Fusarium Head Blight Control and Prevention of Mycotoxin Contamination in Wheat with Botanicals and Tannic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolate Specific Inhibition of Conidia Germination with TA
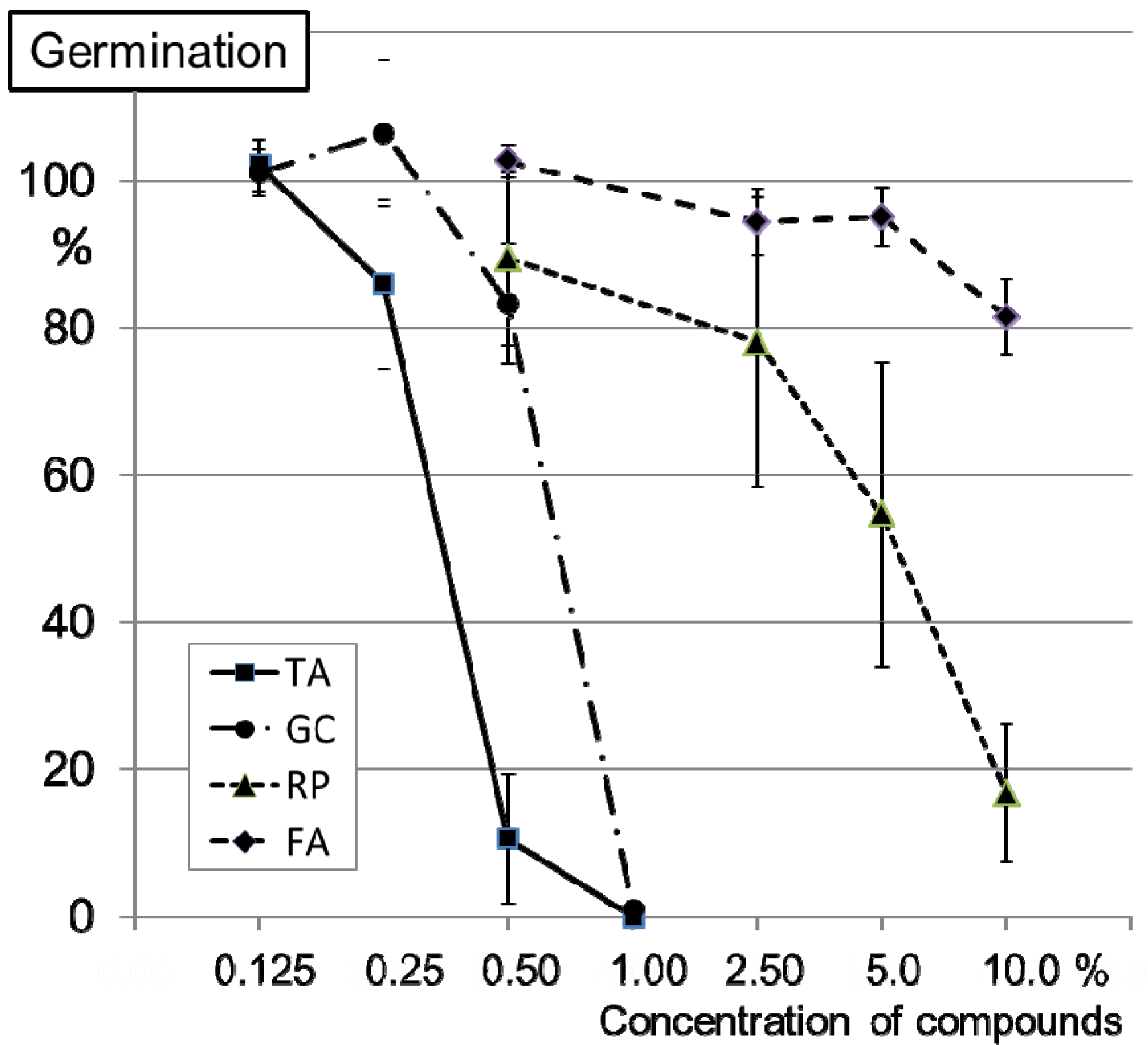
2.2. Inhibition of Conidia Germination of FG0407 with TA and Botanicals

2.3. Inhibition of Mycelial Growth of FG0407 with TA and Botanicals

2.4. Climate Chamber Experiment—Reduction of Disease Severity and Mycotoxins in Artificially Inoculated Wheat
| Treatment Nb. | Ingredient | Identifier | Application vs. inoculation | Concentration | pH | Type of experiment | ||
|---|---|---|---|---|---|---|---|---|
| before | after | % | ±0.2 | A | B | |||
| 1 | Tap water (control 1) | Water | × | - | - | 7.8 | × | × |
| 2 | Acidified water (c. 2) | ac-Water | × | - | - | 4.0 | × | - |
| 3 | Tannic acid | TA b i. | × | - | 5 | 3.8 | × | × |
| 4 | Tannic acid | TA a i. | - | × | 5 | 3.8 | × | - |
| 5 | Tannic acid | TA b+a i. | × | × | 5 | 3.8 | × | × |
| 6 | Galla chinensis | GC b+a i. | × | × | 5 | 3.9 | × | × |
| 7 | Rheum palmatum | RP b+a i. | × | × | 5 | 5.0 | × | × |
| 8 | Frangula alnus | FA b+a i. | × | × | 5 | 5.2 | × | × |
| 9 | Pronto® Plus | PrP b i. | × | - | 0.375 | 8.2 | × | × |

| Parameter | TKW (n = 72) | Disease severity (n = 72) | DON (n = 72) | NIV (n = 36) | AcDON (n = 36) |
|---|---|---|---|---|---|
| Yield | 0.660 | −0.737 | −0.637 | −0.560 | −0.675 |
| TKW | - | −0.919 | −0.932 | −0.664 | −0.814 |
| Disease severity | - | - | 0.948 | 0.877 | 0.907 |
| DON | - | - | - | 0.752 | 0.941 |
| NIV | - | - | - | - | 0.786 |
2.5. Field Experiments with Artificial Inoculation—Reduction of FHB and Mycotoxins in Wheat

| Parameter | Disease severity | FG incidence | FCr incidence | DON | ZEA | NIV |
|---|---|---|---|---|---|---|
| Yield | −0.868 | −0.680 | −0.168 | −0.814 | −0.112 | −0.836 |
| *** | *** | * | *** | NS | *** | |
| Disease severity (FHB) | - | 0.703 | 0.117 | 0.879 | 0.333 | 0.849 |
| *** | NS | *** | *** | *** | ||
| FG incidence | - | - | 0.228 | 0.775 | 0.348 | 0.637 |
| - | - | ** | *** | *** | *** | |
| FCr incidence | - | - | - | 0.254 | 0.133 | 0.370 |
| - | - | - | ** | NS | *** | |
| DON | - | - | - | - | 0.475 | 0.893 |
| - | - | - | - | *** | *** | |
| ZEA | - | - | - | - | - | 0.316 |
| - | - | - | - | - | *** |
2.6. Field Experiments with Semi-Natural Inoculation—Reduction of FHB and Mycotoxins in Wheat

3. Experimental Section
3.1. Fungal Isolates, Growth Conditions and Antifungal Agents
3.2. Isolate Specific Inhibition of Conidia Germination with TA
3.3. Inhibition of Conidia Germination of FG0407 with TA and Botanicals
3.4. Inhibition of Mycelial Growth of FG0407 with TA and Botanicals
3.5. Climate Chamber Experiment—Reduction of Disease Severity and Mycotoxins in Artificially Inoculated Wheat
3.6. Field Experiments with Artificial Inoculation—Reduction of FHB and Mycotoxins in Wheat
3.7. Field Experiments with Semi-Natural Inoculation—Reduction of FHB and Mycotoxins in Wheat
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gale, L.R. Population Biology of Fusarium Species Causing Head Blight of Grain Crops. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; American Phytopathological Society (APS) Press: St. Paul, MN, USA, 2003; pp. 120–143. [Google Scholar]
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Wegulo, S. Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union. 2007, L255, pp. 14–17. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:255:0014:0017:EN:PDF (accessed on 20 February 2011).
- Vogelgsang, S.; Hecker, A.; Musa, T.; Dorn, B.; Forrer, H.R. On-farm experiments over 5 years in a grain maize/winter wheat rotation: Effect of maize residue treatments on Fusarium graminearum infection and deoxynivalenol contamination in wheat. Mycotoxin Res. 2011, 27, 81–96. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals (maize, sorghum, forage grasses and dicotyledonous crops). Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Edwards, S.G.; Godley, N.P. Reduction of Fusarium head blight and deoxynivalenol in wheat with early fungicide applications of prothioconazole. Food Addit. Contam. A 2010, 27, 629–635. [Google Scholar] [CrossRef]
- Forrer, H.-R.; Hecker, A.; Külling, C.; Kessler, P.; Jenny, E.; Krebs, H. Effect of fungicides on fusaria of wheat. Agrarforschung 2000, 7, 258–263. (in German). [Google Scholar]
- Musa, T.; Hecker, A.; Vogelgsang, S.; Forrer, H.R. Forecasting of Fusarium head blight and deoxynivalenol content in winter wheat with FusaProg. EPPO Bull. 2007, 37, 283–289. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium mycotoxin content of uk organic and conventional wheat. Food Addit. Contam. A 2009, 26, 496–506. [Google Scholar] [CrossRef]
- Bernhoft, A.; Clasen, P.; Kristoffersen, A.; Torp, M. Less Fusarium infestation and mycotoxin contamination in organic than in conventional cereals. Food Addit. Contam. A 2010, 27, 842–852. [Google Scholar] [CrossRef]
- Birzele, B.; Meier, A.; Hindorf, H.; Krämer, J.; Dehne, H.-W. Epidemiology of Fusarium infection and deoxynivalenol content in winter wheat in the Rhineland, Germany. Eur. J. Plant Pathol. 2002, 108, 667–673. [Google Scholar] [CrossRef]
- Dierauer, H.; Böhler, D. Direct Seeding of Maize in Organic Farming; Research Institute of Organic Agriculture: Frick, Switzerland, 2012; pp. 1–12. [Google Scholar]
- Peigné, J. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–124. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.E.; Løes, A.K.; Kristoffersen, A.B. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. A 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Dorn, B.; Musa, T.; Krebs, H.; Fried, P.M.; Forrer, H.R. Control of late blight in organic potato production: Evaluation of copper-free preparations under field, growth chamber and laboratory conditions. Eur. J. Plant Pathol. 2007, 119, 217–240. [Google Scholar] [CrossRef]
- Hu, T.; Wang, S.; Cao, K.; Forrer, H.-R. Inhibitory effects of several Chinese medicinal herbs against Phytophthora infestans. ISHS Acta Hortic. 2009, 834, 205–210. [Google Scholar]
- Bassin, S.; Forrer, H. Field screening of copper free fungicides against potato late blight. Agrarforschung 2001, 8, 124–129. (in German). [Google Scholar]
- Krebs, H.; Musa, T.; Forrer, H.-R. Control of Potato Late Blight with Extracts and Suspensions of Buckthorn Bark. In Proceedings of the Zwischen Tradition und Globalisierung-9 Wissenschaftstagung Ökologischer Landbau, Universität Hohenheim, Stuttgart, Germany, 20–23 March 2007. (in German).
- Vogelgsang, S.; Bänziger, I.; Krebs, H.; Legro, R.J.; Sanchez-Sava, V.; Forrer, H.-R. Control of Microdochium majus in winter wheat with botanicals—From laboratory to the field. Plant Pathol. 2013, 62, 1020–1029. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.P.; Zhang, G.Z.; Luo, Y.C. Identification and structure-activity relationship of gallotannins separated from Galla chinensis. Food Sci. Technol. 2009, 42, 1289–1295. [Google Scholar]
- Maleš, Ž.; Kremer, D.; Randić, Z.; Randić, M.; Pilepić, K.; Bojić, M. Quantitative analysis of glucofrangulins and phenolic compounds in Croatian Rhamnus and Frangula species. Acta Biol. Cracoviensia Ser. Bot. 2010, 52, 108–113. [Google Scholar]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar]
- Field, J.A.; Lettinga, G. Toxicity of tannic compounds to microorganisms. Basic Life Sci. 1992, 59, 673–692. [Google Scholar]
- Ahn, Y.J.; Lee, H.S.; Oh, H.S.; Kim, H.T.; Lee, Y.H. Antifungal activity and mode of action of Galla rhois-derived phenolics against phytopathogenic fungi. Pestic. Biochem. Physiol. 2005, 81, 105–112. [Google Scholar] [CrossRef]
- Knudson, L. Tannic acid fermentation. I. J. Biol. Chem. 1913, 14, 159–184. [Google Scholar]
- Henis, Y.; Tagari, H.; Volcani, R. Effect of water extracts of carob pods, tannic acid, and their derivatives on the morphology and growth of microorganisms. Appl. Microbiol. Biotechnol. 1964, 12, 204–209. [Google Scholar]
- McKeehen, J.D.; Busch, R.H.; Fulcher, R.G. Evaluation of wheat (Triticum aestivum L.) phenolic acids during grain development and their contribution to Fusarium resistance. J. Agric. Food Chem. 1999, 47, 1476–1482. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.V.; Arul, J.; Angers, P.; Couture, L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J. Agric. Food Chem. 1999, 47, 1208–1216. [Google Scholar] [CrossRef]
- Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol. Mol. Plant Pathol. 2005, 66, 108–115. [Google Scholar] [CrossRef]
- Veloz-García, R.; Marín-Martínez, R.; Veloz-Rodríguez, R.; Rodríguez-Guerra, R.; Torres-Pacheco, I.; González-Chavira, M.M.; Anaya-López, J.L.; Guevara-Olvera, L.; Feregrino-Pérez, A.A.; Loarca-Piña, G.; et al. Antimicrobial activities of cascalote (Caesalpinia cacalaco) phenolics-containing extract against fungus Colletotrichum lindemuthianum. Ind. Crop. Prod. 2010, 31, 134–138. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Sulyok, M.; Bänziger, I.; Krska, R.; Schuhmacher, R.; Forrer, H.R. Effect of fungal strain and cereal substrate on the in vitro mycotoxin production by Fusarium poae and Fusarium avenaceum. Food Addit. Contam. 2008, 25, 745–757. [Google Scholar] [CrossRef]
- Gindro, K.G.; Godard, S.; De Groote, I.; Viret, O.; Forrer, H.-R.; Dorn, B. Is it possible to induce grapevine defence mechanisms? A new method to evaluate the potential of elicitors. Rev. Suisse Vitic. Arboric. Hortic. 2007, 39, 377–383. [Google Scholar]
- Kessmann, H.; Staub, T.; Hofmann, C.; Maetzke, T.; Herzog, J.; Ward, E.; Uknes, S.; Ryals, J. Induction of systemic acquired disease resistance in plants by chemicals. Annu. Rev. Phytopathol. 1994, 32, 439–459. [Google Scholar] [CrossRef]
- Siranidou, E.; Kang, Z.; Buchenauer, H. Studies on symptom development, phenolic compounds and morphological defence responses in wheat cultivars differing in resistance to Fusarium head blight. J. Phytopathol. 2002, 150, 200–208. [Google Scholar] [CrossRef]
- Mackintosh, C.A.; Garvin, D.F.; Radmer, L.E.; Heinen, S.J.; Muehlbauer, G.J. A model wheat cultivar for transformation to improve resistance to Fusarium Head Blight. Plant Cell Rep. 2006, 25, 313–319. [Google Scholar] [CrossRef]
- Chen, K.-S.; Hsiao, Y.-C.; Kuo, D.-Y.; Chou, M.-C.; Chu, S.-C.; Hsieh, Y.-S.; Lin, T.-H. Tannic acid-induced apoptosis and -enhanced sensitivity to arsenic trioxide in human leukemia HL-60 cells. Leuk. Res. 2009, 33, 297–307. [Google Scholar] [CrossRef]
- Chen, J.C.; Ho, T.Y.; Chang, Y.S.; Wu, S.L.; Hsiang, C.Y. Anti-diarrheal effect of Galla Chinensis on the Escherichia coli heat-labile enterotoxin and ganglioside interaction. J. Ethnopharmacol. 2006, 103, 385–391. [Google Scholar] [CrossRef]
- Vargas, M.H. ED50plus (v1.0): Software to Create and Analyze Dose-Response Curves, 2000. Available online: http://www.softlookup.com/display.asp?-id=2972 (accessed on 20 February 2014).
- Vogelgsang, S.; Sulyok, M.; Hecker, A.; Jenny, E.; Krska, R.; Schuhmacher, R.; Forrer, H.R. Toxigenicity and pathogenicity of Fusarium poae and Fusarium avenaceum on wheat. Eur. J. Plant Pathol. 2008, 122, 265–276. [Google Scholar] [CrossRef]
- Bugbee, B.; Koerner, G. Yield comparisons and unique characteristics of the dwarf wheat cultivar ‘USU-Apogee’. Adv. Space Res. 1997, 20, 1891–1894. [Google Scholar] [CrossRef]
- Pfeiffer, B.; Alt, S.; Schulz, C.; Hein, B.; Kollar, A. Investigations on Alternative Substances for Control of Apple Scab—Results from Conidia Germinating Tests and Experiments with Plant Extracts. In Proceedings of the 11th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 3–5 Febuary 2004; pp. 101–107.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Forrer, H.-R.; Musa, T.; Schwab, F.; Jenny, E.; Bucheli, T.D.; Wettstein, F.E.; Vogelgsang, S. Fusarium Head Blight Control and Prevention of Mycotoxin Contamination in Wheat with Botanicals and Tannic Acid. Toxins 2014, 6, 830-849. https://doi.org/10.3390/toxins6030830
Forrer H-R, Musa T, Schwab F, Jenny E, Bucheli TD, Wettstein FE, Vogelgsang S. Fusarium Head Blight Control and Prevention of Mycotoxin Contamination in Wheat with Botanicals and Tannic Acid. Toxins. 2014; 6(3):830-849. https://doi.org/10.3390/toxins6030830
Chicago/Turabian StyleForrer, Hans-Rudolf, Tomke Musa, Fabienne Schwab, Eveline Jenny, Thomas D. Bucheli, Felix E. Wettstein, and Susanne Vogelgsang. 2014. "Fusarium Head Blight Control and Prevention of Mycotoxin Contamination in Wheat with Botanicals and Tannic Acid" Toxins 6, no. 3: 830-849. https://doi.org/10.3390/toxins6030830






