–2330. [Google Scholar] [CrossRef]
- Belova, E.V.; Dubilei, S.A.; Kravchenko, T.B.; Kolesnikov, A.V.; Zakharova, M.; Shemiakin, I.G. Monoclonal antibodies to B. Anthracis protective antigen are capable to neutralize and to enhance the anthrax lethal toxin action in vitro. Mol. Gen. Mikrobiol. Virusol. 2004, 21–26. [Google Scholar]
- Mohamed, N.; Li, J.; Ferreira, C.S.; Little, S.F.; Friedlander, A.M.; Spitalny, G.L.; Casey, L.S. Enhancement of anthrax lethal toxin cytotoxicity: A subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 2004, 72, 3276–3283. [Google Scholar]
- Little, S.F.; Webster, W.M.; Fisher, D.E. Monoclonal antibodies directed against protective antigen of bacillus anthracis enhance lethal toxin activity in vivo. FEMS Immunol. Med. Microbiol. 2011, 62, 11–22. [Google Scholar] [CrossRef]
- Colombatti, M.; Pezzini, A.; Colombatti, A. Monoclonal antibodies against ricin: Effects on toxin function. Hybridoma 1986, 5, 9–19. [Google Scholar] [CrossRef]
- Maddaloni, M.; Cooke, C.; Wilkinson, R.; Stout, A.V.; Eng, L.; Pincus, S.H. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J. Immunol. 2004, 172, 6221–6228. [Google Scholar]
- Reichert, J.M. Antibody-based therapeutics to watch in 2011. MAbs 2011, 3, 76–99. [Google Scholar] [CrossRef]
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The impact-rsv study group. Pediatrics 1998, 102, 531–537. [CrossRef]
- Storey, S. Respiratory syncytial virus market. Nat. Rev. Drug. Discov. 2010, 9, 15–16. [Google Scholar] [CrossRef]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Griffin, D.E.; Gentry, M.K.; Brown, J.E. Isolation and characterization of monoclonal antibodies to shiga toxin. Infect. Immun. 1983, 41, 430–433. [Google Scholar]
- Donohue-Rolfe, A.; Keusch, G.T.; Edson, C.; Thorley-Lawson, D.; Jacewicz, M. Pathogenesis of shigella diarrhea. IX. Simplified high yield purification of shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J. Exp. Med. 1984, 160, 1767–1781. [Google Scholar] [CrossRef]
- Strockbine, N.A.; Marques, L.R.; Holmes, R.K.; O'Brien, A.D. Characterization of monoclonal antibodies against shiga-like toxin from escherichia coli. Infect. Immun. 1985, 50, 695–700. [Google Scholar]
- Downes, F.P.; Barrett, T.J.; Green, J.H.; Aloisio, C.H.; Spika, J.S.; Strockbine, N.A.; Wachsmuth, I.K. Affinity purification and characterization of shiga-like toxin ii and production of toxin-specific monoclonal antibodies. Infect. Immun. 1988, 56, 1926–1933. [Google Scholar]
- Perera, L.P.; Marques, L.R.; O’Brien, A.D. Isolation and characterization of monoclonal antibodies to shiga-like toxin ii of enterohemorrhagic escherichia coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1988, 26, 2127–2131. [Google Scholar]
- Donohue-Rolfe, A.; Acheson, D.W.; Kane, A.V.; Keusch, G.T. Purification of shiga toxin and shiga-like toxins i and ii by receptor analog affinity chromatography with immobilized p1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect. Immun. 1989, 57, 3888–3893. [Google Scholar]
- Padhye, V.V.; Zhao, T.; Doyle, M.P. Production and characterisation of monoclonal antibodies to verotoxins 1 and 2 from escherichia coli of serotype o 157:H7. J. Med. Microbiol. 1989, 30, 219–226. [Google Scholar] [CrossRef]
- Islam, M.S.; Stimson, W.H. Production and characterization of monoclonal antibodies with therapeutic potential against shiga toxin. J. Clin. Lab. Immunol. 1990, 33, 11–16. [Google Scholar]
- Qadri, F.; Azim, T.; Hossain, A.; Islam, D.; Mondol, G.; Faruque, S.M.; Albert, M.J. A monoclonal antibody to shigella dysenteriae serotype 13 cross-reacting with shiga toxin. FEMS Microbiol. Lett. 1993, 107, 343–347. [Google Scholar] [CrossRef]
- Nakao, H.; Kiyokawa, N.; Fujimoto, J.; Yamasaki, S.; Takeda, T. Monoclonal antibody to shiga toxin 2 which blocks receptor binding and neutralizes cytotoxicity. Infect. Immun. 1999, 67, 5717–5722. [Google Scholar]
- Mukherjee, J.; Chios, K.; Fishwild, D.; Hudson, D.; O'Donnell, S.; Rich, S.M.; Donohue-Rolfe, A.; Tzipori, S. Production and characterization of protective human antibodies against shiga toxin 1. Infect. Immun. 2002, 70, 5896–5899. [Google Scholar]
- Mukherjee, J.; Chios, K.; Fishwild, D.; Hudson, D.; O’Donnell, S.; Rich, S.M.; Donohue-Rolfe, A.; Tzipori, S. Human stx2-specific monoclonal antibodies prevent systemic complications of escherichia coli o157:H7 infection. Infect. Immun. 2002, 70, 612–619. [Google Scholar] [CrossRef]
- Nakao, H.; Kataoka, C.; Kiyokawa, N.; Fujimoto, J.; Yamasaki, S.; Takeda, T. Monoclonal antibody to shiga toxin 1, which blocks receptor binding and neutralizes cytotoxicity. Microbiol. Immunol. 2002, 46, 777–780. [Google Scholar]
- Tanikawa, T.; Ishikawa, T.; Maekawa, T.; Kuronane, K.; Imai, Y. Characterization of monoclonal immunoglobulin a and g against shiga toxin binding subunits produced by intranasal immunization. Scand J. Immunol. 2008, 68, 414–422. [Google Scholar] [CrossRef]
- Sato, H.; Ito, A.; Chiba, J.; Sato, Y. Monoclonal antibody against pertussis toxin: Effect on toxin activity and pertussis infections. Infect. Immun. 1984, 46, 422–428. [Google Scholar]
- Frank, D.W.; Parker, C.D. Interaction of monoclonal antibodies with pertussis toxin and its subunits. Infect. Immun. 1984, 46, 195–201. [Google Scholar]
- Sato, H.; Sato, Y.; Ito, A.; Ohishi, I. Effect of monoclonal antibody to pertussis toxin on toxin activity. Infect. Immun. 1987, 55, 909–915. [Google Scholar]
- Kenimer, J.G.; Kim, K.J.; Probst, P.G.; Manclark, C.R.; Burstyn, D.G.; Cowell, J.L. Monoclonal antibodies to pertussis toxin: Utilization as probes of toxin function. Hybridoma 1989, 8, 37–51. [Google Scholar] [CrossRef]
- Lang, A.B.; Ganss, M.T.; Cryz, S.J., Jr. , Monoclonal antibodies that define neutralizing epitopes of pertussis toxin: Conformational dependence and epitope mapping. Infect. Immun. 1989, 57, 2660–2665. [Google Scholar]
- Sato, H.; Sato, Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect. Immun. 1990, 58, 3369–3374. [Google Scholar]
- Halperin, S.A.; Issekutz, T.B.; Kasina, A. Modulation of bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J. Infect. Dis. 1991, 163, 355–361. [Google Scholar] [CrossRef]
- Kenimer, J.G.; Probst, P.G.; Karpas, A.B.; Burns, D.L.; Kaslow, H.R. Monoclonal antibodies against the enzymatic subunit of both pertussis and cholera toxins. Dev. Biol. Stand 1991, 73, 133–141. [Google Scholar]
- Sato, H.; Sato, Y.; Ohishi, I. Comparison of pertussis toxin (pt)-neutralizing activities and mouse-protective activities of anti-pt mouse monoclonal antibodies. Infect. Immun. 1991, 59, 3832–3835. [Google Scholar]
- Walker, M.J.; Wehland, J.; Timmis, K.N.; Raupach, B.; Schmidt, M.A. Characterization of murine monoclonal antibodies that recognize defined epitopes of pertussis toxin and neutralize its toxic effect on chinese hamster ovary cells. Infect. Immun. 1991, 59, 4249–4251. [Google Scholar]
- Zaccolo, M.; Roggero, S.; Armellini, D.; Pegoraro, L.; Rappuoli, R.; Malavasi, F. Generation of human monoclonal antibodies that confer protection against pertussis toxin. Infect. Immun. 1992, 60, 1258–1260. [Google Scholar]
- Lee, S.J.; Gray, M.C.; Guo, L.; Sebo, P.; Hewlett, E.L. Epitope mapping of monoclonal antibodies against bordetella pertussis adenylate cyclase toxin. Infect. Immun. 1999, 67, 2090–2095. [Google Scholar]
- Pootong, A.; Budhirakkul, P.; Tongtawe, P.; Tapchaisri, P.; Chongsa-nguan, M.; Chaicumpa, W. Monoclonal antibody that neutralizes pertussis toxin activities. Asian Pac. J. Allergy Immunol. 2007, 25, 37–45. [Google Scholar]
- Little, S.F.; Leppla, S.H.; Cora, E. Production and characterization of monoclonal antibodies to the protective antigen component of bacillus anthracis toxin. Infect. Immun. 1988, 56, 1807–1813. [Google Scholar]
- Little, S.F.; Leppla, S.H.; Friedlander, A.M. Production and characterization of monoclonal antibodies against the lethal factor component of bacillus anthracis lethal toxin. Infect. Immun. 1990, 58, 1606–1613. [Google Scholar]
- Little, S.F.; Leppla, S.H.; Burnett, J.W.; Friedlander, A.M. Structure-function analysis of bacillus anthracis edema factor by using monoclonal antibodies. Biochem. Biophys. Res. Commun. 1994, 199, 676–682. [Google Scholar] [CrossRef]
- Little, S.F.; Novak, J.M.; Lowe, J.R.; Leppla, S.H.; Singh, Y.; Klimpel, K.R.; Lidgerding, B.C.; Friedlander, A.M. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of bacillus anthracis using monoclonal antibodies. Microbiology 1996, 142 (Pt 3), 707–715. [Google Scholar]
- Zhao, P.; Liang, X.; Kalbfleisch, J.; Koo, H.M.; Cao, B. Neutralizing monoclonal antibody against anthrax lethal factor inhibits intoxication in a mouse model. Hum. Antibodies 2003, 12, 129–135. [Google Scholar]
- Brossier, F.; Levy, M.; Landier, A.; Lafaye, P.; Mock, M. Functional analysis of bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 2004, 72, 6313–6317. [Google Scholar] [CrossRef]
- Kozel, T.R.; Murphy, W.J.; Brandt, S.; Blazar, B.R.; Lovchik, J.A.; Thorkildson, P.; Percival, A.; Lyons, C.R. Mabs to bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Nat. Acad. Sci. USA 2004, 101, 5042–5047. [Google Scholar]
- Sawada-Hirai, R.; Jiang, I.; Wang, F.; Sun, S.M.; Nedellec, R.; Ruther, P.; Alvarez, A.; Millis, D.; Morrow, P.R.; Kang, A.S. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune. Based Ther. Vaccines 2004, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Lim, N.K.; Kim, J.H.; Oh, M.S.; Lee, S.; Kim, S.Y.; Kim, K.S.; Kang, H.J.; Hong, H.J.; Inn, K.S. An anthrax lethal factor-neutralizing monoclonal antibody protects rats before and after challenge with anthrax toxin. Infect. Immun. 2005, 73, 6547–6551. [Google Scholar]
- Chen, Z.; Moayeri, M.; Zhou, Y.H.; Leppla, S.; Emerson, S.; Sebrell, A.; Yu, F.; Svitel, J.; Schuck, P.; St Claire, M.; et al. Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen. J. Infect. Dis. 2006, 193, 625–633. [Google Scholar] [CrossRef]
- Gubbins, M.J.; Berry, J.D.; Corbett, C.R.; Mogridge, J.; Yuan, X.Y.; Schmidt, L.; Nicolas, B.; Kabani, A.; Tsang, R.S. Production and characterization of neutralizing monoclonal antibodies that recognize an epitope in domain 2 of bacillus anthracis protective antigen. FEMS Immunol. Med. Microbiol. 2006, 47, 436–443. [Google Scholar] [CrossRef]
- Rivera, J.; Nakouzi, A.; Abboud, N.; Revskaya, E.; Goldman, D.; Collier, R.J.; Dadachova, E.; Casadevall, A. A monoclonal antibody to bacillus anthracis protective antigen defines a neutralizing epitope in domain 1. Infect. Immun. 2006, 74, 4149–4156. [Google Scholar]
- Vitale, L.; Blanset, D.; Lowy, I.; O’Neill, T.; Goldstein, J.; Little, S.F.; Andrews, G.P.; Dorough, G.; Taylor, R.K.; Keler, T. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect. Immun. 2006, 74, 5840–5847. [Google Scholar]
- Albrecht, M.T.; Li, H.; Williamson, E.D.; LeButt, C.S.; Flick-Smith, H.C.; Quinn, C.P.; Westra, H.; Galloway, D.; Mateczun, A.; Goldman, S.; et al. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 2007, 75, 5425–5433. [Google Scholar]
- Kozel, T.R.; Thorkildson, P.; Brandt, S.; Welch, W.H.; Lovchik, J.A.; AuCoin, D.P.; Vilai, J.; Lyons, C.R. Protective and immunochemical activities of monoclonal antibodies reactive with the bacillus anthracis polypeptide capsule. Infect. Immun. 2007, 75, 152–163. [Google Scholar]
- Staats, H.F.; Alam, S.M.; Scearce, R.M.; Kirwan, S.M.; Zhang, J.X.; Gwinn, W.M.; Haynes, B.F. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect. Immun. 2007, 75, 5443–5452. [Google Scholar] [CrossRef]
- Abboud, N.; De Jesus, M.; Nakouzi, A.; Cordero, R.J.; Pujato, M.; Fiser, A.; Rivera, J.; Casadevall, A. Identification of linear epitopes in bacillus anthracis protective antigen bound by neutralizing antibodies. J. Biol. Chem. 2009, 284, 25077–25086. [Google Scholar]
- Chen, Z.; Moayeri, M.; Crown, D.; Emerson, S.; Gorshkova, I.; Schuck, P.; Leppla, S.H.; Purcell, R.H. Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody. Infect. Immun. 2009, 77, 3902–3908. [Google Scholar]
- Kelly-Cirino, C.D.; Mantis, N.J. Neutralizing monoclonal antibodies directed against defined linear epitopes on domain 4 of anthrax protective antigen. Infect. Immun. 2009, 77, 4859–4867. [Google Scholar] [CrossRef]
- Rosenfeld, R.; Marcus, H.; Ben-Arie, E.; Lachmi, B.E.; Mechaly, A.; Reuveny, S.; Gat, O.; Mazor, O.; Ordentlich, A. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal bacillus anthracis infection. PLoS One 2009, 4, e6351. [Google Scholar]
- Winterroth, L.; Rivera, J.; Nakouzi, A.S.; Dadachova, E.; Casadevall, A. Neutralizing monoclonal antibody to edema toxin and its effect on murine anthrax. Infect. Immun. 2010, 78, 2890–2898. [Google Scholar] [CrossRef]
- Chen, Z.; Schneerson, R.; Lovchik, J.; Lyons, C.R.; Zhao, H.; Dai, Z.; Kubler-Kielb, J.; Leppla, S.H.; Purcell, R.H. Pre- and postexposure protection against virulent anthrax infection in mice by humanized monoclonal antibodies to bacillus anthracis capsule. Proc. Nat. Acad. Sci. USA 2011, 108, 739–744. [Google Scholar]
- Kulshreshtha, P.; Bhatnagar, R. Inhibition of anthrax toxins with a bispecific monoclonal antibody that cross reacts with edema factor as well as lethal factor of bacillus anthracis. Mol. Immunol. 2011, 48, 1958–1965. [Google Scholar] [CrossRef]
- Leysath, C.E.; Chen, K.H.; Moayeri, M.; Crown, D.; Fattah, R.; Chen, Z.; Das, S.R.; Purcell, R.H.; Leppla, S.H. Mouse monoclonal antibodies to anthrax edema factor protect against infection. Infect. Immun. 2011, 79, 4609–4616. [Google Scholar]
- vor dem Esche, U.; Huber, M.; Zgaga-Griesz, A.; Grunow, R.; Beyer, W.; Hahn, U.; Bessler, W.G. Passive vaccination with a human monoclonal antibody: Generation of antibodies and studies for efficacy in bacillus anthracis infections. Immunobiology 2011, 216, 847–853. [Google Scholar] [CrossRef]
- Colombatti, M.; Johnson, V.G.; Skopicki, H.A.; Fendley, B.; Lewis, M.S.; Youle, R.J. Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. J. Immunol. 1987, 138, 3339–3344. [Google Scholar]
- Chanh, T.C.; Romanowski, M.J.; Hewetson, J.F. Monoclonal antibody prophylaxis against the in vivo toxicity of ricin in mice. Immunol. Invest. 1993, 22, 63–72. [Google Scholar] [CrossRef]
- Lemley, P.V.; Amanatides, P.; Wright, D.C. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma 1994, 13, 417–421. [Google Scholar] [CrossRef]
- Dertzbaugh, M.T.; Rossi, C.A.; Paddle, B.M.; Hale, M.; Poretski, M.; Alderton, M.R. Monoclonal antibodies to ricin: In vitro inhibition of toxicity and utility as diagnostic reagents. Hybridoma (Larchmt) 2005, 24, 236–243. [Google Scholar] [CrossRef]
- Mantis, N.J.; McGuinness, C.R.; Sonuyi, O.; Edwards, G.; Farrant, S.A. Immunoglobulin a antibodies against ricin a and b subunits protect epithelial cells from ricin intoxication. Infect. Immun. 2006, 74, 3455–3462. [Google Scholar]
- McGuinness, C.R.; Mantis, N.J. Characterization of a novel high-affinity monoclonal immunoglobulin g antibody against the ricin b subunit. Infect. Immun. 2006, 74, 3463–3470. [Google Scholar] [CrossRef]
- Pelat, T.; Hust, M.; Hale, M.; Lefranc, M.P.; Dubel, S.; Thullier, P. Isolation of a human-like antibody fragment (scfv) that neutralizes ricin biological activity. BMC Biotechnol 2009, 9, 60. [Google Scholar] [CrossRef]
- Neal, L.M.; O'Hara, J.; Brey, R.N., 3rd; Mantis, N.J. A monoclonal immunoglobulin g antibody directed against an immunodominant linear epitope on the ricin a chain confers systemic and mucosal immunity to ricin. Infect. Immun. 2010, 78, 552–561. [Google Scholar] [CrossRef]
- O'Hara, J.M.; Neal, L.M.; McCarthy, E.A.; Kasten-Jolly, J.A.; Brey, R.N.3rd; Mantis, N.J. Folding domains within the ricin toxin a subunit as targets of protective antibodies. Vaccine 2010, 28, 7035–7046. [Google Scholar]
- Dai, J.; Zhao, L.; Yang, H.; Guo, H.; Fan, K.; Wang, H.; Qian, W.; Zhang, D.; Li, B.; Guo, Y. Identification of a novel functional domain of ricin responsible for its potent toxicity. J. Biol. Chem. 2011, 286, 12166–12171. [Google Scholar]
- Prigent, J.; Panigai, L.; Lamourette, P.; Sauvaire, D.; Devilliers, K.; Plaisance, M.; Volland, H.; Creminon, C.; Simon, S. Neutralising antibodies against ricin toxin. PLoS One 2011, 6, e20166. [Google Scholar]
- Yermakova, A.; Mantis, N.J. Protective immunity to ricin toxin conferred by antibodies against the toxin's binding subunit (rtb). Vaccine 2011, 29, 7925–7935. [Google Scholar] [CrossRef]
- Oguma, K.; Agui, T.; Syuto, B.; Kimura, K.; Iida, H.; Kubo, S. Four different monoclonal antibodies against type c1 toxin of clostridium botulinum. Infect. Immun. 1982, 38, 14–20. [Google Scholar]
- Oguma, K.; Murayama, S.; Syuto, B.; Iida, H.; Kubo, S. Analysis of antigenicity of clostridium botulinum type c1 and d toxins by polyclonal and monoclonal antibodies. Infect. Immun. 1984, 43, 584–588. [Google Scholar]
- Kozaki, S.; Kamata, Y.; Nagai, T.; Ogasawara, J.; Sakaguchi, G. The use of monoclonal antibodies to analyze the structure of clostridium botulinum type e derivative toxin. Infect. Immun. 1986, 52, 786–791. [Google Scholar]
- Ferreira, J.L.; Hamdy, M.K.; Herd, Z.L.; McCay, S.G.; Zapatka, F.A. Monoclonal antibody for the detection of clostridium botulinum type a toxin. Mol. Cell Probes. 1987, 1, 337–345. [Google Scholar] [CrossRef]
- Simpson, L.L.; Kamata, Y.; Kozaki, S. Use of monoclonal antibodies as probes for the structure and biological activity of botulinum neurotoxin. J. Pharmacol. Exp. Ther. 1990, 255, 227–232. [Google Scholar]
- Toratani, S.; Sekine, N.; Katada, T.; Yokosawa, H. Production of monoclonal antibodies that inhibit adp-ribosylation of small gtp-binding proteins catalyzed by clostridium botulinum adp-ribosyltransferase c3. FEBS Lett. 1993, 324, 353–357. [Google Scholar] [CrossRef]
- Cenci Di Bello, I.; Poulain, B.; Shone, C.C.; Tauc, L.; Dolly, J.O. Antagonism of the intracellular action of botulinum neurotoxin type a with monoclonal antibodies that map to light-chain epitopes. Eur. J. Biochem. 1994, 219, 161–169. [Google Scholar] [CrossRef]
- Noah, C.W.; Poteet, S.S.; Ramos, N.C.; Perez, J.C.; Huang, S.Y. Production of monoclonal antibodies specific to clostridium botulinum type b neurotoxin. J. AOAC Int. 1995, 78, 381–385. [Google Scholar]
- Amersdorfer, P.; Wong, C.; Chen, S.; Smith, T.; Deshpande, S.; Sheridan, R.; Finnern, R.; Marks, J.D. Molecular characterization of murine humoral immune response to botulinum neurotoxin type a binding domain as assessed by using phage antibody libraries. Infect. Immun. 1997, 65, 3743–3752. [Google Scholar]
- Brown, D.R.; Lloyd, J.P.; Schmidt, J.J. Identification and characterization of a neutralizing monoclonal antibody against botulinum neurotoxin serotype f, following vaccination with active toxin. Hybridoma 1997, 16, 447–456. [Google Scholar] [CrossRef]
- Pless, D.D.; Torres, E.R.; Reinke, E.K.; Bavari, S. High-affinity, protective antibodies to the binding domain of botulinum neurotoxin type a. Infect. Immun. 2001, 69, 570–574. [Google Scholar] [CrossRef]
- Wu, H.C.; Yeh, C.T.; Huang, Y.L.; Tarn, L.J.; Lung, C.C. Characterization of neutralizing antibodies and identification of neutralizing epitope mimics on the clostridium botulinum neurotoxin type a. Appl. Environ. Microbiol. 2001, 67, 3201–3207. [Google Scholar] [CrossRef]
- Kamata, Y.; Hoshi, H.; Choki, H.; Kozaki, S. Characterization of a neutralizing monoclonal antibody against botulinum adp-ribosyltransferase, c3 exoenzyme. J. Vet. Med. Sci. 2002, 64, 767–771. [Google Scholar] [CrossRef]
- Yang, G.H.; Kim, K.S.; Kim, H.W.; Jeong, S.T.; Huh, G.H.; Kim, J.C.; Jung, H.H. Isolation and characterization of a neutralizing antibody specific to internalization domain of clostridium botulinum neurotoxin type b. Toxicon 2004, 44, 19–25. [Google Scholar] [CrossRef]
- Adekar, S.P.; Jones, R.M.; Elias, M.D.; Al-Saleem, F.H.; Root, M.J.; Simpson, L.L.; Dessain, S.K. A human monoclonal antibody that binds serotype a botulinum neurotoxin. Hybridoma (Larchmt) 2008, 27, 11–17. [Google Scholar] [CrossRef]
- Adekar, S.P.; Takahashi, T.; Jones, R.M.; Al-Saleem, F.H.; Ancharski, D.M.; Root, M.J.; Kapadnis, B.P.; Simpson, L.L.; Dessain, S.K. Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain. PLoS One 2008, 3, e3023. [Google Scholar]
- Adekar, S.P.; Al-Saleem, F.H.; Elias, M.D.; Rybinski, K.A.; Simpson, L.L.; Dessain, S.K. A natural human igm antibody that neutralizes botulinum neurotoxin in vivo. Hybridoma (Larchmt) 2008, 27, 65–69. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, B.; Pellett, S.; Johnson, E.A.; Janda, K.D. Selection and characterization of a human monoclonal neutralizing antibody for clostridium botulinum neurotoxin serotype b. Bioorg. Med. Chem. Lett. 2009, 19, 662–664. [Google Scholar]
- Mazuet, C.; Dano, J.; Popoff, M.R.; Creminon, C.; Volland, H. Characterization of botulinum neurotoxin type a neutralizing monoclonal antibodies and influence of their half-lives on therapeutic activity. PLoS One 2010, 5, e12416. [Google Scholar]
- Corbett, C.R.; Ballegeer, E.; Weedmark, K.A.; Elias, M.D.; Al-Saleem, F.H.; Ancharski, D.M.; Simpson, L.L.; Berry, J.D. Epitope characterization of sero-specific monoclonal antibody to clostridium botulinum neurotoxin type a. Hybridoma (Larchmt) 2011, 30, 503–510. [Google Scholar] [CrossRef]
- Montgomery, V.A.; Smith, L.A. Diagnostic and possible therapeutic application of a monoclonal antibody (14g8) directed against botulinum type c neurotoxin. Hybridoma (Larchmt) 2011, 30, 209–216. [Google Scholar] [CrossRef]
- Lin, Y.S.; Largen, M.T.; Newcomb, J.R.; Rogers, T.J. Production and characterisation of monoclonal antibodies specific for staphylococcal enterotoxin b. J. Med. Microbiol. 1988, 27, 263–270. [Google Scholar] [CrossRef]
- Hamad, A.R.; Herman, A.; Marrack, P.; Kappler, J.W. Monoclonal antibodies defining functional sites on the toxin superantigen staphylococcal enterotoxin b. J. Exp. Med. 1994, 180, 615–621. [Google Scholar]
- Pang, L.T.; Kum, W.W.; Chow, A.W. Inhibition of staphylococcal enterotoxin b-induced lymphocyte proliferation and tumor necrosis factor alpha secretion by mab5, an anti-toxic shock syndrome toxin 1 monoclonal antibody. Infect. Immun. 2000, 68, 3261–3268. [Google Scholar] [CrossRef]
- Tilahun, M.E.; Rajagopalan, G.; Shah-Mahoney, N.; Lawlor, R.G.; Tilahun, A.Y.; Xie, C.; Natarajan, K.; Margulies, D.H.; Ratner, D.I.; Osborne, B.A.; et al. Potent neutralization of staphylococcal enterotoxin b by synergistic action of chimeric antibodies. Infect. Immun. 2010, 78, 2801–2811. [Google Scholar]
- Larkin, E.A.; Stiles, B.G.; Ulrich, R.G. Inhibition of toxic shock by human monoclonal antibodies against staphylococcal enterotoxin b. PLoS One 2010, 5, e13253. [Google Scholar]
- Drozdowski, B.; Zhou, Y.; Kline, B.; Spidel, J.; Chan, Y.Y.; Albone, E.; Turchin, H.; Chao, Q.; Henry, M.; Balogach, J.; et al. Generation and characterization of high affinity human monoclonal antibodies that neutralize staphylococcal enterotoxin b. J. Immun. Based Ther. Vaccines 2010, 8, 9. [Google Scholar] [CrossRef]
- Varshney, A.K.; Wang, X.; Cook, E.; Dutta, K.; Scharff, M.D.; Goger, M.J.; Fries, B.C. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin b-induced lethal shock. J. Biol. Chem. 2011, 286, 9737–9747. [Google Scholar]
- Reichert, J.M.; Dewitz, M.C. Anti-infective monoclonal antibodies: Perils and promise of development. Nat. Rev. Drug. Discov. 2006, 5, 191–195. [Google Scholar] [CrossRef]
- Nelson, A.L.; Dhimolea, E.; Reichert, J.M. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug. Discov. 2010, 9, 767–774. [Google Scholar] [CrossRef]
- Mazumdar, S. Raxibacumab. MAbs 2009, 1, 531–538. [Google Scholar] [CrossRef]
- Froude, J.W.; Stiles, B.; Pelat, T.; Thullier, P. Antibodies for biodefense. MAbs 2011, 3, 517–527. [Google Scholar] [CrossRef]
- Salfeld, J.G. Isotype selection in antibody engineering. Nat. Biotechnol. 2007, 25, 1369–1372. [Google Scholar] [CrossRef]
- Abboud, N.; Chow, S.K.; Saylor, C.; Janda, A.; Ravetch, J.V.; Scharff, M.D.; Casadevall, A. A requirement for fcgammar in antibody-mediated bacterial toxin neutralization. J. Exp. Med. 2010, 207, 2395–2405. [Google Scholar] [CrossRef]
- Jefferis, R. Antibody therapeutics: Isotype and glycoform selection. Expert. Opin. Biol. Ther. 2007, 7, 1401–1413. [Google Scholar] [CrossRef]
- Chow, S.K.; Casadevall, A. Evaluation of cryptococcus neoformans galactoxylomannan-protein conjugate as vaccine candidate against murine cryptococcosis. Vaccine 2011, 29, 1891–1898. [Google Scholar] [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar]
- Presta, L.G. Molecular engineering and design of therapeutic antibodies. Curr. Opin. Immunol. 2008, 20, 460–470. [Google Scholar] [CrossRef]
- Goodner, K.; Horsfall, F.L. The protective action of type i antipneumococcus serum in mice: I. The quantitative aspects of the mouse protection test. J. Exp. Med. 1935, 62, 359–374. [Google Scholar] [CrossRef]
- Smith, K.; Crowe, S.R.; Garman, L.; Guthridge, C.J.; Muther, J.J.; McKee, E.; Zheng, N.Y.; Farris, A.D.; Guthridge, J.M.; Wilson, P.C.; et al. Human monoclonal antibodies generated following vaccination with ava provide neutralization by blocking furin cleavage but not by preventing oligomerization. Vaccine 2012, 30, 4276–4283. [Google Scholar]
- Maynard, J.A.; Maassen, C.B.; Leppla, S.H.; Brasky, K.; Patterson, J.L.; Iverson, B.L.; Georgiou, G. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 2002, 20, 597–601. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, J.H.; Hung, C.F.; Wu, T.C.; Kim, T.W. Enhancement of antibody responses to bacillus anthracis protective antigen domain iv by use of calreticulin as a chimeric molecular adjuvant. Infect. Immun. 2008, 76, 1952–1959. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug. Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef]
- Kimura, A.; Mountzouros, K.T.; Schad, P.A.; Cieplak, W.; Cowell, J.L. Pertussis toxin analog with reduced enzymatic and biological activities is a protective immunogen. Infect. Immun. 1990, 58, 3337–3347. [Google Scholar]
- Zhang, X.; Askins, J.; Fleming, R.; Sturm, B.; Poortman, C.; Viriassov, P.; Peterson, B.; Flynn, M.; Miao, Y.; Zukauskas, D.; et al. Selection of Potent Neutralizing Human Monoclonal Antibodies to Protective Antigen of Bacillus Anthracis. In Proceedings of 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, USA, 14–17 September 2003; p. 277.
- Moayeri, M.; Leppla, S.H. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects. Med. 2009, 30, 439–455. [Google Scholar] [CrossRef]
- Nowakowski, A.; Wang, C.; Powers, D.B.; Amersdorfer, P.; Smith, T.J.; Montgomery, V.A.; Sheridan, R.; Blake, R.; Smith, L.A.; Marks, J.D. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. USA 2002, 99, 11346–11350. [Google Scholar]
- Volk, W.A.; Bizzini, B.; Snyder, R.M.; Bernhard, E.; Wagner, R.R. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infect. Immun. 1984, 45, 604–609. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chow, S.-K.; Casadevall, A. Monoclonal Antibodies and Toxins—A Perspective on Function and Isotype. Toxins 2012, 4, 430-454. https://doi.org/10.3390/toxins4060430
Chow S-K, Casadevall A. Monoclonal Antibodies and Toxins—A Perspective on Function and Isotype. Toxins. 2012; 4(6):430-454. https://doi.org/10.3390/toxins4060430
Chicago/Turabian StyleChow, Siu-Kei, and Arturo Casadevall. 2012. "Monoclonal Antibodies and Toxins—A Perspective on Function and Isotype" Toxins 4, no. 6: 430-454. https://doi.org/10.3390/toxins4060430




