Giant liposomes were prepared from mixtures of phospholipids containing electrically neutral phosphatidylcholine (PC) and various molar ratios of acidic phosphatidylglycerol (PG) [
36]. The lipid compositions of the prepared liposomes were: PC alone (termed PC liposomes), molar ratios between PC and PG of 9:1, 7:3, 1:1 and 3:7 (termed 10%, 30%, 50% and 70% PG liposomes, respectively), and PG alone (termed 100% PG liposomes). The PG used in this study is commonly found in the membranes of bacteria but not in those of eukaryotic cells. However, we used it as a representative of negatively charged phospholipids, e.g., phosphatidylserine and inositol phospholipids, because PG has been frequently used in experiments as a representative of acidic phospholipids, and the preparation of giant liposomes is efficiently improved if their lipid composition includes PG.
Generally, to analyze the effects of proteins or peptides on liposomal behaviors, the concentrations of protein or peptide, rather than the liposome (
i.e., lipid concentration), is varied. However, a number of previous studies have reported that melittin changes its activity, structure (between disordered structure and α-helix) and association state (between monomer and tetramer), depending on its concentration [
23,
24,
25]. However, the aim of this study was to investigate the mechanism of changes in the structure and activity of melittin resulting from interactions with lipid bilayer membranes. Thus, to vary the molecular ratio between the peptide and lipid, we altered the liposome concentration but maintained a constant melittin concentration, unless otherwise noted.
2.1. Melittin-Induced Liposome Transformation
To observe the alteration process from the start, giant liposomes were observed in a concentration gradient of melittin. By observing liposomes in a concentration gradient of melittin, we can observe their behavior during the transformation, and can obtain the same efficacy as if the observations were simultaneously performed at continuously changed melittin concentrations. This method enables us to find novel liposome deformations including transient responses, even if the optimal conditions for observing these are unknown and the deformations occur only under a narrow range of conditions. Finding phased shrinkage and the formation of condensed liposomes is one of the outcomes of this study.
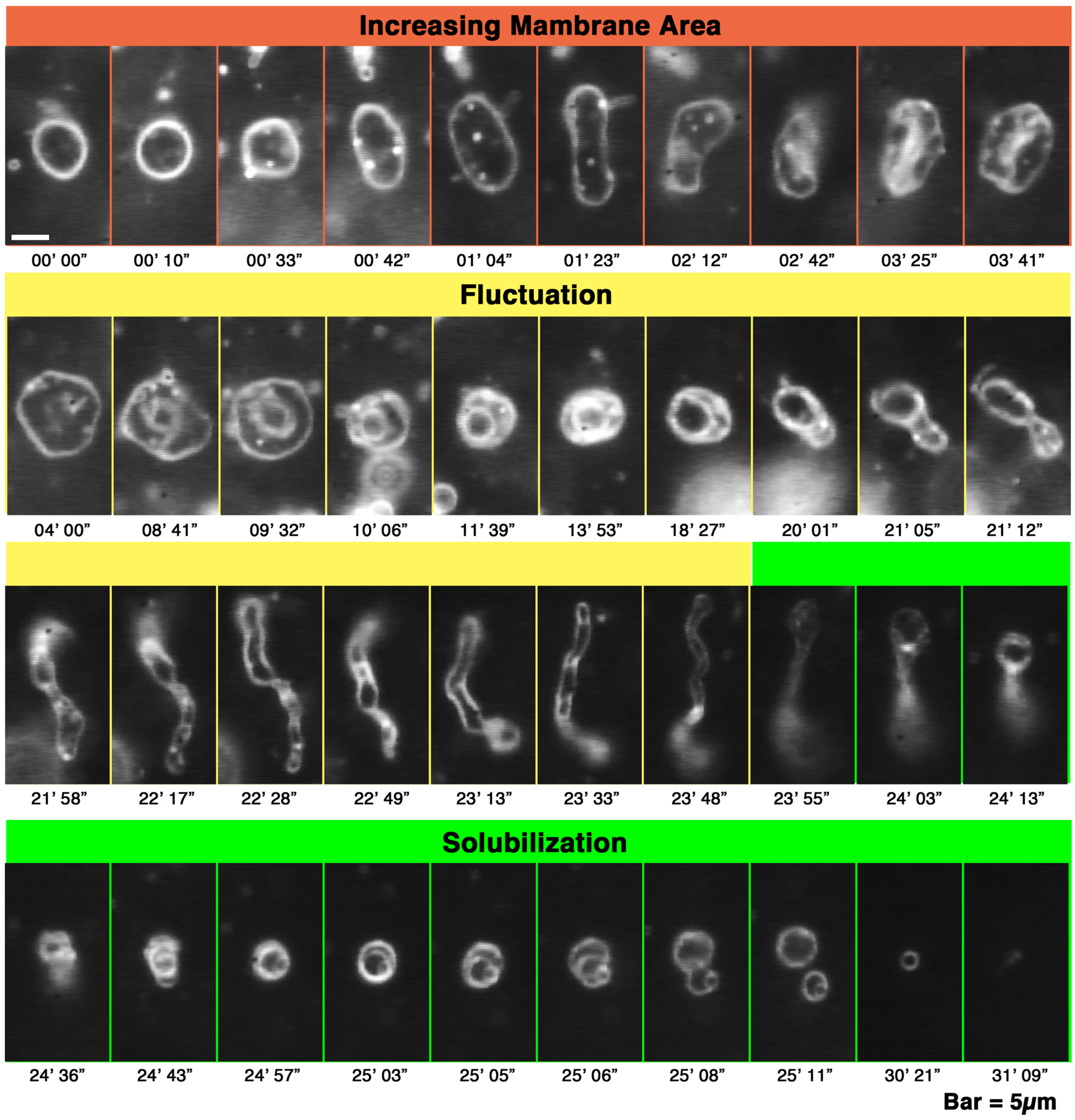
Figure 1.
Deformation processes, increasing membrane area, fluctuation and solubilization of giant liposomes observed in a melittin concentration gradient. Time-lapse images of the deformation of PC liposomes perfused with melittin (final concentration 150 μM). The molecular ratio between melittin and the liposomes (P/L ratio), which is obtained using the ratio between the final concentrations of the peptide and lipids, is 1/4.7. The time after the start of observation is denoted in minutes and seconds under each dark-field image. The bar represents 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
Figure 1.
Deformation processes, increasing membrane area, fluctuation and solubilization of giant liposomes observed in a melittin concentration gradient. Time-lapse images of the deformation of PC liposomes perfused with melittin (final concentration 150 μM). The molecular ratio between melittin and the liposomes (P/L ratio), which is obtained using the ratio between the final concentrations of the peptide and lipids, is 1/4.7. The time after the start of observation is denoted in minutes and seconds under each dark-field image. The bar represents 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
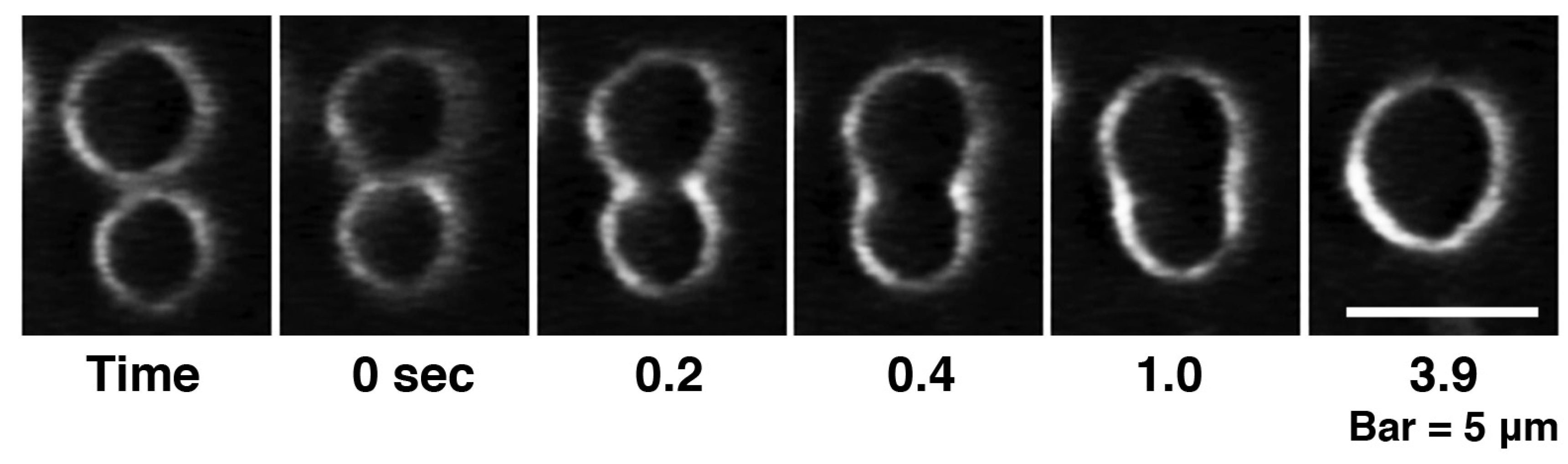
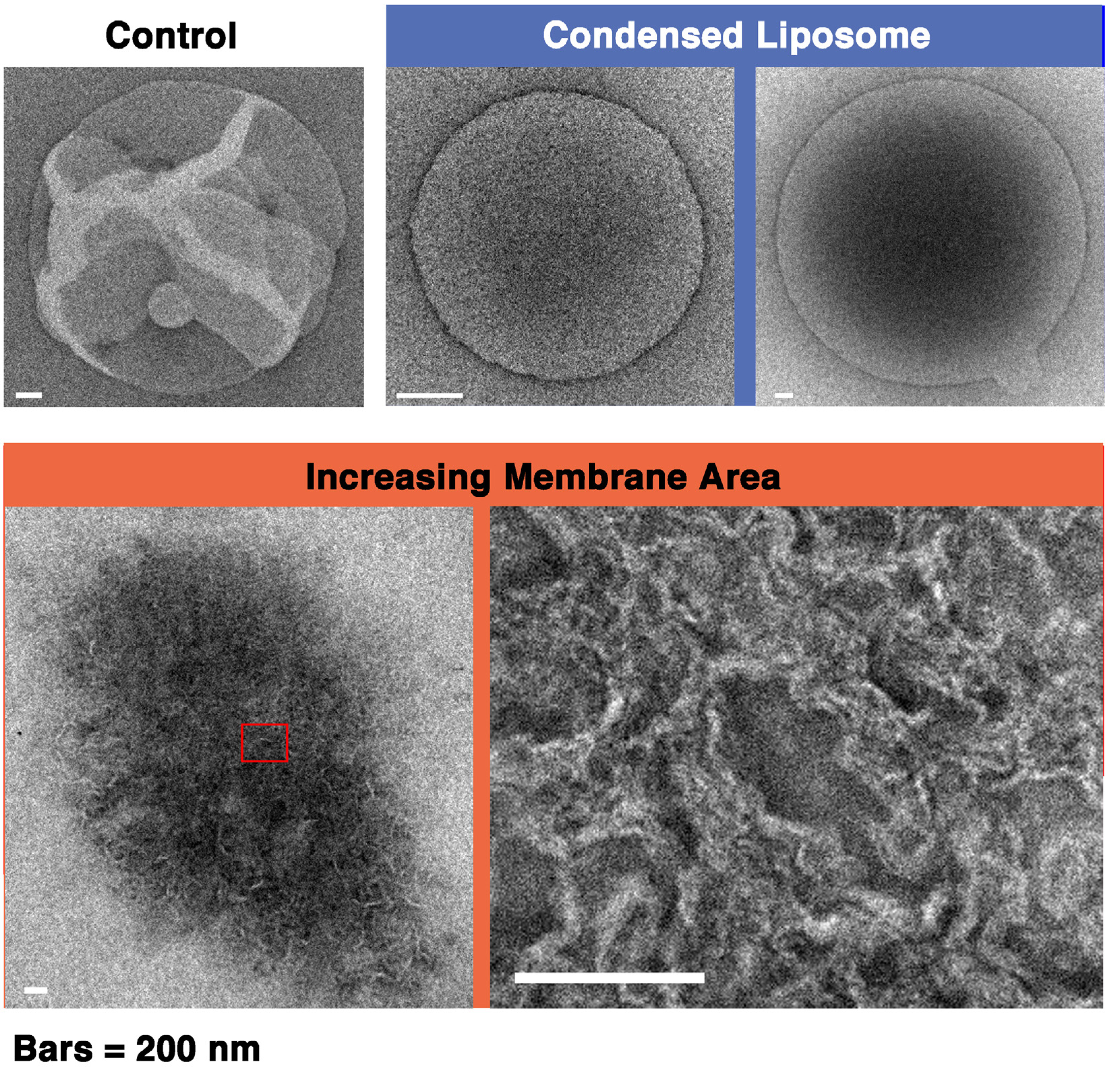
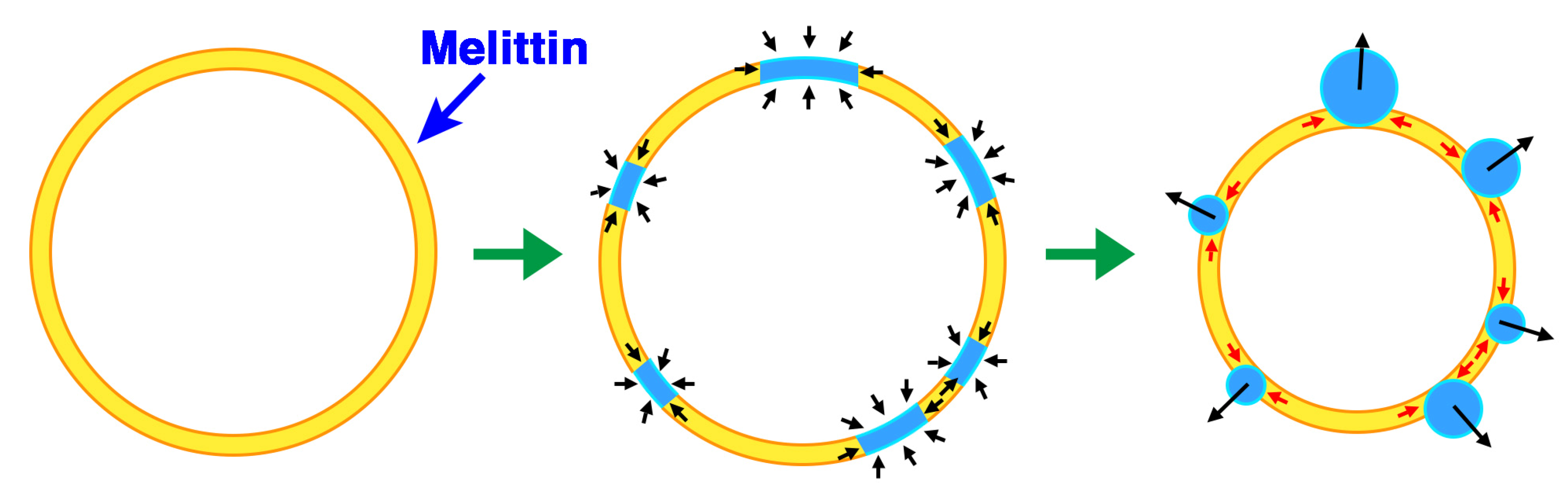
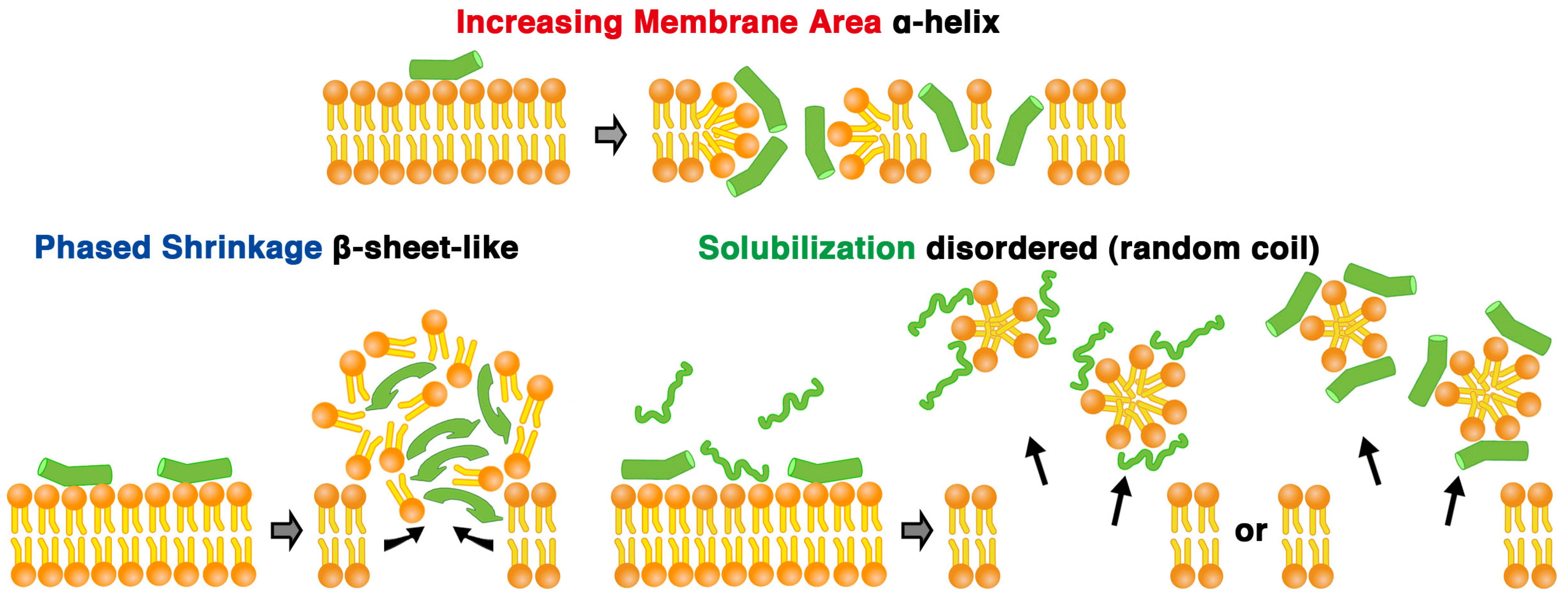
First, the liposome changes induced by melittin were classified. Depending on the experimental conditions, we observed an “increasing membrane area”, “phased shrinkage” or “solubilization” of liposomes. Increasing membrane area is attributable to the intrusion of peptides into the membrane. By increasing membrane area, liposomes unequivocally increased their membrane surface area, but not their internal volume. This imbalance between surface area and volume may decrease tension, thereby resulting in vigorous fluctuations of the membrane. As a result, a spherical liposome changes into a large flabby one (
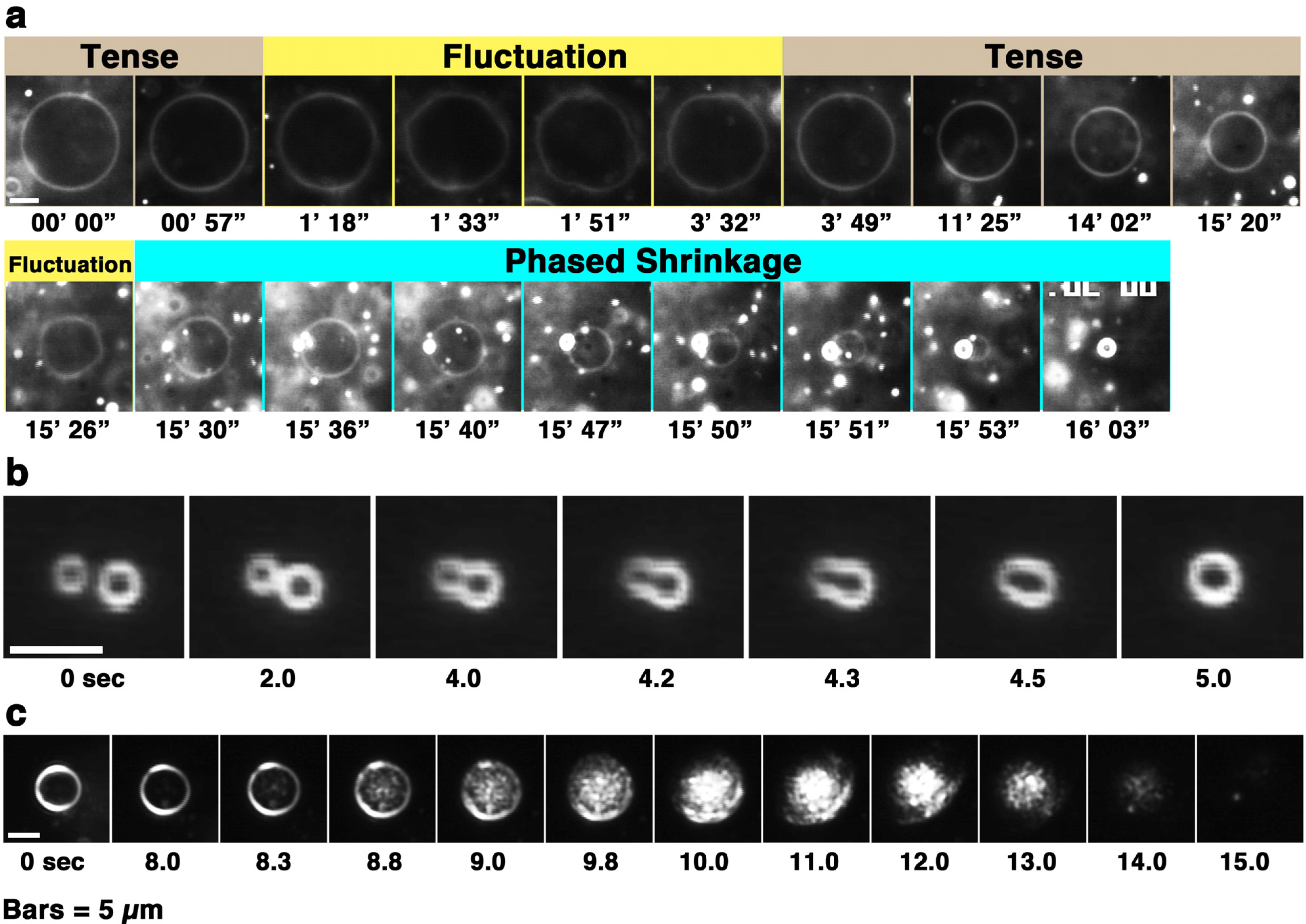
Figure 1, up to approximately 24 min). The phased shrinkage of liposomes, which we consider a newly found activity of melittin, involves the formation of small bright particles on the liposome surface followed by the rapid shrinking of the liposome (
Figure 2a). Usually, before phased shrinkage, liposomes alternate between the fluctuating state and the tense state. During phased shrinkage, because the number of bright particles increased, thereby resulting in an increased light intensity scattered from the liposome, the liposome finally changed into a small bright liposome, which is termed a “condensed liposome”. The bright particles and condensed liposomes are significantly different from usual membrane vesicles in brightness. Because the intensity of light scattered from liposomes and other particles depends on their density of mass [
37], their appearance is consistent with the result of ultrastructural analysis showing that condensed liposomes are densely packed lipid aggregates, as will be mentioned later (
Section 2.6.3). By diffusion, a condensed liposome occasionally reached a region in the specimen where the melittin concentration was much higher. In such cases, fusion between condensed liposomes (
Figure 2b) or disassembly of condensed liposomes (
Figure 2c) could be observed. Through solubilization, liposomes became faded and finally disappeared completely (
Figure 1, times after 24 min). Sometimes, solubilization was accompanied by shrinkage and/or segmentation of the liposome.
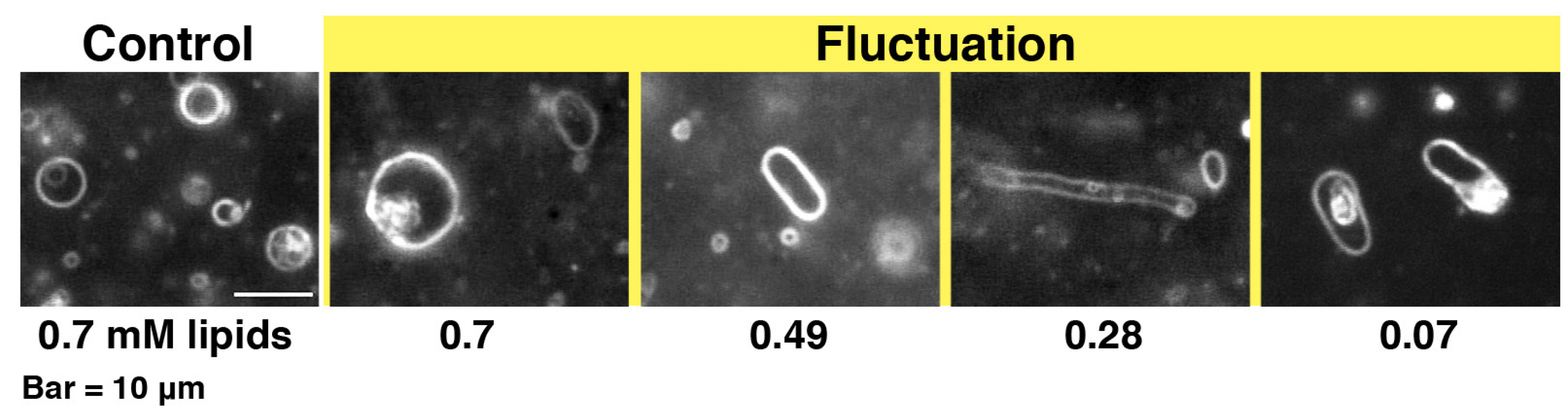
When melittin was added to PC liposomes or to 10% PG liposomes, membrane fluctuations or pearling transformations were usually observed (
Figure 3), such that no drastic deformations accompanying a topological change were found. However, if higher concentrations of melittin (final 0.15–0.30 mM) were perfused, increasing membrane area progressed slowly, and, subsequently, the liposome exhibited solubilization (
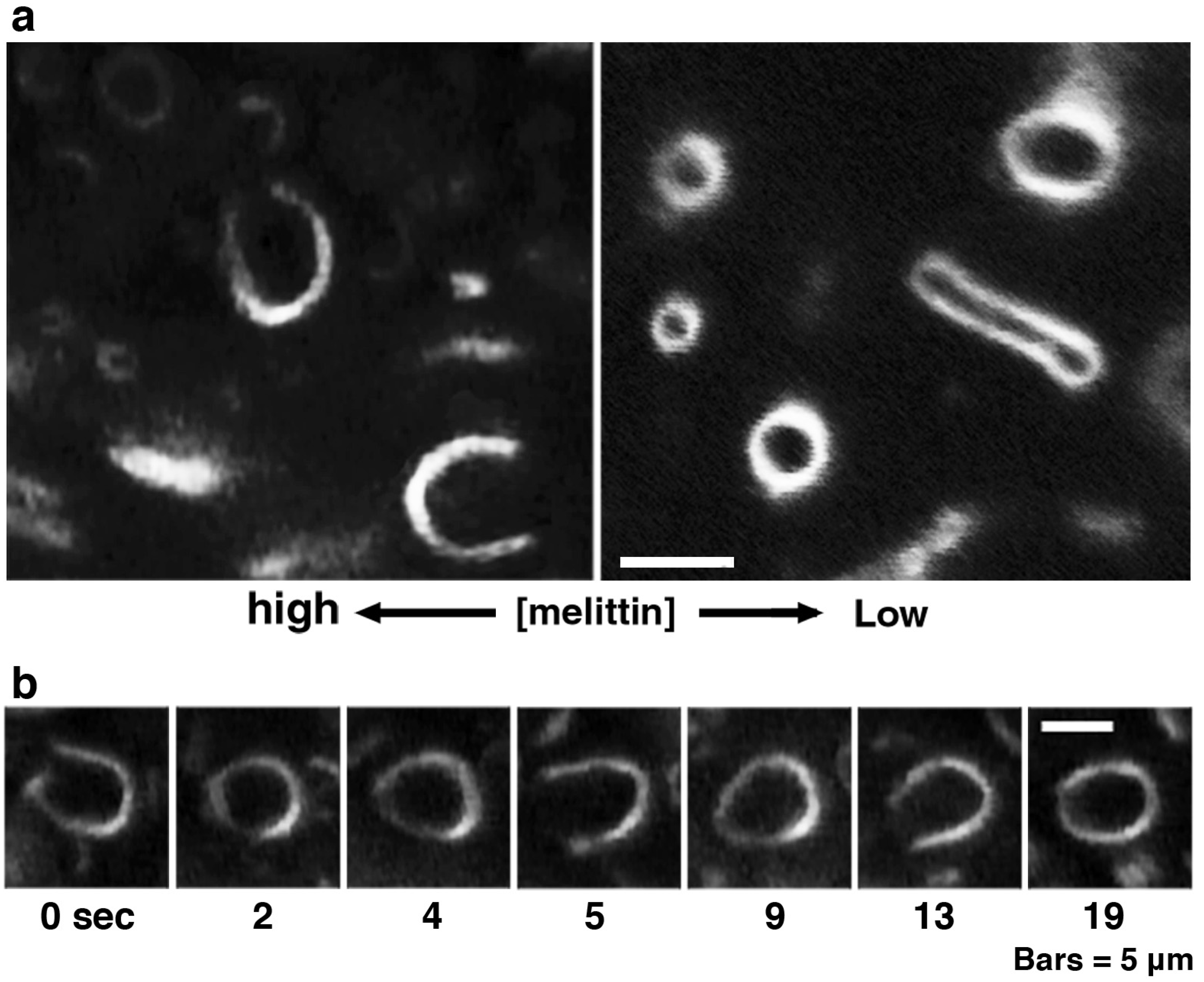
Figure 1). In addition, some PC liposomes opened a large pore (
Figure 4) or fused with other liposomes (
Figure 5) in the presence of such higher concentrations of melittin. The same formation of large pores in liposomes can be caused by proteins belonging to the band 4.1 superfamily, which have the FERM domain at their
N-terminal. It has been reported that the FERM domain has an amphiphilic region that is similar to melittin [
38,
39]. These proteins are localized at the verge of the pore [
40,
41], suggesting that melittin also localizes at the verge of membrane-opening sites. Pores opened with melittin were very unstable, and the pore size and the entire shape of the pore-opened liposome fluctuated vigorously. Most likely, melittin molecules that are participating in pore formation lined up together only loosely at the pore verge, suggesting that the toroidal model, rather than the cylindrical model, better represents the mechanism of melittin-induced membrane pore formation because the latter model supposes tight cylindrical packing among the membrane-bound peptides [
26,
27]. In addition, the barrel stave pore model can also be excluded because the bilayer remains intact and the liposome structure is not perturbed in the barrel stave model [
42]. Other studies, which investigated the membrane-interacting mechanism of melittin or of other pore-forming peptides, such as antimicrobials, also support the toroidal model [
43,
44]. The fusion between PC liposomes mentioned here is distinguishable from that between condensed liposomes because there is no preceding reaction,
i.e., shrinkage or increasing brightness.
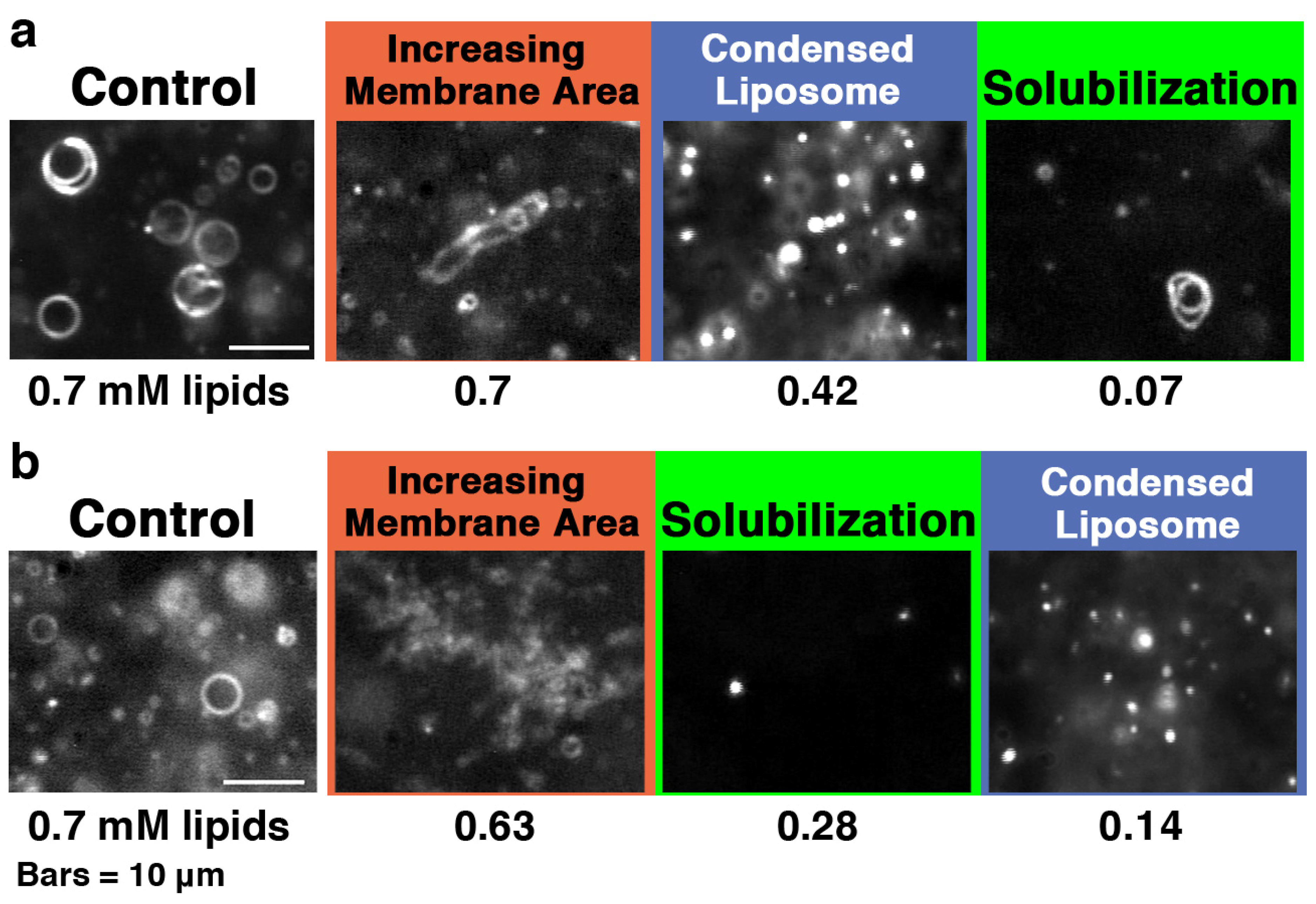
When the concentration of PG in the liposome membrane was in the range from 30% to 100%, we could observe various liposome deformations caused by the addition of melittin. When melittin was added to 30% and 50% PG liposomes with increasing molecular ratios of peptide to lipid, the behavior mainly observed or liposome deformation changed in the order of increasing membrane area, condensed liposome formation and solubilization (
Figure 6a). For 70% and 100% PG liposomes with increasing ratios of peptide to lipid, increasing membrane area, solubilization and condensed liposome formation were mainly observed, in that order (
Figure 6b). In each experimental condition, the upper limit of the molecular ratio of total lipids to melittin required to observe the formation of condensed liposomes decreased with increasing PG concentration in the liposome membrane.
Figure 2.
(a) Phased shrinkage of giant liposomes observed in a melittin concentration gradient (see also supplementary film). The condensed liposome is the product of phased shrinkage deformation; (b) and (c) represent fusions between condensed liposomes and disassembly of a condensed liposome, respectively. Time-lapse images of 50% PG liposomes perfused with melittin (final concentration: 60 μM (the P/L ratio is 1/12)). The video camera sensitivity was decreased arbitrarily according to the increase in brightness of the liposome. The time after the start of observation is denoted in minutes and seconds (a) or as seconds (b and c) under each dark-field image. The bars represent 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
Figure 2.
(a) Phased shrinkage of giant liposomes observed in a melittin concentration gradient (see also supplementary film). The condensed liposome is the product of phased shrinkage deformation; (b) and (c) represent fusions between condensed liposomes and disassembly of a condensed liposome, respectively. Time-lapse images of 50% PG liposomes perfused with melittin (final concentration: 60 μM (the P/L ratio is 1/12)). The video camera sensitivity was decreased arbitrarily according to the increase in brightness of the liposome. The time after the start of observation is denoted in minutes and seconds (a) or as seconds (b and c) under each dark-field image. The bars represent 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
Figure 3.
Dark-field images of 10% PG liposomes in the presence of melittin. The samples were prepared in the same way as those used for CD measurements. The final lipid concentration is denoted in mM under each dark-field image. The final melittin concentration was 60 μM (P/L = 1/12 to 1/1.2). The bar represents 10 μm.
Figure 3.
Dark-field images of 10% PG liposomes in the presence of melittin. The samples were prepared in the same way as those used for CD measurements. The final lipid concentration is denoted in mM under each dark-field image. The final melittin concentration was 60 μM (P/L = 1/12 to 1/1.2). The bar represents 10 μm.
Figure 4.
Large pore formation of giant liposomes observed in a melittin concentration gradient. PC liposomes were perfused with 1 mM melittin (P/L = 1/1). (a) The regions in a microscopic specimen where the melittin concentrations are low (right) and high (left) are shown, respectively; (b) Time-lapse images of a liposome in which a large pore has opened are shown. The time after the start of observation is denoted in seconds under each dark-field image. The cup-like shape of the liposome was unstable, and repeated opening and closing of the pore was observed. The bars represent 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
Figure 4.
Large pore formation of giant liposomes observed in a melittin concentration gradient. PC liposomes were perfused with 1 mM melittin (P/L = 1/1). (a) The regions in a microscopic specimen where the melittin concentrations are low (right) and high (left) are shown, respectively; (b) Time-lapse images of a liposome in which a large pore has opened are shown. The time after the start of observation is denoted in seconds under each dark-field image. The cup-like shape of the liposome was unstable, and repeated opening and closing of the pore was observed. The bars represent 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
Figure 5.
Fusion between PC liposomes observed in the presence of high concentrations of melittin. PC liposomes were perfused with 1 mM melittin (P/L = 1/1). The time after the start of fusion is denoted in seconds under each dark-field image. The bar represents 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
Figure 5.
Fusion between PC liposomes observed in the presence of high concentrations of melittin. PC liposomes were perfused with 1 mM melittin (P/L = 1/1). The time after the start of fusion is denoted in seconds under each dark-field image. The bar represents 5 μm. In figures showing the liposomal behaviors observed in a concentration gradient of melittin, the left side of each photograph is the direction in which the melittin concentration is higher.
Figure 6.
Dark-field images of (a) 30 and (b) 100% PG liposomes in the presence of melittin. The samples were prepared in the same way as those used for CD measurements. The final lipid concentration is denoted in mM under each dark-field image. The final melittin concentration was 60 μM (P/L = 1/12 to 1/1.2). The bars represent 10 μm. The video camera sensitivity was decreased arbitrarily to observe condensed liposomes.
Figure 6.
Dark-field images of (a) 30 and (b) 100% PG liposomes in the presence of melittin. The samples were prepared in the same way as those used for CD measurements. The final lipid concentration is denoted in mM under each dark-field image. The final melittin concentration was 60 μM (P/L = 1/12 to 1/1.2). The bars represent 10 μm. The video camera sensitivity was decreased arbitrarily to observe condensed liposomes.
2.2. Secondary Structure of Melittin Predicted by CD Spectrometry
CD spectrometry, as well as cosedimentation, fluorescence quenching and EM observations, are not useful to study solutions involving concentration gradients. Therefore, in the following experiments, mixtures of liposomes and melittin solutions were examined at 30 min after mixing in test tubes. The solutions were observed by dark-field microscopy after the CD measurements, and we confirmed that the liposome morphologies observed were indistinguishable from those of liposomes that were deformed in a melittin concentration gradient. Additionally, solutions sampled after the fluorescence quenching experiment, aliquots of solutions prepared for the cosedimentation assay and solutions prepared before the EM observation were used to confirm liposome deformations in the same way. We note that condensed liposomes tend to aggregate on the surface of a substrate. However, no adhesion of liposomes to the surface of the cylindrical quartz cell used to measure CD was noted by direct observation using dark-field microscopy.
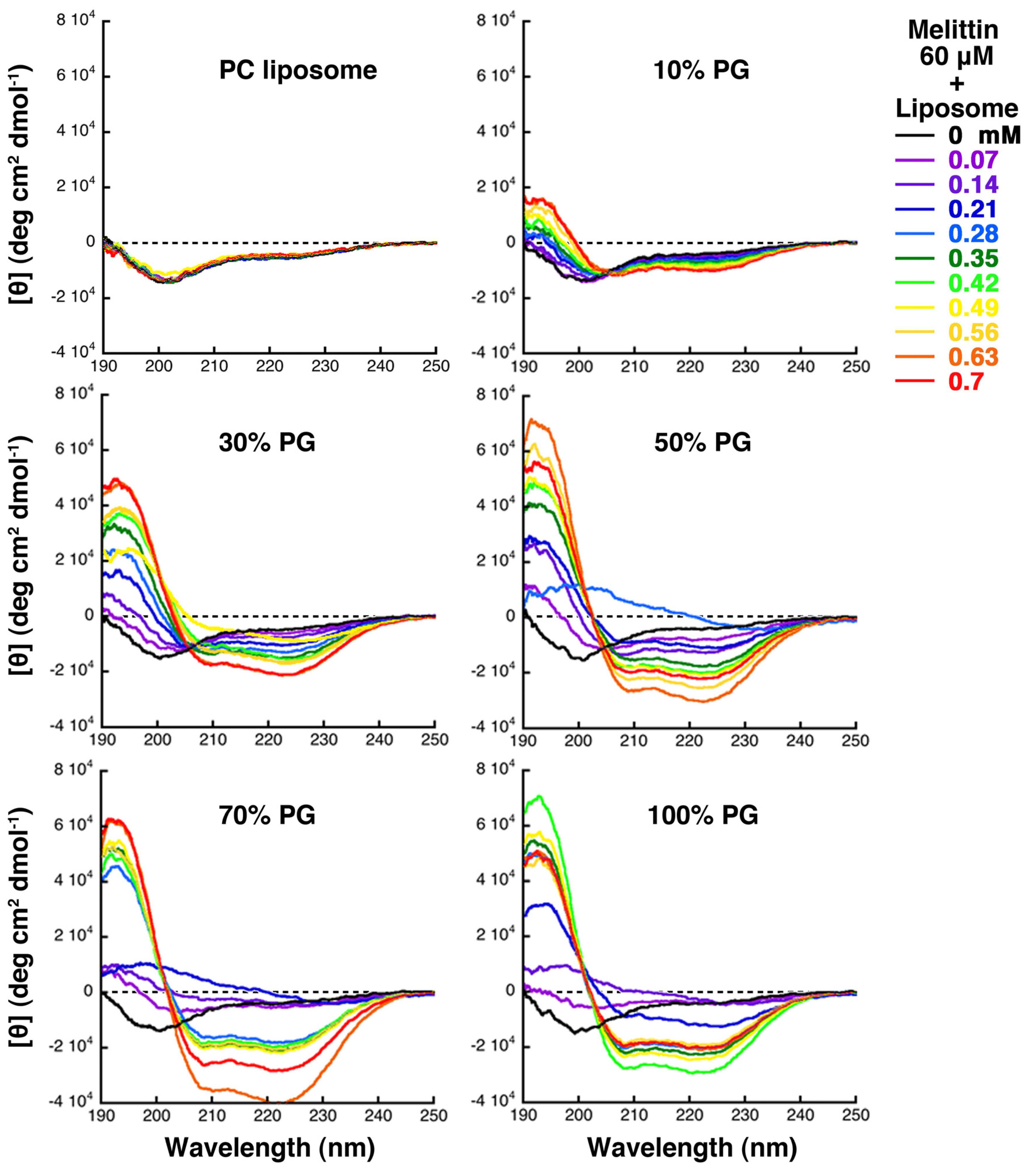
The CD determinations showed that melittin was a mixture of peptides taking various secondary structures, such as a disordered structure and an α-helix, or a disordered structure and an another structure, which exhibited a β-structure-like CD spectrum possessing a positive band at approximately 200 nm in the difference spectrum (spectrum of melittin in the presence of each concentration of the liposomes minus that of melittin alone). However, the majority of melittin exhibited an α-helix structure, a β-structure-like CD spectrum, and a disordered structure, when assessed by CD under conditions where increasing membrane area, condensed liposome formation, and solubilization, respectively, were mainly observed (
Figure 7).
When melittin was added to 10% PG liposomes, the CD spectra showed an isometric point near 205 nm, indicating a two-state transition between a disordered structure and an α-helix, and increasing helicity with increasing lipid concentration. When melittin was added to 30% PG liposomes, even though the isometric point was present, the CD spectra in the presence of 0.42, 0.49, and 0.56 mM concentrations of lipids deviated from that point, suggesting the presence of other states. When the content of PG was greater than 50%, the deviation of the spectra from the isometric point became more prominent, and the CD spectra showed that melittin forming the structure exhibiting the β-structure-like CD spectrum increased transiently. The same results were obtained even when DOPC and DOPG were used in the preparation of the liposomes rather than PC and PG, as will be discussed in the next section (
Figure 8).
The formation of condensed liposomes was observed in parallel to the emergence of the melittin fraction exhibiting the β-structure-like CD spectra. CD spectra obtained under conditions where condensed liposomes are formed indicate that melittin forms the secondary structure showing the β-structure-like CD spectrum possessing a positive band approximately 200 nm in the difference spectrum as described above. It has previously been reported that melittin forms β-structure and shows very similar CD spectral changes while interacting with heparan sulfate or heparin, which is a negatively charged polysaccharide found on the surface of cells [
45]. In addition, similar CD spectral changes have been observed when amyloid β peptide interacts with PG-containing membranes [
46]. Taken together, we describe this alternative structure as a β-like structure hereafter for convenience. Both α-helix and such β-like structures might be commonly seen when amphipathic peptides, such as melittin, interact with lipid bilayer membranes. We note that such a β-like structure has not been reported previously for the membrane-interacting melittin. The very high P/L ratios used here may have affected the finding of the β-like structure in this study. In any case, this finding shows that the direct real-time imaging of liposome behavior is very useful for studying interactions between membranes and peptides.
Figure 7.
CD spectra of melittin (final concentration 60 μM) and giant liposome mixtures. The liposomes examined (PC, 10% PG, 30% PG, 50% PG, 70% PG or 100% PG liposomes) are indicated at the top of each panel. The final lipid concentration for each measurement is indicated by the color of line, as denoted on the right (P/L = 1/12 to 1/1.2).
Figure 7.
CD spectra of melittin (final concentration 60 μM) and giant liposome mixtures. The liposomes examined (PC, 10% PG, 30% PG, 50% PG, 70% PG or 100% PG liposomes) are indicated at the top of each panel. The final lipid concentration for each measurement is indicated by the color of line, as denoted on the right (P/L = 1/12 to 1/1.2).
In this study, we determined the secondary structure of melittin using CD. However, the higher order structure and dynamic behavior of the peptide were not observed. In particular, under conditions where the secondary structure of melittin is predicted to adopt β-like structure (
i.e., phased shrinkage), the study remains insufficient. By varying the experimental conditions more precisely or using NMR and/or calorimetry, the effect of neutralizing the charges on membrane deformation will be more defined [
20,
47,
48,
49]. Notably, large or small unilamellar vesicles should be used rather than giant liposomes when using NMR or calorimetry. Experiments using labeled melittin may also be useful if the natural activities and features of melittin are also maintained after labeling [
50].
Figure 8.
(
a) CD spectra obtained from mixtures of melittin (final concentration 60 μM) and 50% PG liposomes (left) or liposomes prepared from DOPC and DOPG (right). The lipid concentrations are indicated by the color of the lines, as denoted on the right; (
b) Liposomes prepared from DOPC and DOPG in the presence of melittin; the conditions used are similar to those described in
Figure 6. The bar represents 10 μm. The video camera sensitivity was decreased arbitrarily to observe condensed liposomes.
Figure 8.
(
a) CD spectra obtained from mixtures of melittin (final concentration 60 μM) and 50% PG liposomes (left) or liposomes prepared from DOPC and DOPG (right). The lipid concentrations are indicated by the color of the lines, as denoted on the right; (
b) Liposomes prepared from DOPC and DOPG in the presence of melittin; the conditions used are similar to those described in
Figure 6. The bar represents 10 μm. The video camera sensitivity was decreased arbitrarily to observe condensed liposomes.
In general, salt is important for maintaining the structure and activity of proteins and peptides. Therefore, studies performed under conditions that are nearer to physiological salt concentrations are essential. In addition, lipid membrane morphology could be affected by environmental factors such as temperature, osmolarity and pH as well as salt strength [
19,
21,
51,
52]; thus, a considerably wider range of conditions should be examined to understand melittin activities more fully.
2.4. Membrane Affinity of Melittin
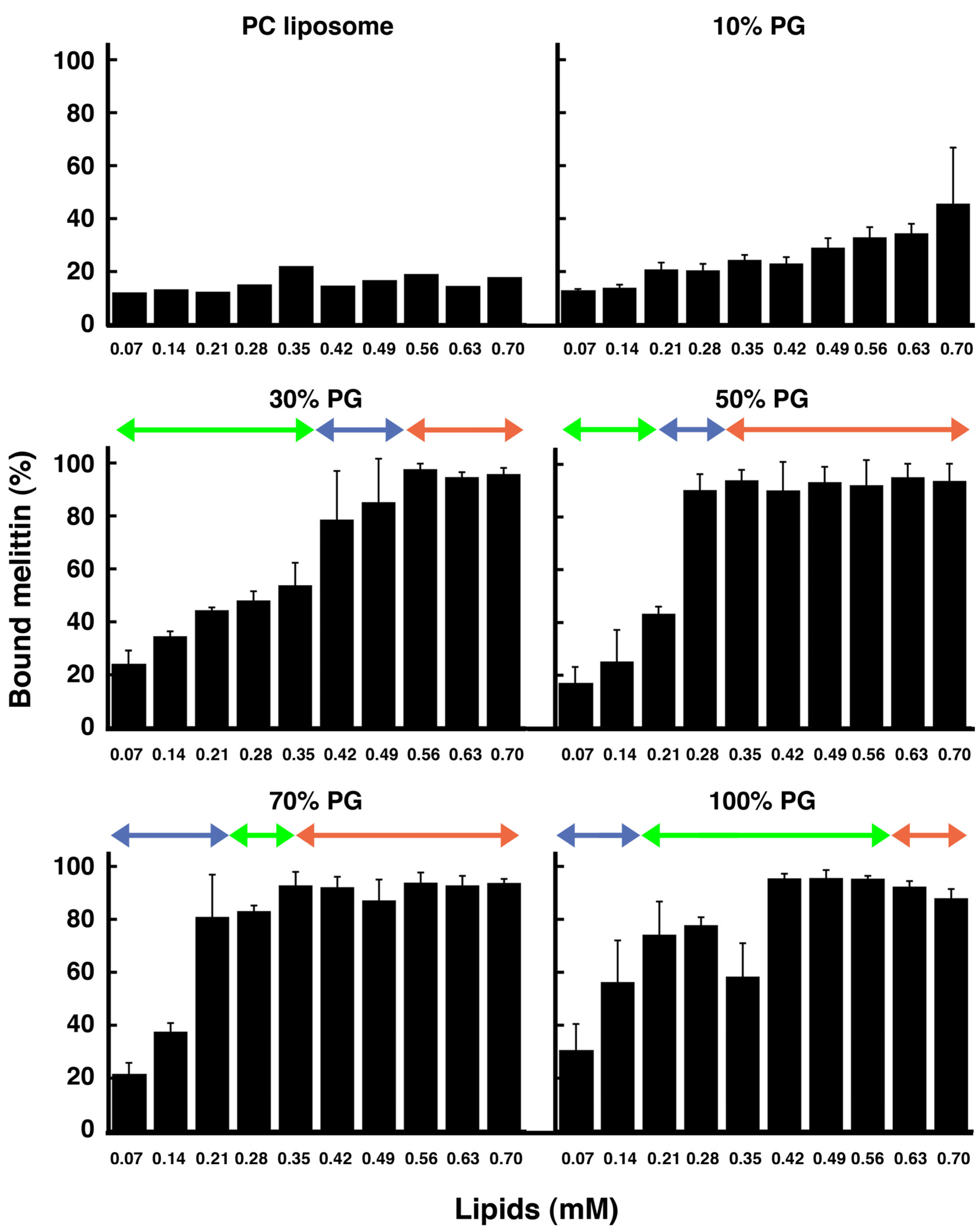
In each condition, the amount of membrane-bound melittin was determined by cosedimentation with liposomes (
Figure 9). For PC liposomes, even though melittin bound to the membrane as reported previously [
44,
49], the membrane-bound fraction was approximately only 10%–20%. For 10% PG liposomes, the membrane-bound fraction slightly increased; however, the amount remained at a lower level than for the 30%, 50%, 70%, and 100% PG liposomes. Conversely, melittin bound substantially to 30%, 50%, 70%, and 100% PG liposomes (approximately 20% of melittin bound to the liposomes even when the concentration of lipids was low, and almost 100% of melittin bound to the liposomes when the concentration of lipids was high). The 30%, 50%, 70%, and 100% PG liposomes could be transformed to condensed liposomes. These membrane-bound melittin fractions, which were determined by the cosedimentation assay, are consistent with the results indicated by CD (
Figure 7) and the fluorescence quenching of the tryptophan residue (
Figure 10, next section). Our results indicate that the affinity of melittin to membranes containing only neutral lipids is very weak and that negatively charged lipids can significantly increase the membrane binding of melittin, as reported previously [
53].
For the 30% and 50% PG liposomes, when the lipid concentration was lower than that required to induce phased shrinkage (i.e., the transformation of liposomes to condensed liposomes), melittin was abundant in the supernatant, indicating that membrane binding of the peptide was saturated under these conditions. Under conditions where increasing membrane area or condensed liposome formation was observed, almost all melittin was bound to liposomes. Therefore, the secondary structure expected by CD under these conditions should be that of the membrane-bound melittin.
For the 70% and 100% PG liposomes, condensed liposome formation could be observed at lower lipid concentrations than for solubilization. Under these conditions, the majority of the peptide was found in the supernatant, although condensed liposomes were formed. This discrepancy between the results of the cosedimentation assay and those of the other methods (the results of real-time observations and CD as described above and the results of the fluorescence-quenching assay as described later) may be because the liposome membranes were collapsed by that deformation, broken into fragments and, thus, no longer precipitated by centrifugation.
Figure 9.
Fraction (%) of liposome-bound melittin. The final melittin concentration was 60 μM. The liposomes examined (PC, 10% PG, 30% PG, 50% PG, 70% PG or 100% PG liposomes) are indicated at the top of each panel. The experimental conditions were the same as those used for the CD measurements. Green, blue and red arrows in each panel show the ranges of concentration of total lipids where solubilization, phased shrinkage and increasing membrane area were mainly observed, respectively. Error bars indicate standard deviations.
Figure 9.
Fraction (%) of liposome-bound melittin. The final melittin concentration was 60 μM. The liposomes examined (PC, 10% PG, 30% PG, 50% PG, 70% PG or 100% PG liposomes) are indicated at the top of each panel. The experimental conditions were the same as those used for the CD measurements. Green, blue and red arrows in each panel show the ranges of concentration of total lipids where solubilization, phased shrinkage and increasing membrane area were mainly observed, respectively. Error bars indicate standard deviations.
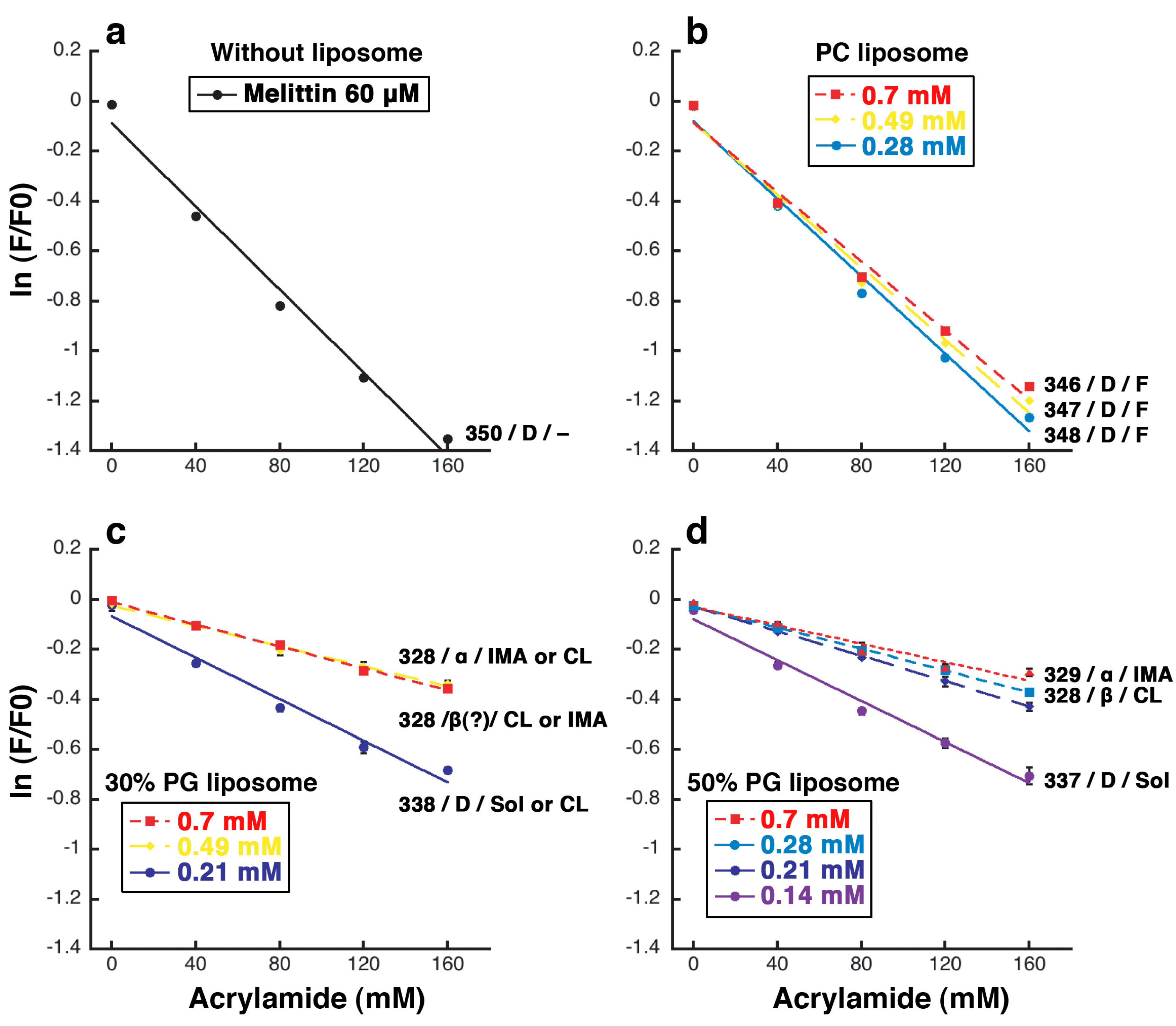
Figure 10.
Fluorescence quenching of tryptophan residue 19 of melittin by acrylamide. The results obtained with melittin alone (a) or mixed with PC (b), 30% PG (c), or 50% PG liposomes (d) are shown. The experimental conditions were the same as those used for the CD measurements. The final melittin concentration was 60 μM. The final lipid concentration is indicated by the color of line, as denoted in the box in each panel. The wavelength (nm) of the emission maximum, the estimated secondary structure of the majority of melittin and the typically observed liposome deformation are indicated at the side of each line. A disordered structure, an α-helix, and a β-like structure are denoted as “D”, “α”, and “β”, respectively. The fluctuation of liposome, increasing membrane area, condensed liposome formation, and solubilization are denoted as “F”, “IMA”, “CL”, and “Sol”, respectively.
Figure 10.
Fluorescence quenching of tryptophan residue 19 of melittin by acrylamide. The results obtained with melittin alone (a) or mixed with PC (b), 30% PG (c), or 50% PG liposomes (d) are shown. The experimental conditions were the same as those used for the CD measurements. The final melittin concentration was 60 μM. The final lipid concentration is indicated by the color of line, as denoted in the box in each panel. The wavelength (nm) of the emission maximum, the estimated secondary structure of the majority of melittin and the typically observed liposome deformation are indicated at the side of each line. A disordered structure, an α-helix, and a β-like structure are denoted as “D”, “α”, and “β”, respectively. The fluctuation of liposome, increasing membrane area, condensed liposome formation, and solubilization are denoted as “F”, “IMA”, “CL”, and “Sol”, respectively.
2.5. Fluorescence Quenching of the Tryptophan 19 Residue
Melittin has a tryptophan residue at position 19. If the indole ring of that residue exists in a hydrophilic environment,
i.e., if melittin does not penetrate the lipid bilayer membrane, then the fluorescence from the residue would be quenched by acrylamide in an aqueous solution. Conversely, if melittin penetrates the membrane, then the fluorescence would remain and be detectable because acrylamide cannot access the indole ring in a hydrophobic environment. Therefore, based on the fluorescence intensity of the tryptophan residue, we can estimate the position of melittin in the membrane under each condition [
54]. It should be noted that we have confirmed that acrylamide does not cause any changes in the morphology or topology of giant liposomes (data not shown).
When melittin is alone in solution, the fluorescence intensity from the tryptophan residue at position 19 was exponentially decreased depending on the concentration of acrylamide. The efficiency of quenching was 8.3 M
−1 (
Figure 10a), and the emission maximum was observed at approximately 350 nm. In the presence of PC liposomes, the quenching profile was similar to that of melittin alone, whereas the efficiency of quenching was slightly decreased to 6.9 M
−1 at 0.70 mM PC liposomes (the efficiency is 17% lower than for melittin alone,
Figure 10a,b), and the emission maximum was slightly changed. When the final lipid concentrations were 0.28, 0.49, and 0.70 mM, the emission maxima were approximately 348, 347, and 346 nm, respectively. Because acrylamide quenched the fluorescence intensity even in the presence of PC liposomes, the tryptophan residue was assumed to be in a hydrophilic environment, such as in solution or on the surface of the membrane. To reduce the acrylamide-induced fluorescence quenching, it appears that much higher concentrations of PC liposomes would be required. The cosedimentation assay indicated that the affinity between melittin and PC liposomes is weak and showed that approximately only 10%–20% of melittin is bound to the membrane (
Figure 9). These results indicate that most melittin does not bind to membranes prepared from neutral PC alone. However, from another point of view, the results indicate that a small fraction of melittin binds to and most likely penetrates into the membrane because the non-quenching fraction in the fluorescence-quenching assay and the membrane-bound fraction in the cosedimentation assay were very similar. This binding and penetration of melittin may be a reason for the fluctuation of PC liposomes in the presence of melittin (
Figure 1). In previous studies, the interaction between melittin and membranes made from PC only was reported [
2,
26,
27,
55]. In contrast, our study shows only weak binding between melittin and PC liposomes, and a high concentration of melittin is required to induce the solubilization of PC liposomes. It has been reported that melittin has a tendency to form aggregates on membranes [
26,
27]. Even if the membrane affinity of melittin is weak, upon binding to the membrane, melittin may develop the ability to form an aggregate on the membrane. As the amount of membrane-bound melittin exceeds a threshold, melittin immediately would form an aggregate involving the surrounding lipids and dissociate from the membrane, a process that results in the solubilization of the liposome.
When melittin was added to 30% PG liposomes (
Figure 10c), different results were obtained depending on the lipid concentration. When the final lipid concentration was 0.21 mM (the condition where solubilization or the formation of condensed liposomes can be observed), the fluorescence was obviously quenched and the emission maximum shifted to 338 nm. When the final lipid concentrations were 0.49 or 0.70 mM, conditions where the formation of condensed liposomes or increasing membrane area can be observed, only a little quenching was observed, and the emission maximum was shifted to 328 nm. When the final lipid concentration was 0.35 mM, the reproducibility of the measurements was very low, suggesting that this condition (a final lipid concentration of 0.35 mM) represents a transition between the above two conditions (lipid concentration ranges: ≤ 0.21 and ≥ 0.49 mM).
When melittin was added to 50% PG liposomes (
Figure 10d), the results obtained were dependent on the lipid concentration, similar to when 30% PG liposomes were used. When the final lipid concentration was 0.14 mM (the condition where solubilization can be observed) the fluorescence was obviously quenched. In contrast, when the final lipid concentrations were 0.28 or 0.70 mM, conditions where the formation of condensed liposomes and increasing membrane area can be observed, respectively, only a little quenching was observed. When the final lipid concentrations were 0.14, 0.28, or 0.70 mM, the emission maxima were shifted to 337, 328, and 329 nm, respectively. In the presence of 50% PG liposomes, the efficiency of quenching was significantly decreased to 1.9 M
−1 at 0.70 mM liposomes (the efficiency is approximately 80% lower than for melittin alone,
Figure 10a,d). The results of the cosedimentation assay indicated that the affinity between melittin and PG liposomes is strong, as described above, and showed that approximately 90% of melittin bound to the membrane at 0.28 mM liposomes (
Figure 9). Therefore, it appears that approximately 80% of melittin is incorporated into the membrane, 10% is bound to the surface, and the remaining 10% remains in solution. We note that when melittin was added to 70% or 100% PG liposomes, the reproducibility of the measurements was very low at any lipid concentration (data not shown), suggesting that a complex interaction took place between melittin and these liposomes.
Taken together, when increasing membrane area or phased shrinkage occurred, melittin reached more hydrophobic regions of the lipid bilayer than when solubilization occurred. In view of the facts that increasing membrane area causes a large increase in membrane area and melittin opens large pores in membranes, we consider that melittin inserts not only into the outer leaflet but also translocates to the inner leaflet of the lipid bilayer. The degree of penetration depended on the concentration of negatively charged PG, but not on that of total phospholipids.