Porcine/Chicken or Human Nephropathy as the Result of Joint Mycotoxins Interaction
Abstract
:1. Introduction
2. A Known Etiology of Classic Mycotoxic Nephropathy as Described for the First Time in Denmark
3. The Joint Mycotoxin Interaction and the Complex Etiology of Mycotoxic Nephropathy
| Mycotoxins | Producing species | Reference |
|---|---|---|
| OTA (ochratoxin A) | P. viridicatum, P. Commune, A. niger, A. ochraceus, A. wentii, A. fumigatus and others | [1] |
| PA (penicillic acid) | P. polonicum, P. aurantiogriseum, P. viridicatum, P. Crustosum, A. ochraceus and others | [1] |
| FB1 (fumonisin B1) | Gibberella fujikuroi, F. verticillioides | [1] |
| CIT (citrinin) | P. citrinum, P. viridicatum, P. expansum, A. candidus and others | [1] |
| UM (unknown metabolite) | P. polonicum, P. aurantiogriseum, P. crustosum, P. viridicatum, P. chrysogenum, P. commune, P. citrinum, P. expansum, A. fumigatus, A. flavus, A. candidus and others | [1] |
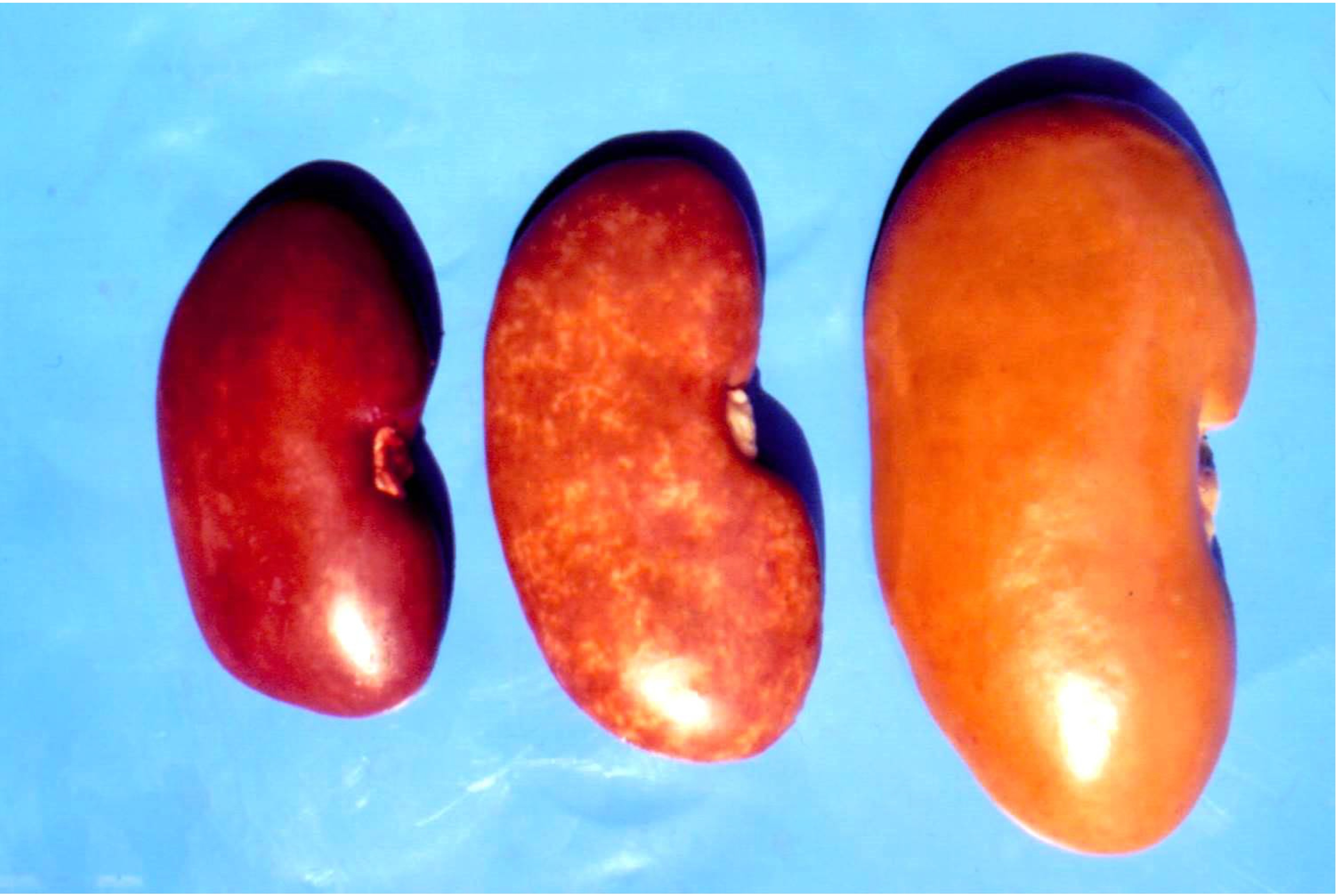
4. The Joint Mycotoxin Interaction and Its Relationship to Balkan Endemic Nephropathy
| Areas (farms) | Year | Food or feed | Number of samples | Number of positives | % of positive | Range/mean (µg/kg) | Reference |
|---|---|---|---|---|---|---|---|
| Endemic of BEN | 1986 | Beans | 34 | 13 | 38.2 | 25–200 | [129] |
| Maize | 34 | 14 | 41.7 | 25–250 | |||
| 1989 | Beans | 30 | 11 | 36.6 | 25–240 | ||
| Maize | 32 | 14 | 43.7 | 25–900 | |||
| 1990 | Beans | 25 | 10 | 40.0 | 85–260 | ||
| Maize | 25 | 11 | 44.0 | 25–890 | |||
| Nonendemic | 1986 | Beans | 24 | 2 | 8.3 | 20–150 | [129] |
| Maize | 24 | 2 | 8.3 | 20–180 | |||
| 1989 | Beans | 25 | 2 | 8.0 | 25–200 | ||
| Maize | 25 | 2 | 8.0 | 10–230 | |||
| 1990 | Beans | 40 | 2 | 5.0 | 10–220 | ||
| Maize | 40 | 2 | 5.0 | 20–235 | |||
| Farms with MPN | 1993 | feed | 7 | 7 | 100 | 38–552 | [3] |
| 1994 | feed | 10 | 10 | 100 | 42–427 | ||
| 2006 | feed | 25 | 25 | 100 | 188 ± 27 | [1] | |
| 2007 | feed | 25 | 25 | 100 | 376 ± 63 | ||
| Farms without MPN | 1993 | feed | 5 | - | - | - | [3] |
| 2006 | feed | 25 | - | - | - | [1] |
| Areas (farms) | Year (season) | Number of samples | Number of positives | % of positive | Mean ± SEM (µg/kg) | References |
|---|---|---|---|---|---|---|
| Endemic of BEN | 1986 | 30 | 7 | 23.3 | 20.0 ± 2.0 | [130] |
| 1989 | 24 | 7 | 29.2 | 27.2 ± 11.9 | ||
| 1990 | 20 | 5 | 25.0 | 25.0 ± 10.6 | ||
| Nonendemic | 1986 | 52 | 4 | 7.7 | 10.0 | [130] |
| 1989 | 30 | 2 | 6.6 | 0 < 2 | ||
| 1990 | 30 | 2 | 6.6 | 8.0 | ||
| Farms with MPN | autumn 1993 | 25 | 16 | 64 | 4.8 ± 0.9 | [5] |
| spring 1994 | 25 | 25 | 100 | 60.9 ± 9.2 | ||
| autumn 1994 | 25 | 12 | 48 | 21.9 ± 14.2 | ||
| 2006 | 10 | 8 | 80 | 28.8 ± 25.1 | [1] | |
| 2007 | 10 | 9 | 90 | 6.3 ± 4.9 | ||
| Farms without MPN | 1993 | 5 | - | - | - | [5] |
| Parameters | Bulgarian MPN | BEN |
|---|---|---|
| Size of kidneys: | ||
| ✓ in early stages | enlarged | no information |
| ✓ in later stages | reduced towards former stages | strongly reduced |
| Degenerative changes mainly in proximal tubules | + (mostly in early stages) | + (mostly in early stages) |
| Granular and hyalin casts or necrotic debris in the tubules | + (mostly in early stages) | + (mostly in early stages) |
| Dilated atrophic tubules (retention cysts) | + (in later stages) | + (in later stages) |
| Interstitial fibrosis and hyalinization/sclerosis of glomeruli | + (in later stages) | + (in later stages) |
| Mononuclear (inflammatory) cell infiltration | + (moderate) | + (slight) |
| Dilated lymphatics (lymphatic cysts) | + (mostly in later stages) | + (mostly in later stages) |
| Vascular lesions | + | + |
| Neoplastic changes | + (benign tumour in kidneys) | + (malignant tumours in ureters/pelvis) |
| Ultrastructural damages in proximal tubules: | ||
| ✓ Reduced brush border in high and density | + | + |
| ✓ Diminution or disappearance of mitochondrial cristae | + | + |
| ✓ Swollen and distorted mitochondria | + | + |
| ✓ Myelin-like figures and lipid droplets in the mitochondria | + | + |
| ✓ Electron dense formations in the nuclei and mitochondria | + | + |
| ✓ Large number apical vesicles | + | + |
| ✓ Lost membrane integrity of cell organelles | + | + |
| ✓ Increased number of phagolysosomes | + | + |
| ✓ Thickening of basement tubular membranes | + (in later stages) | + (in later stages) |
5. The Implication of Some Fungi and New Fungal Metabolites in Etiology of MPN/MCN/BEN
6. The Significance of the Masked Target Mycotoxins and Their Binding to Some Serum Macromolecules in the Etiology of MPN/MCN/BEN
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Stoev, S.D.; Dutton, M.; Njobeh, P.; Mosonik, J.; Steenkamp, P. Mycotoxic nephropathy in Bulgarian pigs and chickens: Complex aetiology and similarity to Balkan Enedemic Nephropathy. Food Addit. Contam. A 2010, 27, 72–88. [Google Scholar] [CrossRef]
- Stoev, S.D.; Denev, S.; Dutton, M.F.; Njobeh, P.B.; Mosonik, J.S.; Steenkamp, P.A.; Petkov, I. Complex etiology and pathology of mycotoxic nephropathy in South African pigs. Mycotox. Res. 2010, 26, 31–46. [Google Scholar] [CrossRef]
- Stoev, S.D.; Hald, B.; Mantle, P. Porcine nephropathy in Bulgaria: A progressive syndrome of complex of uncertain (mycotoxin) etiology. Vet. Rec. 1998, 142, 190–194. [Google Scholar] [CrossRef]
- Stoev, S.D.; Grozeva, N.; Hald, B. Ultrastructural and toxicological investigations in spontaneous cases of porcine nephropathy in Bulgaria. Vet. Arhiv. 1998, 68, 39–49. [Google Scholar]
- Stoev, S.D.; Stoeva, J.; Anguelov, G.; Hald, B.; Creppy, E.E.; Radic, B. Haematological, biochemical and toxicological investigations in spontaneous cases with different frequency of porcine nephropathy in Bulgaria. J. Vet. Med. Series A 1998, 45, 229–236. [Google Scholar] [CrossRef]
- Stoev, S.D.; Daskalov, H.; Radic, B.; Domijan, A.; Peraica, M. Spontaneous mycotoxic nephropathy in Bulgarian chickens with unclarified mycotoxin aetiology. Vet. Res. 2002, 33, 83–94. [Google Scholar] [CrossRef]
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. 2013, 53, 887–901. [Google Scholar]
- Krogh, P.; Hasselager, E. Studies on fungal nephrotoxicity. Arsskr. K. Vet. Land-bohojsk 1968, 198–214. [Google Scholar]
- Friis, P.; Hasselager, E.; Krogh, P. Isolation of citrinin and oxalic acid from P. viridicatum westling and their nephrotoxicity in rats and pigs. Acta Path. Microb. Scand. 1969, 77, 559–560. [Google Scholar] [CrossRef]
- Krogh, P.; Axelsen, N.H.; Elling, F.; Gyrd-Hansen, N.; Hald, B.; Hyldgaard-Jensen, J.; Larsen, A.E.; Madsen, A.; Mortensen, H.P.; Moller, T.; et al. Experimental porcine nephropathy: Changes of renal function and structure induced by ochratoxin A-contaminated feed. Acta Path. Microb. Scand. Sect. A 1974, 246, 1–21. [Google Scholar]
- Krogh, P. Ochratoxin A residues in tissues of slaughter pigs with nephropathy. Nord. Vet. Med. 1977, 29, 402–405. [Google Scholar]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef]
- Van der Merwe, K.J.; Steyn, P.; Fourie, L. Mycotoxins. Part II. The constitution of ochratoxin A, B, and C, metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc. 1965, 7083–7088. [Google Scholar] [CrossRef]
- Steyn, P.S. Chemical studies related to A. ochraceus Wilh. Ph.D. Thesis, University of South Africa, Pretoria, South Africa, 1966. [Google Scholar]
- Steyn, P.S.; Holzapfel, C.W. The isolation of the methyl and ethyl esters of ochratoxin A and B, metabolites of Aspergillus ochraceus Wilh. J. S. Afr. Chem. Inst. 1967, 20, 186–189. [Google Scholar]
- Varga, J.; Kevei, E.; Rinyu, E.; Teren, J.; Kozakiewic, Z. Ochratoxin production by Aspergillus species. Appl. Environ. Microb. 1996, 62, 4461–4464. [Google Scholar]
- Rizzo, A.; Eskola, M.; Atroshi, F. Ochratoxin A in cereals, foodstuffs and human plasma. Eur. J. Plant Pathol. 2002, 108, 631–637. [Google Scholar] [CrossRef]
- Sage, L.; Krivobok, S.; Delbos, E.; Seigle-Murandi, F.; Creppy, E.E. Fungal Flora and ochratoxin A production in grapes and musts from France. J. Agric. Food Chem. 2002, 50, 1306–1311. [Google Scholar] [CrossRef]
- Van Walbeck, W. Microbial Food-Borne Infections and Intoxication. In Symposium of Department of National Health and Welfare; Ontario, Ottawa, Canada, 1973, pp. 78–87.
- Cook, W.O.; Osweiler, G.D.; Anderson, T.D.; Richard, J.L. Ochratoxicosis in Iowa swine. Am. Vet. Med. Assn. 1986, 188, 1399–1402. [Google Scholar]
- Pohland, A.E.; Nesheim, S.; Friedman, L. Ochratoxin A, a review. Pure Appl. Chem. 1992, 64, 1029–1046. [Google Scholar] [CrossRef]
- Stoev, S.D. Mycotoxic Nephropathies in Farm Animals—Diagnostics, Risk Assessment and Preventive Measures. In Mycotoxins in Farm Animals; Oswald, I., Taranu, I., Pandalai, S.G., Eds.; Transworld Research Network: Trivandrum, Kerala, India, 2008; pp. 155–195. [Google Scholar]
- Stoev, S.D. Complex etiology, prophylaxis and hygiene control in mycotoxic nephropathies in farm animals and humans. Int. J. Mol. Sci. 2008, 9, 578–605. [Google Scholar] [CrossRef]
- Battaglia, R.; Hatzold, T.; Kroes, R. Conclusions from the workshop on ochratoxin in food, organized by ILSI Europe in Aix-en-Provence. Food Addit. Contam. 1996, 13, 1–3. [Google Scholar] [CrossRef]
- CAST (Council for Agricultural Science and Technology), Mycotoxins: Economic and Health Risk. In Task Force Report 116; Ames, IA, USA, 1989; pp. 1–91.
- Krogh, P. Ochratoxin in Food. In Mycotoxins in Food; Krogh, P., Ed.; Academic press: London, UK, 1987; pp. 97–121. [Google Scholar]
- Pfohl-Leszkowicz, A.; Petkova-Bocharova, T.; Chernozemsky, I.N.; Castegnaro, M. Balkan endemic nephropathy and the associated urinary tract tumours: Review on etiological causes, potential role of mycotoxins. Food Addit. Contam. 2002, 19, 282–302. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Roubal, T. Ochratoxin A exposure biomarkers in the Czech Republic and comparison with foreign countries. Biomarkers 2012, 17, 577–589. [Google Scholar] [CrossRef]
- MAFF (Ministry of Agriculture Fisheries and Foods). Survey of Aflatoxins and Ochratoxin A in Cereals and Retail Products. Food Surveillance Information Sheet No 130. November 1997. Available online: http://archive.food.gov.uk/maff/archive/food/infsheet/1997/no130/130afla.htm (accessed on 24 June 2013).
- Van Egmond, H.P.; Speijers, G.J.A. Survey of data on the incidence and levels of ochratoxin A in food and animal feed world-wide. J. Nat. Toxins 1994, 3, 125–144. [Google Scholar]
- MAFF (Ministry of Agriculture Fisheries and Foods). Surveillance of Ochratoxin A in Green (Unroasted) Coffee Beans. Food Surveillance Information Sheet No 80. March 1996. Available online: http://archive.food.gov.uk/maff/archive/food/infsheet/1996/no80/80oacoff.htm (accessed on 24 June 2013).
- Nakajima, M.; Tsubouchi, H.; Miyabe, M.; Ueno, Y. Syrvey of aflatoxin B1 and ochratoxin A in commercial green coffee beans by HPLC linked with immunoaffinity chromatography. Food Agric. Immunol. 1997, 9, 77–83. [Google Scholar] [CrossRef]
- Mantle, P.G. Ochratoxin A in coffee. J. Food Mycol. 1998, 1, 63–65. [Google Scholar]
- Mantle, P.G. Uptake of radiolabelled ochratoxin A from soil by coffee plants. Phytochemistry 2000, 53, 377–378. [Google Scholar] [CrossRef]
- Mantle, P.G.; Chow, A.M. Ochratoxin formation in Aspergillus ochraceus with particular reference to spoilage of coffee. Int. J. Food Microbiol. 2000, 56, 105–109. [Google Scholar] [CrossRef]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in roasted coffee from French supermarkets and transfer in coffee beverages: Comparison of analysis methods. Toxins 2010, 2, 1928–1942. [Google Scholar] [CrossRef] [Green Version]
- Majerus, P.; Otteneder, H. Nachweis und Vorkommen von Ochratoxin A in Wein und Traubensaft. Dtsch. Lebensm. Rundsch. 1996, 92, 388–390. [Google Scholar]
- Zimmerli, B.; Dick, R. Ochratoxin A in table wine and grape-juice: Occurrence and risk assessment. Food Addit. Contam. 1996, 13, 655–668. [Google Scholar] [CrossRef]
- Ospital, M.; Cazabeil, J.; Betbeder, A.; Tricard, C.; Creppy, E.E.; Medina, B. L’ochratoxine A dans les vins. Rev. Fr. Oenol. 1998, 169, 16–18. [Google Scholar]
- Budaspal, P.A.; Legarda, T.M. Ochratoxin A in wines and grape products originated from Spain and other European countries. Alimentaria 1999, 299, 107–113. [Google Scholar]
- Rosa, C.A.R.; Santana, D.M.N.; Fraga, M.E.; Sabaa-Srur, A.O.U. Ochratoxin A Detection by Immunoaffinity Clean-Up and HPLC in Grape Juice and Wine Samples Sold in Rio de Janeiro Brazil. In Proceedings of 10th International IUPAC symposium on mycotoxins and phycotoxins, Guaruja, Sao Paulo, Brazil, 2000; p. 85.
- Scott, P.M.; Kanhere, S.R. Determination of ochratoxin A in beer. Food Addit. Contam. 1995, 12, 591–598. [Google Scholar] [CrossRef]
- Fiali, A.; Ouammi, L.; Betbeder, A.M.; Baudrimont, I.; Benayada, R.; Creppy, E.E. Ochratoxin A in beverages from Morocco: A preliminary survey. Food Addit. Contam. 2001, 18, 565–568. [Google Scholar]
- Patel, S.; Hazel, C.M.; Winterton, A.G.M.; Mortbay, E. Survey of ethnic foods for mycotoxins. Food Addit. Contam. 1996, 13, 833–841. [Google Scholar] [CrossRef]
- Vrabcheva, T.; Gareis, M.; Bresch, H.; Bodechtel, C.; Engel, G.; Majerus, P.; Rosner, H.; Wolff, J. Occurrence of ochratoxin A and B in spice and herbs. Revue Med. Vet. 1998, 149, 533. [Google Scholar]
- Walker, R. Mycotoxins of Growing Interest: Ochratoxins. In 3rd Joint FAO/UNEP International Conference on Mycotoxins, Tunis, Tunisia, March 1999; p. 5.
- Ottender, H.; Majerus, P. Occurrence of ochratoxin A (OTA) in wines: Influence of the type of wine and its geographical origin. Food Addit. Contam. 2000, 17, 793–798. [Google Scholar] [CrossRef]
- Madhyastha, M.S.; Marquardt, R.R.; Frohlich, A.A.; Platford, G.; Abramson, D. Effects of different cereals and oilseed substrates on the growth and production of toxins by A. alutaceus and P. Verrucosum. J. Agric. Food. Chem. 1990, 38, 1506–1510. [Google Scholar] [CrossRef]
- Northolt, M.D.; van Egmond, H.P.; Paulsch, W.E. Ochratoxin A production by some fungal species in relation to water activity and temperature. J. Food Protect. 1979, 42, 485–490. [Google Scholar]
- Trenk, H.L.; Butz, M.E.; Chu, F. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl. Microbiol. 1971, 21, 1032–1035. [Google Scholar]
- Sansing, G.A.; Davis, N.D.; Diener, U.L. Effect of time and temperature on ochratoxin A production by A. Ochraceus. Can. J. Microbiol. 1973, 19, 1259–1263. [Google Scholar] [CrossRef]
- Lillehoj, E.B.; Aalund, O.; Hald, B. Bioproduction of [14C] ochratoxin A in submerged culture. Appl. Environ. Microbiol. 1978, 36, 720–723. [Google Scholar]
- Lillehoj, E.B.; Elling, F. Environmental conditions that facilitate ochratoxin contamination of agricultural commodities. Acta Agric. Scand. 1983, 33, 113–128. [Google Scholar] [CrossRef]
- Stoev, S.D.; Vitanov, S.; Anguelov, G.; Petkova-Bocharova, T.; Creppy, E.E. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and penicillic acid. Vet. Res. Commun. 2001, 25, 205–223. [Google Scholar] [CrossRef]
- Stoev, S.D.; Stefanov, M.; Denev, S.; Radic, B.; Domijan, A.M.; Peraica, M. Experimental mycotoxicosis in chickens induced by ochratoxin A and penicillic acid and intervention by natural plant extracts. Vet. Res. Commun. 2004, 28, 727–746. [Google Scholar] [CrossRef]
- Stoev, S.D.; Anguelov, G.; Ivanov, I.; Pavlov, D. Influence of ochratoxin A and an extract of artichoke on the vaccinal immunity and health in broiler chicks. Exp. Toxicol. Pathol. 2000, 52, 43–55. [Google Scholar] [CrossRef]
- Stoev, S.D.; Goundasheva, D.; Mirtcheva, T.; Mantle, P. Susceptibility to secondary bacterial infections in growing pigs as an early response in ochratoxicosis. Exp. Toxicol. Pathol. 2000, 52, 287–296. [Google Scholar] [CrossRef]
- Oswald, I.P.; Desautels, C.; Laffitte, J.; Fournout, S.; Peres, S.Y.; Odin, M.; Le Bars, P.; Le Bars, J.; Fairbrother, J.M. Mycotoxin fumonisin B-1 increases intestinal colonization by pathogenic Escherichia coli in pigs. Appl. Environ. Microb. 2003, 69, 5870–5874. [Google Scholar] [CrossRef]
- Oswald, I.P.; Bouhet, S.; Marin, D.E.; Pinton, P.; Taranu, I. Mycotoxin Effects on the Pig Immune System. In Proceedings of the European Mycotoxin Seminar Series “Evaluating the Impact of Mycotoxins in Europe” European Lecture Tour, Sofia, Bulgaria, February 2005; pp. 78–93.
- Oswald, I.P.; Marin, D.E.; Bouhet, S.; Pinton, P.; Taranu, I.; Accensi, F. Immunotoxicological risk of mycotoxins for domestic animals. Food Addit. Contam. 2005, 22, 354–360. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Norred, W.P.; Bacon, C.W.; Meredith, F.I.; Howard, P.C. An overview of rodent toxicities: Liver and kidney effects of fumonisins and Fusarium moniliforme. Environ. Health Persp. 2001, 109, 259–266. [Google Scholar]
- Pfohl-Leszkowicz, A.; Tozlovanu, M.; Manderville, R.; Peraica, M.; Castegnaro, M.; Stefanovic, V. New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in Human Nephropathy and Urinary tract tumor. Mol. Nutr. Food Res. 2007, 51, 1131–1146. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A. Ochratoxin A and aristolochic acid involvement in nephropathies and associated urothelial tract tumours. Arh. Hig. Rada. Toksikol. 2009, 60, 465–483. [Google Scholar] [CrossRef]
- Dutton, M.F.; Kinsey, A. Incidence of mycotoxins and fungi in feedstuffs in Natal in 1995. Mycopathologia 1995, 131, 31–36. [Google Scholar] [CrossRef]
- Krogh, P. Mycotoxic Nephropathy. In Advances in Veterinary Science and Comparative Medicine; Academic Press: New York, NY, USA, 1976; Volume 20, pp. 147–170. [Google Scholar]
- Krogh, P.; Elling, F.; Gyrd-Hansen, N.; Hald, B.; Larsen, A.E.; Lillehoj, E.B.; Madsen, A.; Mortensen, H.P.; Ravnskov, U. Experimental porcine nephropathy: Changes of renal function and structure perorally induced by cristalline ochratoxin A. Acta Path. Microbiol. Scand. Sect. A 1976, 84, 429–434. [Google Scholar]
- Klaric, M.S.; Rumora, L.; Ljubanovic, D.; Pepeljnjak, S. Cytotoxicity and apoptosis induced by fumonisin B1, beauvericin and ochratoxin A in porcine kidney PK15 cells: Effects of individual and combined treatment. Arch. Toxicol. 2007, 82, 247–255. [Google Scholar]
- Kubena, L.F.; Edrington, T.S.; Harvey, R.B.; Phillips, T.D.; Sarr, A.B.; Rottinghaus, G.E. Individual and combined effects of fumonisin B1 present in fusarium moniliforme culture material and diacetoxyscirpenol or ochratoxin A in turkey poults. Poultry Sci. 1997, 76, 256–264. [Google Scholar]
- Manderville, R.A.; Pfohl-Leszkowicz, A. Bioactivation and DNA adduction as a rationale for ochratoxin A carcinogenesis. World Mycotoxin J. 2008, 1, 357–367. [Google Scholar] [CrossRef]
- Stoev, S.D. Some metric, antidote and pathomorphological investigations in experimental intoxication with ochratoxin A and penicillic acid in chicks. Bulg. J. Agric. Sci. 1998, 4, 551–563. [Google Scholar]
- Stoev, S.D. Ultrastructural and antidote investigations into the experimental intoxication of chickens with ochratoxin A and penicillic acid. Folia Vet. 2000, 44, 85–90. [Google Scholar]
- Stoev, S.; Anguelov, G.; Pavlov, D.; Pirovski, L. Some antidotes and paraclinical investigations in experimental intoxication with ochratoxin A and penicillic acid in chicks. Vet. Arhiv. 1999, 69, 179–189. [Google Scholar]
- Dickens, F.; Jones, H.E.H. Carcinogenic activity of a series of reactive lactones and related substances. Br. J. Cancer 1961, 15, 85–100. [Google Scholar] [CrossRef]
- Palmgren, M.S.; Ciegler, A. Toxicity and Carcinogenicity of Fungal Lactones: Patulin and Penicillic Acid. In Handbook of Natural Toxins. Plant and Fungal Toxins; Keeler, R.F., Tu, A.T., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1983; Volume 1, pp. 325–341. [Google Scholar]
- Ueno, Y.; Kubota, K. DNA-attacking ability of carcinogenic mycotoxins in recombination deficient mutant cells of Bacillus subtilis. Cancer Res. 1976, 36, 445–451. [Google Scholar]
- International Agency for Research on Cancer (IARC), Ochratoxin A. Some Naturally Occurring Substances; Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; IARC Press: Lyon, France, 1993; Volume 56, pp. 489–521.
- Huff, W.E.; Hamilton, P.B.; Ciegler, A. Evaluation of penicillic acid for toxicity in broiler chickens. Poultry Sci. 1980, 59, 1203–1207. [Google Scholar] [CrossRef]
- Umeda, M.; Yamamoto, T.; Saito, M. DNA-strand breakage of HeLa cells induced by several mycotoxins. Jpn. J. Exp. Med. 1972, 42, 527–539. [Google Scholar]
- Umeda, M. Cytotoxicity of Mycotoxins. In Mycotoxins in Human and Animal Health; Rodricks, J.V., Hesseltine, C.W., Mehlman, M.A., Eds.; Pathotox Publ. Inc.: Park Forest South, IL, USA, 1977; pp. 712–729. [Google Scholar]
- Dierickx, P.J.; De Beer, J.O. Interaction of the mycotoxin penicillic acid with glutathione and rat liver glutathione S-transferases. Mycopathologia 1984, 86, 137–141. [Google Scholar] [CrossRef]
- Chan, P.K.; Hayes, A.W. Effect of penicillic acid on biliary excretion of indocyanine green in the mouse and rat. J. Toxicol. Environ. Health. 1981, 7, 169–179. [Google Scholar] [CrossRef]
- Hayes, A.W.; Unger, P.P.; Williams, W.L. Acute toxicity of penicillic acid and rubratoxin B in dogs. Ann. Nutr. Aliment. 1977, 31, 711–721. [Google Scholar]
- Sansing, G.A.; Lillehof, E.B.; Detroy, R.W.; Muller, M.A. Synergistic toxic effects of citrinin, ochratoxin A and penicillic acid in mice. Toxicon 1976, 14, 213–220. [Google Scholar] [CrossRef]
- Shepherd, E.C.; Philips, T.D.; Joiner, G.N.; Kubena, L.F.; Heidelbaugh, N.D. Ochratoxin A and penicillic acid interaction in mice. J. Environ. Sci. Health B 1981, 16, 557–573. [Google Scholar] [CrossRef]
- Micco, C.; Miraglia, M.; Onori, R.; Libanori, A.; Brera, C.; Mantovani, A.; Macri, C. Effect of combined exposure to ochratoxin A and penicillic acid on residues and toxicity in broilers. Int. J. Food Sci. Nutr. 1991, 20, 101–108. [Google Scholar]
- Doster, R.C.; Sinhuber, R.O. Comparative rates of hydrolysis of ochratoxin A and B in vitro. Food Cosmet. Toxicol. 1972, 10, 389–394. [Google Scholar] [CrossRef]
- Suzuki, S.; Satoh, T.; Yamazaki, M. The pharmacokinetics of ochratoxin A in rats. Jpn. J. Pharmacol. 1977, 27, 735–744. [Google Scholar] [CrossRef]
- Parker, R.W.; Phillips, T.D.; Kubena, L.E.; Russell, L.H.; Heidelbaugh, N.D. Inhibition of pancreatic carboxypeptidase A: A possible mechanism of interaction between penicillic acid and ochratoxin A. J. Environ. Sci. Health B 1982, 17, 77–91. [Google Scholar] [CrossRef]
- Stoev, S.D.; Petkova-Bocharova, T.K. A Possible Role of Ochratoxin A in a Disease Causation in Connection With Balkan Endemic Nephropathy. In Proceedings of the 8th International Congress on Animal Hygiene, St. Paul, MN, USA, September 1994; pp. 61–64.
- Stoev, S.D. The role of ochratoxin A as a possible cause of Balkan endemic nephropathy and its risk evaluation. Vet. Human Toxicol. 1998, 40, 352–360. [Google Scholar]
- Kubena, L.F.; Phillips, T.D.; Witzel, D.A.; Heidelbaugh, N.D. Toxicity of ochratoxin A and penicillic acid to chicks. Bull. Environ. Contam. Toxicol. 1984, 32, 711–716. [Google Scholar] [CrossRef]
- Dwivedi, P.; Burns, R.B. Pathology of ochratoxicosis A in young broiler chicks. Res. Vet. Sci. 1984, 36, 92–103. [Google Scholar]
- Manning, R.O.; Wyatt, D. Toxicity of Aspergillus ochraceus contaminated wheat and different chemical forms of ochratoxin A in broiler chicks. Poult. Sci. 1984, 63, 458–465. [Google Scholar] [CrossRef]
- Mohiudin, S.M.; Warasi, S.M.A.; Reddy, M.V. Haematological and biochemical changes in broiler chicken. Indian Vet. J. 1993, 70, 613–617. [Google Scholar]
- Kozaczynski, W. Experimental ochratoxicosis A in chickens. Histopathological and histochemical study. Arch. Vet. Pol. 1996, 34, 205–219. [Google Scholar]
- Elling, F.; Nielsen, J.P.; Lillehoj, E.B.; Thomassen, M.S.; Stormer, F.C. Ochratoxin A-induced porcine nephropathy: Enzyme and ultrastructural changes after short-term exposure. Toxicon 1985, 23, 247–254. [Google Scholar] [CrossRef]
- Stoev, S.D.; Paskalev, M.; MacDonald, S.; Mantle, P. Experimental one year ochratoxin A toxicosis in pigs. Exp. Toxicol. Pathol. 2002, 53, 481–487. [Google Scholar] [CrossRef]
- Elling, F. Morphological Aspects of Mycotoxic Nephropathy. In Mycotoxins in Human and Animal Health; Rodricks, J.V., Hesseltine, C.W., Mehlman, M.A., Eds.; Pathotox Publishers, Inc.: Park Forest South, IL, USA, 1977; pp. 499–506. [Google Scholar]
- Dragan, Y.P.; Bidlack, W.R.; Cohen, S.M.; Goldsworthy, T.L.; Hard, G.C.; Howard, P.C.; Riley, R.T.; Voss, K.A. Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: Fumonisin B1 as an example. Toxicol. Sci. 2001, 61, 6–17. [Google Scholar] [CrossRef]
- Petrik, J.; Zanic-Grubišic, T.; Barišic, K.; Pepeljnjak, S.; Radic, B.; Fereničic, Z.; Cepelak, I. Apoptosis and oxidative stress induced by ochratoxin A in rat kidney. Arch. Toxicol. 2003, 77, 685–693. [Google Scholar] [CrossRef]
- Creppy, E.E.; Chiarappa, P.; Baudrimont, I.; Borracci, P.; Moukha, S.; Carratù, M.R. Synergistic effects of fumonisin B1 and ochratoxin A: Are “in vitro” cytotoxicity data predictive of “in vivo” acute toxicity. Toxicology 2004, 201, 115–123. [Google Scholar]
- Stoev, S.D.; Gundasheva, D.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Mitev, Y.; Daskalov, H.; Dutton, M.; Mwanza, M.; et al. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp. Toxicol. Pathol. 2012, 64, 733–741. [Google Scholar] [CrossRef]
- Domijan, A.; Zeljezic, D.; Kopjar, N.; Peraica, M. Standard and Fpg-modifed comet assay in kidney cells of ochratoxin A-and fumonisin B1-treated rats. Toxicology 2006, 222, 53–59. [Google Scholar] [CrossRef]
- Lillehoj, E.B.; Ciegler, A. Mycotoxin Synergism. In Microbiology; Schlessinger, D., Ed.; American Society of Microbiology: Washington, DC, USA, 1975; pp. 344–358. [Google Scholar]
- Bernhoft, A.; Keblys, M.; Morrison, E.; Larsen, H.J.S.; Flåøyen, A. Combined effects of selected Penicillium mycotoxins on “in vitro” proliferation of porcine lymphocytes. Mycopathologia 2004, 158, 441–450. [Google Scholar] [CrossRef]
- Koshinsky, H.A.; Khachatourians, G.G. Mycotoxicoses: The Effects of Mycotoxin Combinations. In Foodborne Disease Handbook. Diseases Caused by Viruses, Parasites, and Fungi; Hui, Y.H., Gorham, J.R., Murrell, K.D., Cliver, D.O., Eds.; University of Saskatchewan: Saskatoon, Canada, 1994; Volume 2, pp. 463–520. [Google Scholar]
- Pfohl-Leszkowicz, A.; Molinié, A.; Tozlovanu, M.; Manderville, R.A. Combined Toxic Effects of Ochratoxin A and Citrinin, In Vitro and In Vivo. In Food Contaminants, Mycotoxins and Food Allergen; Siantar, D.P., Trucksess, M.W., Scott, P.M., Herman, E.M., Eds.; ACS Symposium Series: Washington, DC, USA, 2008; pp. 56–80. [Google Scholar]
- Kanisawa, M. Synergistic effect of citrinin on hepatorenal carcinogenesis of ochratoxin A in mice. Dev. Food Sci. 1984, 7, 245–254. [Google Scholar]
- Rahimtula, A.D.; Bereziat, J.C.; Eussacchini-Griot, V.; Bartsch, H. Lipid peroxidation as a possible cause of ochratoxin A toxicity. Biochem. Pharmacol. 1988, 37, 4469–4477. [Google Scholar] [CrossRef]
- Abado-Becognee, K.; Mobio, T.K.; Ennamany, R.; Fleurat-Lessard, F.; Shier, W.T.; Badria, F.; Creppy, E.E. Cytotoxicity of fumonisin B1: Implication of lipid peroxidation and inhibition of protein and DNA syntheses. Arch. Toxicol. 1998, 72, 233–236. [Google Scholar] [CrossRef]
- Stoev, S.D.; Djuvinov, D.; Mirtcheva, T.; Pavlov, D.; Mantle, P. Studies on some feed additives giving partial protection against ochratoxin A toxicity in chicks. Toxicol. Lett. 2002, 135, 33–50. [Google Scholar] [CrossRef]
- Stoev, S.D. Studies on some feed additives and materials giving partial protection against the suppressive effect of ochratoxin A on egg production of laying hens. Res. Vet. Sci. 2010, 88, 486–491. [Google Scholar] [CrossRef]
- Stoev, S.D.; Denev, S.; Dutton, M.F.; Nkosi, B. Cytotoxic effect of mycotoxins Ochratoxin A, Citrinin, Penicillic acid, Fumonisin B1 and their combinations on human peripheral blood mononuclear cells as measured by MTT assy. Open Toxinol. J. 2009, 2, 1–8. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Marasas, W.F.O.; Farber, E. The cancer initiating potential of the fumonisin B mycotoxins. Carcinogenesis 1992, 13, 433–437. [Google Scholar] [CrossRef]
- Howard, P.C.; Warbritton, A.; Voss, K.A.; Lorenzen, R.J.; Thurman, J.D.; Kovach, R.M.; Bucci, T.J. Compensatory regeneration as a mechanism for renal tubule carcinogenesis of fumonisin B1 in F344/N/Nctr BR rat. Environ. Health Persp. 2001, 109, 309–314. [Google Scholar]
- Stoev, S.D. Studies on carcinogenic and toxic effects of ochratoxin A in chicks. Toxins 2010, 2, 649–664. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R. Review on Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R. An update on direct genotoxicity as a molecular mechanism of ochratoxin A carcinogenicity. Chem. Res. Toxicol. 2012, 25, 252–262. [Google Scholar] [CrossRef]
- Bucci, .T.J.; Howard, P.C.; Tolleson, W.H.; Laborde, J.B.; Hansen, D.K. Renal effects of fumonisin mycotoxins in animals. Toxicol. Pathol. 1998, 26, 190–194. [Google Scholar]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A. Fundamentals of Toxicologic Pathology; Elsevier Inc.: San Diego, CA, USA, 2009; pp. 1–691. [Google Scholar]
- Pósa, R.; Magyar, T.; Stoev, S.D.; Glávits, R.; Donkó, T.; Repa, I.; Kovács, M. Use of computed tomography and histopathologic review for lung lesions produced by the interaction between Mycoplasma hyopneumoniae and fumonisin mycotoxins in pigs. Vet. Pathol. 2013. [Google Scholar] [CrossRef]
- Petkova-Bocharova, T.; Stoichev, I.; Chernozemsky, I.; Castegnaro, M.; Pfohl-Leszkowicz, A. Formation of DNA adducts in tissues of mice progeny through transplacental contamination after administration of a single dose of ochratoxin A to the pregnant mother. Environ. Mol. Mutagen. 1998, 32, 155–162. [Google Scholar] [CrossRef]
- Jennings-Gee, J.; Tozlovanu, M.; Manderville, R.; Miller, M.; Pfohl-Leszkowicz, A.; Schwartz, G. Ochratoxin A: In utero exposure in mice induces adducts in testicular DNA. Toxins 2010, 2, 1428–1444. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.; Manderville, R.; Pfohl-Leszkowicz, A. Response to comments of Peter G. Mantle. Toxins 2010, 2, 2337–2339. [Google Scholar] [CrossRef] [Green Version]
- Jurjevic, Z.; Solfrizzo, M.; Cvjetkovic, B.; Avantaggiato, G.; Visconti, A. Ochratoxin A and fumonisins (B1 and B2) in maize from Balkan nephropathy endemic and non endemic areas of Croatia. Mycotox. Res. 1999, 15, 67–80. [Google Scholar] [CrossRef]
- Jurjevic, L.; Solfrizzo, M.; Cvjetkovic, B.; De Girolamo, A.; Visconti, A. Occurrence of beauvericin in corn from Croatia. Food Technol. Biotechnol. 2002, 40, 91–94. [Google Scholar]
- Domijan, A.; Peraica, M.; Jurjevic, Z.; Ivic, D.; Cvjetkovic, B. Fumonisin B1, fumonisin B2, zearalenone and ochratoxin A contamination of maize in Croatia. Food Add. Contam. 2005, 22, 677–680. [Google Scholar] [CrossRef]
- Diaz, C.T.; Sogbe, E.; Ascanio, E.; Hernandez, M. Ochratoxin A and fumonisin B1 natural interaction in pigs. Clinical and pathological studies. Rev. Cient. Fac. Cien. V. 2001, 314–321. [Google Scholar]
- Petkova-Bocharova, T.; Castegnaro, M.; Michelon, J.; Maru, V. Ochratoxin A and Other Mycotoxins in Cereals from an Area of Balkan Endemic Nephropathy and Urinary Tract Tumours in Bulgaria. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours, Scientific Publication 115; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC Press: Lyon, France, 1991; pp. 83–87. [Google Scholar]
- Petkova-Bocharova, T.; Castegnaro, M. Ochratoxin A in Human Blood in Relation to Balkan Endemic Nephropathy and Urinary Tract Tumours in Bulgaria. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours, Scientific Publication 115; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; International Agency for Research on Cancer: Lyon, France, 1991; pp. 135–137. [Google Scholar]
- Vrabcheva, T.; Usleber, E.; Dietrich, R.; Martlbauer, E. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J. Agric. Food Chem. 2000, 48, 2483–2488. [Google Scholar] [CrossRef]
- Castegnaro, M.; Canadas, D.; Vrabcheva, T.; Petkova-Bocharova, T.; Chernozemsky, I.N.; Pfohl-Leszkowicz, A. Balkan endemic nephropathy: Role of ochratoxin A through biomarkers. Mol. Nutr. Food Res. 2006, 50, 519–529. [Google Scholar] [CrossRef]
- Fuchs, R.; Peraica, M. Ochratoxin A in human kidney diseases. Food Addit. Contam. 2005, 22, 53–57. [Google Scholar] [CrossRef]
- Fuchs, R.; Hult, K. Ochratoxin A in blood and its pharmacokinetic properties. Food Chem. Toxicol. 1992, 30, 201–204. [Google Scholar] [CrossRef]
- Mantle, P.G.; McHugh, K.M. Nephrotoxic fungi in foods from nephropathy households in Bulgaria. Mycol. Res. 1993, 97, 205–212. [Google Scholar] [CrossRef]
- Rubiales, M.V.; Bragulat, M.R.; Cabanes, F.J. Mycotoxin production by Penicillium species in liquid medium. Revue Med. Vet. 1998, 149, 526. [Google Scholar]
- Le Bars, J. Enhancement factors of penicillic acid production by Penicillium verrucosum var. cyclopium in foodstuffs [animal feeds]. Ann. Rech. Vet. 1980, 11, 321–326. [Google Scholar]
- Lieu, F.Y.; Bullerman, L.B. Production and stability of aflatoxins, penicillic acid and patulin in several substrates. J. Food Sci. 1977, 42, 1222–1224. [Google Scholar] [CrossRef]
- Lieu, F.Y.; Bullerman, L.B. Binding of patulin and penicillic acid to glutathione and cysteine and toxicity of the resulting adducts. Milchwissensch 1978, 33, 16–20. [Google Scholar]
- Miljkovic, A.; Pfohl-Leszkowicz, A.; Dobrota, M.; Mantle, P.G. Comparative responses to mode of oral administration and dose of ochratoxin A or nephrotoxic extract of Penicillium polonicum in rats. Exp. Toxicol. Pathol. 2003, 54, 305–312. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Chuturgoon, A.A.; Koch, S.H.; Steenkamp, P.A.; Stoev, S.D. Identification of novel metabolite and its cytotoxic effect on human lymphocyte cells in comparison to other mycotoxins. Int. J. Biol. Chem. Sci. 2009, 3, 524–531. [Google Scholar]
- Mantle, P.; Miljkovic, A.; Udupa, V.; Dobrota, M. Does apoptosis cause renal atrophy in Balkan endemic nephropathy. Lancet 1998, 352, 1118–1119. [Google Scholar] [CrossRef]
- Obrecht-Pflumio, S.; Dirheimer, G. In vitro DNA and dGMP adducts formation caused by ochratoxin A. Chem-Biol. Interact. 2000, 127, 29–44. [Google Scholar] [CrossRef]
- Obrecht-Pflumio, S.; Dirheimer, G. Horseradish peroxidase mediates DNA and deoxyguanosine 3'-monophosphate adduct formation in the presence of ochratoxin A. Arch. Toxicol. 2001, 75, 583–590. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Chakor, K.; Creppy, E.; Dirheimer, G. DNA Adduct Formation in Mice Treated with Ochratoxin A. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours, Scientific Publications No. 115; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC Press: Lyon, France, 1991; pp. 245–253. [Google Scholar]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Kane, A.; Creppy, E.E.; Dirheimer, G. Differential DNA adduct formation and disappearance in three mice tissues after treatment by the mycotoxin, ochratoxin A. Mutat. Res. 1993, 289, 265–273. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Gabryelski, W.; Manderville, W. Formation of 2'-deoxyguanosine-carbon-8-bound ochratoxin A adduct in rat kidney DNA. Mol. Nutr. Food Res. 2009, 53, 154–155. [Google Scholar] [CrossRef]
- Mantle, P.G.; Faucet-Marquis, V.; Manderville, R.A.; Squillaci, B.; Pfohl-Leszkowicz, A. Structures of covalent adducts between DNA and ochratoxin A: A new factor in debate about genotoxicity and human risk assessment. Chem. Res. Toxicol. 2010, 23, 89–98. [Google Scholar] [CrossRef]
- Atroshi, F.; Biese, I.; Saloniemi, H.; Ali-Vehmas, T.; Saari, S.; Rizzo, A.; Veijalainen, P. Significance of apoptosis and its relationship to antioxidants after ochratoxin A administration in mice. J. Pharm. Pharmaceut. Sci. 2000, 3, 281–291. [Google Scholar]
- Faucet, V.; Pfohl-Leszkowicz, A.; Dai, J.; Castegnaro, M.; Manderville, R.A. Evidence for covalent DNA adduction by ochratoxin A following chronic exposure to rat and subacute exposure to pig. Chem. Res. Toxicol. 2004, 17, 1289–1296. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Castegnaro, M.; Nikolov, I.G.; Chernozemski, I.N.; Bartsch, H.; Betbeder, A.M.; Creppy, E.E.; Dirheimer, G. Ochratoxin A-Related DNA Adducts in Urinary Tract Tumours of Bulgarian Subjects. In Postlabelling Methods for Detection of DNA Adducts, Scientific Publication 124; Phillips, D.H., Castegnaro, M., Bartsch, H., Eds.; International Agency for Research on Cancer: Lyon, France, 1993; pp. 141–148. [Google Scholar]
- Macgeorge, K.M.; Mantle, P.G. Nephrotoxicity of Penicillium aurantiogriseum and P. commune from an endemic nephropathy areas of Yugoslavia. Mycopathologia 1990, 112, 139–145. [Google Scholar] [CrossRef]
- Yeulet, S.E.; Mantle, P.G.; Rudge, M.S.; Greig, J.B. Nephrotoxiciy of Penicillium aurantiogriseum, a possible factor in the aetiology of Balkan Endemic Nephropathy. Mycopathologia 1988, 102, 21–30. [Google Scholar] [CrossRef]
- Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. Cleavage of zearalenone-glycoside, a masked mycotoxin, during digestion in swine. J. Vet. Med. B. 1990, 37, 236–240. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [Green Version]
- Berthiller, F.; Schuhmacher, R.; Adam, G.; Krska, R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009, 395, 1243–1252. [Google Scholar] [CrossRef]
- Sewram, V.; Shephard, G.S.; Marasas, W.F.O.; Penteado, M.; De Castro, M.F. Improving extraction of Fumonisin mycotoxins from Brazilian corn-based infant foods. J. Food Prot. 2003, 66, 85–859. [Google Scholar]
- Kim, E.K.; Scott, P.M.; Lau, B.P. Hidden fumonisin in corn flakes. Food Addit. Contam. 2003, 20, 161–169. [Google Scholar] [CrossRef]
- Park, J.W.; Scott, P.M.; Lau, B.P.; Lewis, D.A. Analysis of heat processed corn foods for fumonisins and bound fumonisins. Food Addit. Contam. 2004, 21, 1168–1178. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Mangia, M.; Berthiller, F.; Molinelli, A.; Sulyok, M.; Schuhmacher, R.; Krska, R.; Galaverna, G.; Dossena, A.; Marchelli, R. Difficulties in fumonisin determination: The issue of hidden fumonisins. Anal. Bioanal. Chem. 2009, 395, 1335–1345. [Google Scholar] [CrossRef]
- Ruhland, M.; Engelhardt, G.; Schaefer, W.; Wallnoefer, P.R. Transformation of the mycotoxin ochratoxin A in plants: 1. Isolation and identification of metabolites formed in cell suspension cultures of wheat and maize. Nat. Tox. 1996, 4, 254–260. [Google Scholar]
- Stormer, F.C.; Pederson, J.I. Formation of 4-hydroxy-ochratoxin A from ochratoxin A by rat liver microsomes. Appl. Environ. Microbiol. 1980, 39, 971–975. [Google Scholar]
- Storen, O.; Holm, H.; Stormer, F.C. Metabolism of ochratoxin A by rats. Appl. Environ. Microbiol. 1982, 44, 785–789. [Google Scholar]
- Petkova-Bocharova, T.; Faucet, V.; Pfohl-Leszkowicz, A.; Mantle, P.G. Analysis for DNA adducts, ochratoxin A content and enzymes expression in kidneys of pigs exposed to mild experimental chronic ochratoxicosis. Facta Univers. Series: Med. Biol. 2003, 10, 111–115. [Google Scholar]
- Tozlovanu, M.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Manderville, R. Genotoxicity of the hydroquinone metabolite of ochratoxin A: Structure-activity relationships for covalent DNA adduction. Chem. Res. Toxicol. 2006, 19, 1241–1247. [Google Scholar] [CrossRef]
- Tozlovanu, M.; Canadas, D.; Pfohl-Leszkowicz, A.; Frenette, C.; Paugh, R.; Manderville, R. Glutathione conjugates of ochratoxin A as biomarkers of exposure. Arh. Hig. Rada Toksikol. 2012, 63, 417–427. [Google Scholar]
- The Nordic Working Group on Food Toxicology and Risk Evaluation(NNT), Health Evaluation of Ochratoxin A in Food Products. In Nordiske Seminar-og Arbeidsrapporter; Nordic Council of Ministers: Copenhagen, Denmark, 1991; pp. 1–29.
- Stojkovic, R.; Hult, K.; Gamulin, S.; Plestina, R. High affinity binding of ochratoxin A to plasma constituent. Biochem. Int. 1984, 9, 33–38. [Google Scholar]
- Dai, J.; Park, G.; Perry, J.; Ilichev, Y.; Bow, D.; Pritchard, J.; Faucet, V.; Pfohl-Leszkowicz, A.; Manderville, R.; Simon, J. Molecular aspects of the transport and toxicity of ochratoxin A. Accounts Chem. Res. 2004, 37, 874–881. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T.; Scott, P.M. Risk assessment of the mycotoxin ochratoxin A. Biomed. Environ. Sci. 1989, 2, 179–248. [Google Scholar]
- Hagelberg, S.; Fuchs, R.; Hult, K. Toxicokinetics of ochratoxin A in several species and its plasma-binding properities. J. Appl. Toxicol. 1989, 9, 91–96. [Google Scholar] [CrossRef]
- Macanovic, M.; Rezakovic, D.; Basagic, E.; Brkic, N. Endemic (Balkan) Nephropathy. Lancet 1976, 307, 541. [Google Scholar]
- Ferluga, D.; Vizjak, A.; Hvala, A.; Vodovnic, A.; Trnacevic, S.; Halibassic, A. A Kidney Biopsy Study of Early Endemic Nephropathy. In Endemic Nephropathy in Croatia; Cvoriscec, D., Ceovic, S., Stavljenic-Rukavina, A., Eds.; Academia Croatica Scientiarum Medicarum: Zagreb, Yugoslavia, 1996; pp. 43–72. [Google Scholar]
- Aleraj, B. A Study of the Viral Etiology of Endemic Nephropathy. In Endemic Nephropathy in Croatia; Cvoriscec, D., Ceovic, S., Stavljenic-Rukavina, A., Eds.; Academia Croatica Scientiarum Medicarum: Zagreb, Yugoslavia, 1996; pp. 23–29. [Google Scholar]
- Dimitrov, T. Balkan endemic nephropathy; Nedijalkov, S., Ed.; Publishing House of the Bulgarian Academy of Sciences: Sofia, Bulgaria, 1984; pp. 1–84. [Google Scholar]
- Suzuki, S.; Kozuka, Y.; Satoh, T.; Yamazaki, M. Studies of the nephrotoxicity of ochratoxin A in rats. Toxicol. Appl. Pharm. 1975, 34, 479–490. [Google Scholar] [CrossRef]
- Hult, K.; Fuchs, R. Analysis and Dynamics of Ochratoxin A in Biological Systems. In Mycotoxins and Phycotoxins. Sixth International IUPAC Symposium on Mycotoxins and Phycotoxins; Steyn, P.S., Vleggaar, R., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1985; pp. 365–376. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stoev, S.D.; Denev, S.A. Porcine/Chicken or Human Nephropathy as the Result of Joint Mycotoxins Interaction. Toxins 2013, 5, 1503-1530. https://doi.org/10.3390/toxins5091503
Stoev SD, Denev SA. Porcine/Chicken or Human Nephropathy as the Result of Joint Mycotoxins Interaction. Toxins. 2013; 5(9):1503-1530. https://doi.org/10.3390/toxins5091503
Chicago/Turabian StyleStoev, Stoycho D., and Stefan A. Denev. 2013. "Porcine/Chicken or Human Nephropathy as the Result of Joint Mycotoxins Interaction" Toxins 5, no. 9: 1503-1530. https://doi.org/10.3390/toxins5091503




