Prevalence, Biogenesis, and Functionality of the Serine Protease Autotransporter EspP
Abstract
:1. Introduction
| Protein | Organism | Function/Effects | Reference |
|---|---|---|---|
| EatA (ETEC autotransporter A) | ETEC | Mucosal destruction | [21] |
| EpeA (EHEC plasmid-encoded autotransporter) | EHEC | Mucinase activity | [22] |
| EspC (EPEC secreted protein C) | EPEC | Mediation of EPEC lysozyme resistance, vacuolation, cell rounding and detachment, cytoskeletal damage | [23,24,25] |
| EspI (E. coli secreted protease, island-encoded) | STEC | Unknown | [26] |
| EspP/PssA (extracellular serine protease, plasmid-encoded/protein secreted by STEC A) | EHEC | See this review | [1,3] |
| Hbp/Tsh (hemoglobin protease/temperature-sensitive hemagglutinin) | Human septic E. coli/APEC/UPEC | Binding of hemoglobin/heme, degradation of hemoglobin, hemagglutinin, adhesion, mucinase activity | [27,28,29,30,31] |
| Pet (plasmid-encoded toxin) | EAEC | Inflammation, mucus secretion, tissue damage | [32,33] |
| Pic/PicU (protease involved in intestinal colonization) | EAEC/UPEC | Hemagglutinin, serum resistance mediator, mucinase activity | [34,35] |
| Sat (secreted autotransporter toxin) | DAEC/EAEC/UPEC | Causes autophagy, vacuolating toxicity, cell detachment and elongation, formation of lesions in tight-junctions | [18,36,37,38,39] |
| SepA (Shigella extracellular protein) | EAEC | Tissue inflammation, mucosal atrophy, fluid accumulation | [40,41] |
| SigA (Shigella IgA-like protease homolog) | EAEC | Cell rounding and detachment | [40,42] |
| Vat (vacuolating autotransporter toxin) | APEC | Vacuolating toxicity | [43] |
2. Extracellular Serine Protease Plasmid-Encoded
2.1. Organization of EspP

2.2. Autotransport

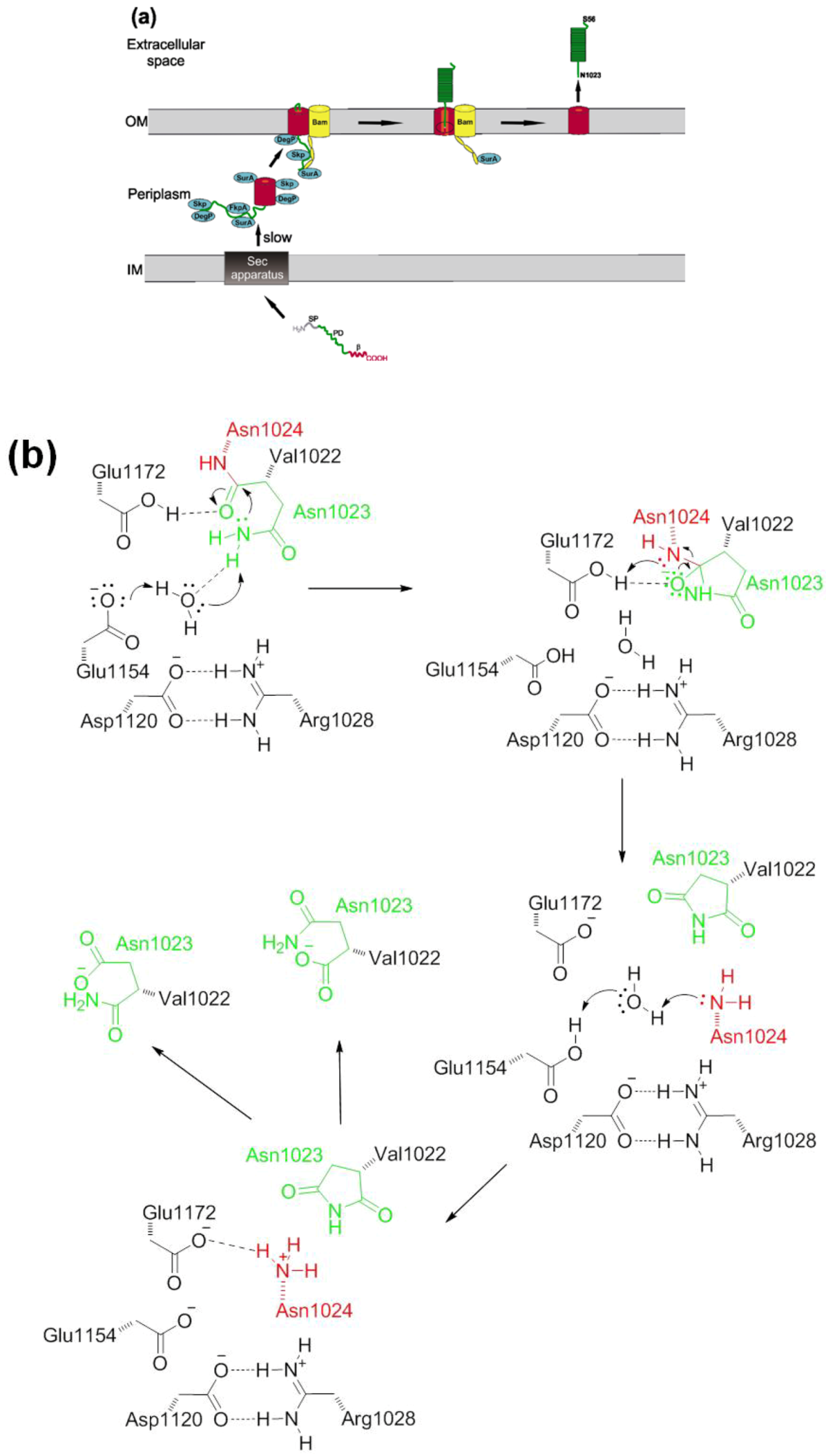
2.3. Prevalence and Distribution of EspP
2.4. Proteolytic Activity and Structure of the Passenger Domain
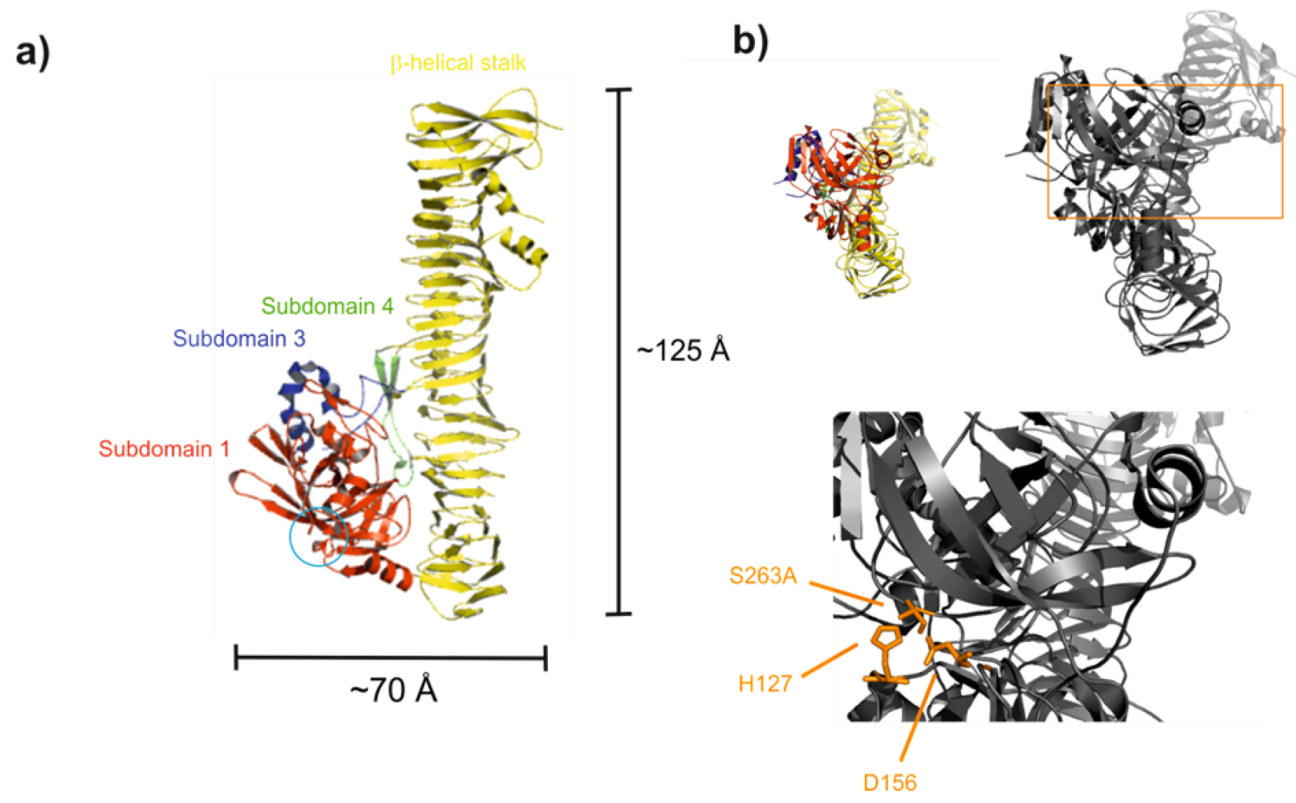
2.5. Cytotoxicity and Cellular Adherence
2.6. Gene Expression
3. Conclusions
Conflict of Interest
References
- Brunder, W.; Schmidt, H.; Karch, H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 1997, 24, 767–778. [Google Scholar]
- Brockmeyer, J.; Spelten, S.; Kuczius, T.; Bielaszewska, M.; Karch, H. Structure and function relationship of the autotransport and proteolytic activity of EspP from Shiga toxin-producing Escherichia coli. PLoS One 2009, 4, e6100. [Google Scholar]
- Djafari, S.; Ebel, F.; Deibel, C.; Kramer, S.; Hudel, M.; Chakraborty, T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol. Microbiol. 1997, 25, 771–784. [Google Scholar] [CrossRef]
- Pohlner, J.; Halter, R.; Beyreuther, K.; Meyer, T.F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 1987, 325, 458–462. [Google Scholar]
- Desvaux, M.; Parham, N.J.; Henderson, I.R. The autotransporter secretion system. Res. Microbiol. 2004, 155, 53–60. [Google Scholar] [CrossRef]
- Henderson, I.R.; Navarro-Garcia, F.; Desvaux, M.; Fernandez, R.C.; Ala’Aldeen, D. Type V protein secretion pathway: The autotransporter story. Microbiol. Mol. Biol. Rev. 2004, 68, 692–744. [Google Scholar] [CrossRef]
- Ieva, R.; Bernstein, H.D. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc. Natl. Acad. Sci. USA 2009, 106, 19120–19125. [Google Scholar] [CrossRef]
- Ruiz-Perez, F.; Henderson, I.R.; Leyton, D.L.; Rossiter, A.E.; Zhang, Y.; Nataro, J.P. Roles of periplasmic chaperone proteins in the biogenesis of serine protease autotransporters of Enterobacteriaceae. J. Bacteriol. 2009, 191, 6571–6583. [Google Scholar]
- Ruiz-Perez, F.; Henderson, I.R.; Nataro, J.P. Interaction of FkpA, a peptidyl-prolyl cis/trans isomerase with EspP autotransporter protein. Gut Microbes 2010, 1, 339–344. [Google Scholar] [CrossRef]
- Ieva, R.; Tian, P.; Peterson, J.H.; Bernstein, H.D. Sequential and spatially restricted interactions of assembly factors with an autotransporter beta domain. Proc. Natl. Acad. Sci. USA 2011, 108, E383–E391. [Google Scholar]
- Selkrig, J.; Mosbahi, K.; Webb, C.T.; Belousoff, M.J.; Perry, A.J.; Wells, T.J.; Morris, F.; Leyton, D.L.; Totsika, M.; Phan, M.D.; et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat. Struct. Mol. Biol. 2012, 19, 506–510. [Google Scholar] [CrossRef]
- Leyton, D.L.; Rossiter, A.E.; Henderson, I.R. From self sufficiency to dependence: Mechanisms and factors important for autotransporter biogenesis. Nat. Rev. 2012, 10, 213–225. [Google Scholar] [CrossRef]
- Celik, N.; Webb, C.T.; Leyton, D.L.; Holt, K.E.; Heinz, E.; Gorrell, R.; Kwok, T.; Naderer, T.; Strugnell, R.A.; Speed, T.P.; et al. A bioinformatic strategy for the detection, classification and analysis of bacterial autotransporters. PLoS One 2012, 7, e43245. [Google Scholar]
- Wells, T.J.; Totsika, M.; Schembri, M.A. Autotransporters of Escherichia coli: A sequence-based characterization. Microbiology 2010, 156, 2459–2469. [Google Scholar] [CrossRef]
- Henderson, I.R.; Nataro, J.P. Virulence functions of autotransporter proteins. Infect. Immun. 2001, 69, 1231–1243. [Google Scholar] [CrossRef]
- Grozdanov, L.; Raasch, C.; Schulze, J.; Sonnenborn, U.; Gottschalk, G.; Hacker, J.; Dobrindt, U. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 2004, 186, 5432–5441. [Google Scholar]
- Parham, N.J.; Pollard, S.J.; Desvaux, M.; Scott-Tucker, A.; Liu, C.; Fivian, A.; Henderson, I.R. Distribution of the serine protease autotransporters of the Enterobacteriaceae among extraintestinal clinical isolates of Escherichia coli. J. Clin. Microbiol. 2005, 43, 4076–4082. [Google Scholar] [CrossRef]
- Boisen, N.; Ruiz-Perez, F.; Scheutz, F.; Krogfelt, K.A.; Nataro, J.P. Short report: High prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am. J. Trop. Med. Hyg. 2009, 80, 294–301. [Google Scholar]
- Yen, Y.T.; Kostakioti, M.; Henderson, I.R.; Stathopoulos, C. Common themes and variations in serine protease autotransporters. Trends Microbiol. 2008, 16, 370–379. [Google Scholar] [CrossRef]
- Dautin, N. Serine protease autotransporters of enterobacteriaceae (SPATEs): Biogenesis and function. Toxins 2010, 2, 1179–1206. [Google Scholar] [CrossRef]
- Patel, S.K.; Dotson, J.; Allen, K.P.; Fleckenstein, J.M. Identification and molecular characterization of EatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect. Immun. 2004, 72, 1786–1794. [Google Scholar] [CrossRef]
- Leyton, D.L.; Sloan, J.; Hill, R.E.; Doughty, S.; Hartland, E.L. Transfer region of pO113 from enterohemorrhagic Escherichia coli: Similarity with R64 and identification of a novel plasmid-encoded autotransporter, EpeA. Infect. Immun. 2003, 71, 6307–6319. [Google Scholar]
- Navarro-Garcia, F.; Canizalez-Roman, A.; Sui, B.Q.; Nataro, J.P.; Azamar, Y. The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli. Infect. Immun. 2004, 72, 3609–3621. [Google Scholar] [CrossRef]
- Stein, M.; Kenny, B.; Stein, M.A.; Finlay, B.B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 1996, 178, 6546–6554. [Google Scholar]
- Mellies, J.L.; Navarro-Garcia, F.; Okeke, I.; Frederickson, J.; Nataro, J.P.; Kaper, J.B. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 2001, 69, 315–324. [Google Scholar] [CrossRef]
- Schmidt, H.; Zhang, W.L.; Hemmrich, U.; Jelacic, S.; Brunder, W.; Tarr, P.I.; Dobrindt, U.; Hacker, J.; Karch, H. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 2001, 69, 6863–6873. [Google Scholar]
- Otto, B.R.; van Dooren, S.J.; Nuijens, J.H.; Luirink, J.; Oudega, B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 1998, 188, 1091–1103. [Google Scholar] [CrossRef]
- Provence, D.L.; Curtiss, R., III. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 1994, 62, 1369–1380. [Google Scholar]
- Kostakioti, M.; Stathopoulos, C. Functional analysis of the Tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect. Immun. 2004, 72, 5548–5554. [Google Scholar] [CrossRef]
- Heimer, S.R.; Rasko, D.A.; Lockatell, C.V.; Johnson, D.E.; Mobley, H.L. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 2004, 72, 593–597. [Google Scholar] [CrossRef]
- Kobayashi, R.K.; Gaziri, L.C.; Venancio, E.J.; Vidotto, M.C. Detection of Tsh protein mucinolytic activity by SDS-PAGE. J. Microbiol. Methods 2007, 68, 654–655. [Google Scholar] [CrossRef]
- Eslava, C.; Navarro-Garcia, F.; Czeczulin, J.R.; Henderson, I.R.; Cravioto, A.; Nataro, J.P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 1998, 66, 3155–3163. [Google Scholar]
- Navarro-Garcia, F.; Eslava, C.; Villaseca, J.M.; Lopez-Revilla, R.; Czeczulin, J.R.; Srinivas, S.; Nataro, J.P.; Cravioto, A. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 1998, 66, 3149–3154. [Google Scholar]
- Henderson, I.R.; Czeczulin, J.; Eslava, C.; Noriega, F.; Nataro, J.P. Characterization of pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 1999, 67, 5587–5596. [Google Scholar]
- Parham, N.J.; Srinivasan, U.; Desvaux, M.; Foxman, B.; Marrs, C.F.; Henderson, I.R. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 2004, 230, 73–83. [Google Scholar] [CrossRef]
- Guignot, J.; Chaplais, C.; Coconnier-Polter, M.H.; Servin, A.L. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 2007, 9, 204–221. [Google Scholar] [CrossRef]
- Guyer, D.M.; Henderson, I.R.; Nataro, J.P.; Mobley, H.L. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 2000, 38, 53–66. [Google Scholar] [CrossRef]
- Guyer, D.M.; Radulovic, S.; Jones, F.E.; Mobley, H.L. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 2002, 70, 4539–4546. [Google Scholar] [CrossRef]
- Lievin-Le Moal, V.; Comenge, Y.; Ruby, V.; Amsellem, R.; Nicolas, V.; Servin, A.L. Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell. Microbiol. 2011, 13, 992–1013. [Google Scholar] [CrossRef]
- Karch, H.; Denamur, E.; Dobrindt, U.; Finlay, B.B.; Hengge, R.; Johannes, L.; Ron, E.Z.; Tonjum, T.; Sansonetti, P.; Vicente, M. The enemy within us: Lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol. Med. 2012, 4, 841–848. [Google Scholar] [CrossRef]
- Benjelloun-Touimi, Z.; Sansonetti, P.J.; Parsot, C. SepA, the major extracellular protein of Shigella flexneri: Autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 1995, 17, 123–135. [Google Scholar] [CrossRef]
- Al-Hasani, K.; Henderson, I.R.; Sakellaris, H.; Rajakumar, K.; Grant, T.; Nataro, J.P.; Robins-Browne, R.; Adler, B. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 2000, 68, 2457–2463. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Yano, T.; Carvalho, H.E.; Parreira, V.R.; Gyles, C.L. Vacuolating cytotoxin produced by avian pathogenic Escherichia coli. Avian Dis. 2001, 45, 43–51. [Google Scholar]
- Navarro-Garcia, F.; Sears, C.; Eslava, C.; Cravioto, A.; Nataro, J.P. Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 1999, 67, 2184–2192. [Google Scholar]
- Dutta, P.R.; Cappello, R.; Navarro-Garcia, F.; Nataro, J.P. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect. Immun. 2002, 70, 7105–7113. [Google Scholar]
- Harrington, S.M.; Sheikh, J.; Henderson, I.R.; Ruiz-Perez, F.; Cohen, P.S.; Nataro, J.P. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 2009, 77, 2465–2473. [Google Scholar] [CrossRef]
- Law, D. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. J. Appl. Microbiol. 2000, 88, 729–745. [Google Scholar] [CrossRef]
- Karmali, M.A. Infection by Shiga toxin-producing Escherichia coli: An overview. Mol. Biotechnol. 2004, 26, 117–122. [Google Scholar] [CrossRef]
- Brockmeyer, J.; Bielaszewska, M.; Fruth, A.; Bonn, M.L.; Mellmann, A.; Humpf, H.U.; Karch, H. Subtypes of the plasmid-encoded serine protease EspP in Shiga toxin-producing Escherichia coli: Distribution, secretion, and proteolytic activity. Appl. Environ. Microbiol. 2007, 73, 6351–6359. [Google Scholar]
- Khan, A.B.; Naim, A.; Orth, D.; Grif, K.; Mohsin, M.; Prager, R.; Dierich, M.P.; Wurzner, R. Serine protease espP subtype alpha, but not beta or gamma, of Shiga toxin-producing Escherichia coli is associated with highly pathogenic serogroups. Int. J. Med. Microbiol. 2009, 299, 247–254. [Google Scholar] [CrossRef]
- Oomen, C.J.; van Ulsen, P.; van Gelder, P.; Feijen, M.; Tommassen, J.; Gros, P. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 2004, 23, 1257–1266. [Google Scholar] [CrossRef]
- De, E.; Saint, N.; Glinel, K.; Meli, A.C.; Levy, D.; Jacob-Dubuisson, F. Influence of the passenger domain of a model autotransporter on the properties of its translocator domain. Mol. Membr. Biol. 2008, 25, 192–202. [Google Scholar] [CrossRef]
- Ieva, R.; Skillman, K.M.; Bernstein, H.D. Incorporation of a polypeptide segment into the β-domain pore during the assembly of a bacterial autotransporter. Mol. Microbiol. 2008, 67, 188–201. [Google Scholar]
- Oliver, D.C.; Huang, G.; Nodel, E.; Pleasance, S.; Fernandez, R.C. A conserved region within the Bordetella pertussis autotransporter BrkA is necessary for folding of its passenger domain. Mol. Microbiol. 2003, 47, 1367–1383. [Google Scholar]
- Velarde, J.J.; Nataro, J.P. Hydrophobic residues of the autotransporter EspP linker domain are important for outer membrane translocation of its passenger. J. Biol. Chem. 2004, 279, 31495–31504. [Google Scholar] [CrossRef]
- Economou, A.; Christie, P.J.; Fernandez, R.C.; Palmer, T.; Plano, G.V.; Pugsley, A.P. Secretion by numbers: Protein traffic in prokaryotes. Mol. Microbiol. 2006, 62, 308–319. [Google Scholar]
- Leo, J.C.; Grin, I.; Linke, D. Type V secretion: Mechanism(s) of autotransport through the bacterial outer membrane. Philos. Trans. R. Soc. Lond. B 2012, 367, 1088–1101. [Google Scholar]
- Peterson, J.H.; Szabady, R.L.; Bernstein, H.D. An unusual signal peptide extension inhibits the binding of bacterial presecretory proteins to the signal recognition particle, trigger factor, and the SecYEG complex. J. Biol. Chem. 2006, 281, 9038–9048. [Google Scholar]
- von Heijne, G. Signal sequences. The limits of variation. J. Mol. Biol. 1985, 184, 99–105. [Google Scholar] [CrossRef]
- Henderson, I.R.; Navarro-Garcia, F.; Nataro, J.P. The great escape: Structure and function of the autotransporter proteins. Trends Microb. 1998, 6, 370–378. [Google Scholar] [CrossRef]
- Szabady, R.L.; Peterson, J.H.; Skillman, K.M.; Bernstein, H.D. An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proc. Natl. Acad. Sci. USA 2005, 102, 221–226. [Google Scholar]
- Barnard, T.J.; Dautin, N.; Lukacik, P.; Bernstein, H.D.; Buchanan, S.K. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat. Struct. Mol. Biol. 2007, 14, 1214–1220. [Google Scholar]
- Peterson, J.H.; Tian, P.; Ieva, R.; Dautin, N.; Bernstein, H.D. Secretion of a bacterial virulence factor is driven by the folding of a C-terminal segment. Proc. Natl. Acad. Sci. USA 2010, 107, 17739–17744. [Google Scholar]
- Dautin, N.; Barnard, T.J.; Anderson, D.E.; Bernstein, H.D. Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J. 2007, 26, 1942–1952. [Google Scholar] [CrossRef]
- Barnard, T.J.; Gumbart, J.; Peterson, J.H.; Noinaj, N.; Easley, N.C.; Dautin, N.; Kuszak, A.J.; Tajkhorshid, E.; Bernstein, H.D.; Buchanan, S.K. Molecular basis for the activation of a catalytic asparagine residue in a self-cleaving bacterial autotransporter. J. Mol. Biol. 2012, 415, 128–142. [Google Scholar]
- Dautin, N.; Bernstein, H.D. Residues in a conserved α-helical segment are required for cleavage but not secretion of an Escherichia coli serine protease autotransporter passenger domain. J. Bacteriol. 2011, 193, 3748–3756. [Google Scholar] [CrossRef]
- Kostakioti, M.; Stathopoulos, C. Role of the α-helical linker of the C-terminal translocator in the biogenesis of the serine protease subfamily of autotransporters. Infect. Immun. 2006, 74, 4961–4969. [Google Scholar] [CrossRef]
- Tajima, N.; Kawai, F.; Park, S.Y.; Tame, J.R. A novel intein-like autoproteolytic mechanism in autotransporter proteins. J. Mol. Biol. 2010, 402, 645–656. [Google Scholar] [CrossRef]
- Restieri, C.; Garriss, G.; Locas, M.C.; Dozois, C.M. Autotransporter-encoding sequences are phylogenetically distributed among Escherichia coli clinical isolates and reference strains. Appl. Environ. Microbiol. 2007, 73, 1553–1562. [Google Scholar]
- Bugarel, M.; Martin, A.; Fach, P.; Beutin, L. Virulence gene profiling of enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli strains: A basis for molecular risk assessment of typical and atypical EPEC strains. BMC Microbiol. 2011, 11, 142. [Google Scholar] [CrossRef]
- Schmidt, H.; Geitz, C.; Tarr, P.I.; Frosch, M.; Karch, H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: Phenotypic and genetic profiling of virulence traits and evidence for clonality. J. Infect. Dis. 1999, 179, 115–123. [Google Scholar] [CrossRef]
- Welinder-Olsson, C.; Badenfors, M.; Cheasty, T.; Kjellin, E.; Kaijser, B. Genetic profiling of enterohemorrhagic Escherichia coli strains in relation to clonality and clinical signs of infection. J. Clin. Microbiol. 2002, 40, 959–964. [Google Scholar]
- Aktan, I.; Carter, B.; Wilking, H.; La Ragione, R.M.; Wieler, L.; Woodward, M.J.; Anjum, M.F. Influence of geographical origin, host animal and stx gene on the virulence characteristics of Escherichia coli O26 strains. J. Med. Microbiol. 2007, 56, 1431–1439. [Google Scholar] [CrossRef]
- Posse, B.; De Zutter, L.; Heyndrickx, M.; Herman, L. Metabolic and genetic profiling of clinical O157 and non-O157 Shiga-toxin-producing Escherichia coli. Res. Microbiol. 2007, 158, 591–599. [Google Scholar] [CrossRef]
- Fratamico, P.M.; Yan, X.; Caprioli, A.; Esposito, G.; Needleman, D.S.; Pepe, T.; Tozzoli, R.; Cortesi, M.L.; Morabito, S. The complete DNA sequence and analysis of the virulence plasmid and of five additional plasmids carried by Shiga toxin-producing Escherichia coli O26:H11 strain H30. Int. J. Med. Microbiol. 2011, 301, 192–203. [Google Scholar] [CrossRef]
- Brunder, W.; Karch, H.; Schmidt, H. Complete sequence of the large virulence plasmid pSFO157 of the sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H-strain 3072/96. Int. J. Med. Microbiol. 2006, 296, 467–474. [Google Scholar] [CrossRef]
- Nagano, H.; Okui, T.; Fujiwara, O.; Uchiyama, Y.; Tamate, N.; Kumada, H.; Morimoto, Y.; Yano, S. Clonal structure of Shiga toxin (Stx)-producing and β-D-glucuronidase-positive Escherichia coli O157:H7 strains isolated from outbreaks and sporadic cases in Hokkaido, Japan. J. Med. Microbiol. 2002, 51, 405–416. [Google Scholar]
- Rump, L.V.; Meng, J.; Strain, E.A.; Cao, G.; Allard, M.W.; Gonzalez-Escalona, N. Complete DNA sequence analysis of enterohemorrhagic Escherichia coli plasmid pO157_2 in β-glucuronidase-positive E. coli O157:H7 reveals a novel evolutionary path. J. Bacteriol. 2012, 194, 3457–3463. [Google Scholar]
- Brunder, W.; Schmidt, H.; Frosch, M.; Karch, H. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 1999, 145, 1005–1014. [Google Scholar]
- Bielaszewska, M.; Stoewe, F.; Fruth, A.; Zhang, W.; Prager, R.; Brockmeyer, J.; Mellmann, A.; Karch, H.; Friedrich, A.W. Shiga toxin, cytolethal distending toxin, and hemolysin repertoires in clinical Escherichia coli O91 isolates. J. Clin. Microbiol. 2009, 47, 2061–2066. [Google Scholar]
- Boerlin, P.; McEwen, S.A.; Boerlin-Petzold, F.; Wilson, J.B.; Johnson, R.P.; Gyles, C.L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 1999, 37, 497–503. [Google Scholar]
- Pradel, N.; Bertin, Y.; Martin, C.; Livrelli, V. Molecular analysis of shiga toxin-producing Escherichia coli strains isolated from hemolytic-uremic syndrome patients and dairy samples in France. Appl. Environ. Microbiol. 2008, 74, 2118–2128. [Google Scholar]
- Toszeghy, M.; Phillips, N.; Reeves, H.; Wu, G.; Teale, C.; Coldham, N.; Randall, L. Molecular and phenotypic characterisation of Extended Spectrum β-lactamase CTX-M Escherichia coli from farm animals in Great Britain. Res. Vet. Sci. 2012, 1142–1150. [Google Scholar]
- Horcajo, P.; Dominguez-Bernal, G.; de la Fuente, R.; Ruiz-Santa-Quiteria, J.A.; Blanco, J.E.; Blanco, M.; Mora, A.; Dahbi, G.; Lopez, C.; Puentes, B.; et al. Comparison of ruminant and human attaching and effacing Escherichia coli (AEEC) strains. Vet. Microbiol. 2012, 155, 341–348. [Google Scholar] [CrossRef]
- Geue, L.; Segura-Alvarez, M.; Conraths, F.J.; Kuczius, T.; Bockemühl, J.; Karch, H.; Gallien, P. A long-term study on the prevalence of shiga toxin-producing Escherichia coli (STEC) on four German cattle farms. Epidemiol. Infect. 2002, 129, 173–185. [Google Scholar]
- Khan, A.; Yamasaki, S.; Sato, T.; Ramamurthy, T.; Pal, A.; Datta, S.; Chowdhury, N.R.; Das, S.C.; Sikdar, A.; Tsukamoto, T.; et al. Prevalence and genetic profiling of virulence determinants of non-O157 Shiga toxin-producing Escherichia coli isolated from cattle, beef, and humans, Calcutta, India. Emerg. Infect. Dis. 2002, 8, 54–62. [Google Scholar] [CrossRef]
- Nielsen, E.M.; Andersen, M.T. Detection and characterization of verocytotoxin-producing Escherichia coli by automated 5' nuclease PCR assay. J. Clin. Microbiol. 2003, 41, 2884–2893. [Google Scholar] [CrossRef]
- Ewers, C.; Schuffner, C.; Weiss, R.; Baljer, G.; Wieler, L.H. Molecular characteristics of Escherichia coli serogroup O78 strains isolated from diarrheal cases in bovines urge further investigations on their zoonotic potential. Mol. Nutr. Food Res. 2004, 48, 504–514. [Google Scholar] [CrossRef]
- Geue, L.; Selhorst, T.; Schnick, C.; Mintel, B.; Conraths, F.J. Analysis of the clonal relationship of shiga toxin-producing Escherichia coli serogroup O165:H25 isolated from cattle. Appl. Environ. Microbiol. 2006, 72, 2254–2259. [Google Scholar] [CrossRef]
- Karama, M.; Johnson, R.P.; Holtslander, R.; McEwen, S.A.; Gyles, C.L. Prevalence and characterization of verotoxin-producing Escherichia coli (VTEC) in cattle from an Ontario abattoir. Can. J. Vet. Res. 2008, 72, 297–302. [Google Scholar]
- Cookson, A.L.; Bennett, J.; Nicol, C.; Thomson-Carter, F.; Attwood, G.T. Molecular subtyping and distribution of the serine protease from shiga toxin-producing Escherichia coli among atypical enteropathogenic E. coli strains. Appl. Environ. Microbiol. 2009, 75, 2246–2249. [Google Scholar] [CrossRef]
- De Verdier, K.; Nyman, A.; Greko, C.; Bengtsson, B. Antimicrobial resistance and virulence factors in Escherichia coli from Swedish dairy calves. Acta Vet. Scand. 2012, 54, 2. [Google Scholar] [CrossRef]
- Madic, J.; Vingadassalon, N.; de Garam, C.P.; Marault, M.; Scheutz, F.; Brugere, H.; Jamet, E.; Auvray, F. Detection of Shiga toxin-producing Escherichia coli serotypes O26:H11, O103:H2, O111:H8, O145:H28, and O157:H7 in raw-milk cheeses by using multiplex real-time PCR. Appl. Environ. Microbiol. 2011, 77, 2035–2041. [Google Scholar]
- Slanec, T.; Fruth, A.; Creuzburg, K.; Schmidt, H. Molecular analysis of virulence profiles and Shiga toxin genes in food-borne Shiga toxin-producing Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 6187–6197. [Google Scholar] [CrossRef]
- Bugarel, M.; Beutin, L.; Martin, A.; Gill, A.; Fach, P. Micro-array for the identification of Shiga toxin-producing Escherichia coli (STEC) seropathotypes associated with hemorrhagic colitis and hemolytic uremic syndrome in humans. Int. J. Food Microbiol. 2010, 142, 318–329. [Google Scholar] [CrossRef]
- Bosilevac, J.M.; Koohmaraie, M. Prevalence and characterization of non-O157 shiga toxin-producing Escherichia coli isolates from commercial ground beef in the United States. Appl. Environ. Microbiol. 2011, 77, 2103–2112. [Google Scholar] [CrossRef]
- Monaghan, A.; Byrne, B.; Fanning, S.; Sweeney, T.; McDowell, D.; Bolton, D.J. Serotypes and virulence profiles of non-O157 Shiga toxin-producing Escherichia coli isolates from bovine farms. Appl. Environ. Microbiol. 2011, 77, 8662–8668. [Google Scholar]
- Polifroni, R.; Etcheverria, A.I.; Sanz, M.E.; Cepeda, R.E.; Kruger, A.; Lucchesi, P.M.; Fernandez, D.; Parma, A.E.; Padola, N.L. Molecular characterization of Shiga toxin-producing Escherichia coli isolated from the environment of a dairy farm. Curr. Microbiol. 2012, 65, 337–343. [Google Scholar]
- Orth, D.; Ehrlenbach, S.; Brockmeyer, J.; Khan, A.B.; Huber, G.; Karch, H.; Sarg, B.; Lindner, H.; Wurzner, R. EspP, a serine protease of enterohemorrhagic Escherichia coli, impairs complement activation by cleaving complement factors C3/C3b and C5. Infect. Immun. 2010, 78, 4294–4301. [Google Scholar] [CrossRef]
- Brockmeyer, J.; Aldick, T.; Soltwisch, J.; Zhang, W.; Tarr, P.I.; Weiss, A.; Dreisewerd, K.; Muthing, J.; Bielaszewska, M.; Karch, H. Enterohaemorrhagic Escherichia coli haemolysin is cleaved and inactivated by serine protease EspPalpha. Environ. Microbiol. 2011, 13, 1327–1341. [Google Scholar]
- Yamazaki, T.; Miyamoto, M.; Yamada, S.; Okuda, K.; Ishihara, K. Surface protease of Treponema denticola hydrolyzes C3 and influences function of polymorphonuclear leukocytes. Microbes Infect. 2006, 8, 1758–1763. [Google Scholar] [CrossRef]
- Potempa, M.; Potempa, J.; Kantyka, T.; Nguyen, K.A.; Wawrzonek, K.; Manandhar, S.P.; Popadiak, K.; Riesbeck, K.; Eick, S.; Blom, A.M. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009, 5, e1000316. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.M.; Lee, J.H.; Seo, S.J.; Lee, I.H. Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect. Immun. 2007, 75, 1861–1869. [Google Scholar] [CrossRef]
- Kuo, C.F.; Lin, Y.S.; Chuang, W.J.; Wu, J.J.; Tsao, N. Degradation of complement 3 by streptococcal pyrogenic exotoxin B inhibits complement activation and neutrophil opsonophagocytosis. Infect. Immun. 2008, 76, 1163–1169. [Google Scholar]
- Schenkein, H.A.; Fletcher, H.M.; Bodnar, M.; Macrina, F.L. Increased opsonization of a prtH-defective mutant of Porphyromonas gingivalis W83 is caused by reduced degradation of complement-derived opsonins. J. Immunol. 1995, 154, 5331–5337. [Google Scholar]
- Hong, Y.Q.; Ghebrehiwet, B. Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin. Immunol. Immunopathol. 1992, 62, 133–138. [Google Scholar] [CrossRef]
- Oda, T.; Kojima, Y.; Akaike, T.; Ijiri, S.; Molla, A.; Maeda, H. Inactivation of chemotactic activity of C5a by the serratial 56-kilodalton protease. Infect. Immun. 1990, 58, 1269–1272. [Google Scholar]
- Discipio, R.G.; Daffern, P.J.; Kawahara, M.; Pike, R.; Travis, J.; Hugli, T.E.; Potempa, J. Cleavage of human complement component C5 by cysteine proteinases from Porphyromonas (Bacteroides) gingivalis. Prior oxidation of C5 augments proteinase digestion of C5. Immunology 1996, 87, 660–667. [Google Scholar]
- Wexler, D.E.; Chenoweth, D.E.; Cleary, P.P. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 1985, 82, 8144–8148. [Google Scholar]
- Duga, S.; Asselta, R.; Tenchini, M.L. Coagulation factor V. Int. J. Biochem. Cell Biol. 2004, 36, 1393–1399. [Google Scholar] [CrossRef]
- Aldick, T.; Bielaszewska, M.; Uhlin, B.E.; Humpf, H.U.; Wai, S.N.; Karch, H. Vesicular stabilization and activity augmentation of enterohaemorrhagic Escherichia coli haemolysin. Mol. Microbiol. 2009, 71, 1496–1508. [Google Scholar] [CrossRef]
- Brewer, H.B., Jr.; Fairwell, T.; LaRue, A.; Ronan, R.; Houser, A.; Bronzert, T.J. The amino acid sequence of human APOA-I, an apolipoprotein isolated from high density lipoproteins. Biochem. Biophys. Res. Commun. 1978, 80, 623–630. [Google Scholar] [CrossRef]
- Concha, M.I.; Molina, S.A.; Oyarzún, C.; Villanueva, J.; Amthauer, R. Local expression of apolipoprotein A-I gene and a possible role for HDL in primary defence in the carp skin. Fish Shellfish Immunol. 2003, 14, 259–273. [Google Scholar] [CrossRef]
- Burger, D.; Dayer, J.M. High-density lipoprotein-associated apolipoprotein A-I: The missing link between infection and chronic inflammation? Autoimmun. Rev. 2002, 1, 111–117. [Google Scholar] [CrossRef]
- Massamiri, T.; Tobias, P.S.; Curtiss, L.K. Structural determinants for the interaction of lipopolysaccharide binding protein with purified high density lipoproteins: Role of apolipoprotein A-I. J. Lipid Res. 1997, 38, 516–525. [Google Scholar]
- Flegel, W.A.; Baumstark, M.W.; Weinstock, C.; Berg, A.; Northoff, H. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect. Immun. 1993, 61, 5140–5146. [Google Scholar]
- Emancipator, K.; Csako, G.; Elin, R.J. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect. Immun. 1992, 60, 596–601. [Google Scholar]
- Schmidt, H.; Beutin, L.; Karch, H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 1995, 63, 1055–1061. [Google Scholar]
- Bauer, M.E.; Welch, R.A. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 1996, 64, 167–175. [Google Scholar]
- Aldick, T.; Bielaszewska, M.; Zhang, W.; Brockmeyer, J.; Schmidt, H.; Friedrich, A.W.; Kim, K.S.; Schmidt, M.A.; Karch, H. Hemolysin from Shiga toxin-negative Escherichia coli O26 strains injures microvascular endothelium. Microbes Infect. 2007, 9, 282–290. [Google Scholar] [CrossRef]
- Welch, R.A. RTX toxin structure and function: A story of numerous anomalies and few analogies in toxin biology. Curr. Topics Microbiol. Immunol. 2001, 257, 85–111. [Google Scholar] [CrossRef]
- Nagamune, K.; Yamamoto, K.; Naka, A.; Matsuyama, J.; Miwatani, T.; Honda, T. In vitro proteolytic processing and activation of the recombinant precursor of El Tor cytolysin/hemolysin (pro-HlyA) of Vibrio cholerae by soluble hemagglutinin/protease of V. cholerae, trypsin, and other proteases. Infect. Immun. 1996, 64, 4655–4658. [Google Scholar]
- Garred, O.; van Deurs, B.; Sandvig, K. Furin-induced cleavage and activation of Shiga toxin. J. Biol. Chem. 1995, 270, 10817–10821. [Google Scholar] [CrossRef]
- Melton-Celsa, A.R.; Kokai-Kun, J.F.; O’Brien, A.D. Activation of Shiga toxin type 2d (Stx2d) by elastase involves cleavage of the C-terminal two amino acids of the A2 peptide in the context of the appropriate B pentamer. Mol. Microbiol. 2002, 43, 207–215. [Google Scholar] [CrossRef]
- Khan, S.; Mian, H.S.; Sandercock, L.E.; Chirgadze, N.Y.; Pai, E.F. Crystal structure of the passenger domain of the Escherichia coli autotransporter EspP. J. Mol. Biol. 2011, 413, 985–1000. [Google Scholar] [CrossRef]
- Otto, B.R.; Sijbrandi, R.; Luirink, J.; Oudega, B.; Heddle, J.G.; Mizutani, K.; Park, S.Y.; Tame, J.R. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J. Biol. Chem. 2005, 280, 17339–17345. [Google Scholar]
- Johnson, T.A.; Qiu, J.; Plaut, A.G.; Holyoak, T. Active-site gating regulates substrate selectivity in a chymotrypsin-like serine protease the structure of haemophilus influenzae immunoglobulin A1 protease. J. Mol. Biol. 2009, 389, 559–574. [Google Scholar] [CrossRef]
- Leyton, D.L.; Sevastsyanovich, Y.R.; Browning, D.F.; Rossiter, A.E.; Wells, T.J.; Fitzpatrick, R.E.; Overduin, M.; Cunningham, A.F.; Henderson, I.R. Size and conformation limits to secretion of disulfide-bonded loops in autotransporter proteins. J. Biol. Chem. 2011, 286, 42283–42291. [Google Scholar]
- Xicohtencatl-Cortes, J.; Saldana, Z.; Deng, W.; Castaneda, E.; Freer, E.; Tarr, P.I.; Finlay, B.B.; Puente, J.L.; Giron, J.A. Bacterial macroscopic rope-like fibers with cytopathic and adhesive properties. J. Biol. Chem. 2010, 285, 32336–32342. [Google Scholar]
- Toth, I.; Cohen, M.L.; Rumschlag, H.S.; Riley, L.W.; White, E.H.; Carr, J.H.; Bond, W.W.; Wachsmuth, I.K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect. Immun. 1990, 58, 1223–1231. [Google Scholar]
- Sheng, H.; Lim, J.Y.; Knecht, H.J.; Li, J.; Hovde, C.J. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immun. 2006, 74, 4685–4693. [Google Scholar]
- Lim, J.Y.; La, H.J.; Sheng, H.; Forney, L.J.; Hovde, C.J. Influence of plasmid pO157 on Escherichia coli O157:H7 Sakai biofilm formation. Appl. Environ. Microbiol. 2010, 76, 963–966. [Google Scholar] [CrossRef]
- Puttamreddy, S.; Cornick, N.A.; Minion, F.C. Genome-wide transposon mutagenesis reveals a role for pO157 genes in biofilm development in Escherichia coli O157:H7 EDL933. Infect. Immun. 2010, 78, 2377–2384. [Google Scholar] [CrossRef]
- Ebel, F.; Deibel, C.; Kresse, A.U.; Guzman, C.A.; Chakraborty, T. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect. Immun. 1996, 64, 4472–4479. [Google Scholar]
- Al-Hasani, K.; Navarro-Garcia, F.; Huerta, J.; Sakellaris, H.; Adler, B. The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells. PLoS One 2009, 4, e8223. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Weiss, A.; Brockmeyer, J. Prevalence, Biogenesis, and Functionality of the Serine Protease Autotransporter EspP. Toxins 2013, 5, 25-48. https://doi.org/10.3390/toxins5010025
Weiss A, Brockmeyer J. Prevalence, Biogenesis, and Functionality of the Serine Protease Autotransporter EspP. Toxins. 2013; 5(1):25-48. https://doi.org/10.3390/toxins5010025
Chicago/Turabian StyleWeiss, André, and Jens Brockmeyer. 2013. "Prevalence, Biogenesis, and Functionality of the Serine Protease Autotransporter EspP" Toxins 5, no. 1: 25-48. https://doi.org/10.3390/toxins5010025




