Differences in Susceptibility to Okadaic Acid, a Diarrhetic Shellfish Poisoning Toxin, between Male and Female Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lethality after OA Inoculation
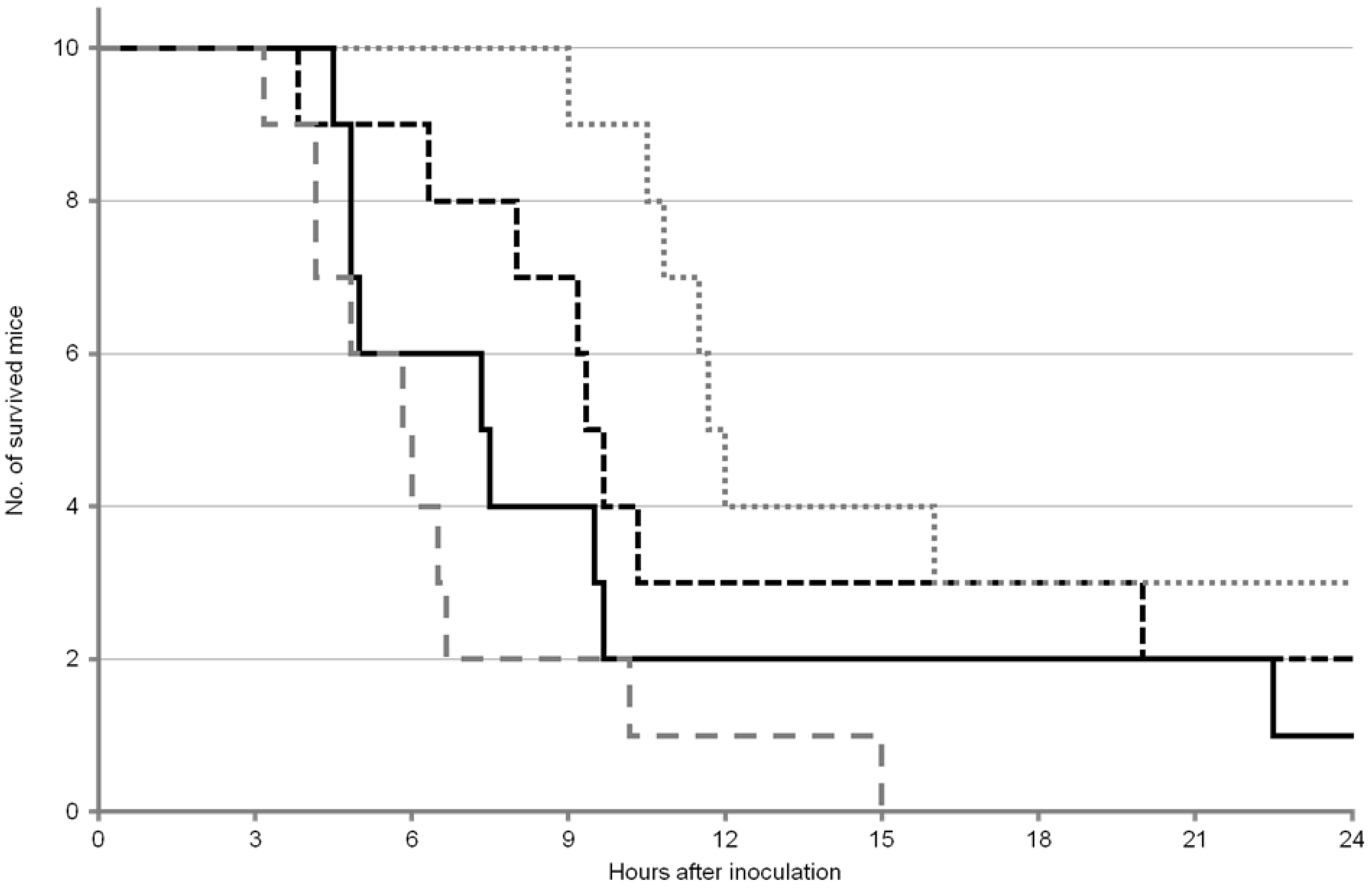
2.2. Survival Analysis of the Mice after OA Inoculation
2.3. Comparison of the Results of the First and Second Experiments
| Strain | lethality | survived | dead | median survival time (h) |
|---|---|---|---|---|
| ICR male | 90% | 1 | 9 | 8.75 |
| ICR female | 90% | 1 | 9 | 8.17 |
| ddY male | 80% | 2 | 8 | 6.17 |
| ddY female | 80% | 2 | 8 | 6.33 |
| Strain | lethality | survived | dead | median survival time (h) |
|---|---|---|---|---|
| ICR male | 80% | 2 | 8 | 9.50 |
| ICR female | 70% | 3 | 7 | 11.83 |
| ddY male | 90% | 1 | 9 | 7.42 |
| ddY female | 100% | 0 | 10 | 5.92 |
| Strain | Strain | Log-Rank test | Gehan-Wilcoxon test | |
|---|---|---|---|---|
| among all strains | NS | NS | ||
| ICR male | vs | ICR female | NS | NS |
| ddY male | vs | ddY female | NS | NS |
| ICR male | vs | ddY male | NS | NS |
| ICR female | vs | ddY female | NS | NS |
| Strain | Strain | Log-Rank test | Gehan-Wilcoxon test | |
|---|---|---|---|---|
| among all strains | *** | *** | ||
| ICR male | vs | ICR female | NS | NS |
| ddY male | vs | ddY female | NS | NS |
| ICR male | vs | ddY male | NS | NS |
| ICR female | vs | ddY female | *** | *** |
| Strain | Log-Rank test | Gehan-Wilcoxon test |
|---|---|---|
| ICR male | NS | NS |
| ddY male | NS | NS |
| ICR female | * | ** |
| ddY female | NS | NS |
 ; ICR female:
; ICR female:  ; ddY male:
; ddY male:  ; ddY female:
; ddY female:  .
.
 ; ICR female:
; ICR female:  ; ddY male:
; ddY male:  ; ddY female:
; ddY female:  .
.
 ; ICR female:
; ICR female:  ; ddY male:
; ddY male:  ; ddY female:
; ddY female:  .
.
 ; ICR female:
; ICR female:  ; ddY male:
; ddY male:  ; ddY female:
; ddY female:  .
.
3. Experimental Section
3.1. Animals
3.2. Chemicals
3.3. Experiment Design
3.4. Statistics
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Yasumoto, T.; Murata, M.; Oshima, Y.; Matsumoto, G.K. Diarrhetic shellfish poisoning. In Seafood Toxins; Ragelis, E.P., Ed.; American Chemical Society: Washington, D.C., USA, 1984; pp. 207–214. [Google Scholar]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bul. Jpn. Soc. Sci. Fish. 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Yasumoto, T. Method for the bioassay of diarrhetic shellfish toxin (in Japanese). Food Sanit. Res. 1981, 31, 515–522. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Diarrhoeic Shellfish Poisoning (DSP). Marine Biotoxins; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 53–95, chapter 3. Available online: http://www.fao.org/docrep/007/y5486e/y5486e00.htm (accessed on 24 June 2011).
- EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Toxins by Mouse Bioassay, version 5; Community Reference Laboratory for Marine Biotoxins (CRLMB): Vigo, Spain, 2009. Available online: http://www.aesan.msps.es/CRLMB/docs/docs/metodos_analiticos_de_desarrollo/EU-Harmonised-SOP-MBA-Lipophilic-Version5-June2009.pdf (accessed on 24 June 2011).
- James, K.J.; Bishop, A.G.; Carmody, E.P.; Kelly, S.S. Detection Methods for Okadaic Acid and Analogues. In Seafood and Freshwater Toxins; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 217–238, chapter 11. [Google Scholar]
- Tubaro, A.; Sosa, S.; Bornancin, A.; Hungerford, J. Pharmacology and Toxicology of Diarrheic Shellfish Toxins. In Seafood and Freshwater Toxins, 2nd; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 229–253, chapter 11. [Google Scholar]
- Tanaka, S.; Matsuzawa, A. The history of the “deutsche maus”, the origin of the dd mouse group (in Japanese). Jikken Dobutsu 1990, 39, 141–153. [Google Scholar]
- Suzuki, H. Susceptibility of different mice strains to okadaic acid, a diarrhetic shellfish poisoning toxin. Food Addit. Contam. Part A 2012, 29, 1307–1310. [Google Scholar] [CrossRef]
- Fernández, M.L.; Richard, D.J.A.; Cembella, A.D. In vivo assays for phycotoxins. In Manual in Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO Publishing: Paris, France, 2004; pp. 347–380. [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria. R: A language and environment for statistical computing. 2011. Available online: http://www.R-project.org/ (accessed on 24 June 2011).
- AOAC Official Method 959.08, Paralytic Shellfish Poison, Biological Method, First Action 1959, Final Action; Chapter 49. In Official Methods of Analysis of AOAC INTERNATIONAL, 18th; AOAC INTERNATIONAL (Ed.) AOAC INTERNATIONAL: Gaithersburg, MD, USA, 2005; pp. 79–81.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Suzuki, H. Differences in Susceptibility to Okadaic Acid, a Diarrhetic Shellfish Poisoning Toxin, between Male and Female Mice. Toxins 2013, 5, 9-15. https://doi.org/10.3390/toxins5010009
Suzuki H. Differences in Susceptibility to Okadaic Acid, a Diarrhetic Shellfish Poisoning Toxin, between Male and Female Mice. Toxins. 2013; 5(1):9-15. https://doi.org/10.3390/toxins5010009
Chicago/Turabian StyleSuzuki, Hodaka. 2013. "Differences in Susceptibility to Okadaic Acid, a Diarrhetic Shellfish Poisoning Toxin, between Male and Female Mice" Toxins 5, no. 1: 9-15. https://doi.org/10.3390/toxins5010009




