Inhibitory Effects of Respiration Inhibitors on Aflatoxin Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibitory Activity of Natural Respiration Inhibitors on Aflatoxin Production

| Classification | Target | Compound | IC50 (µM) * |
|---|---|---|---|
| Natural product | complex I | rotenone | 13 |
| complex II | siccanin | 13 | |
| atpenin A5 | 9.7 | ||
| complex III | antimycin A | 7.2 | |
| Synthetic miticide | complex I | pyridaben | 0.01 |
| tolfenpyrad | 0.18 | ||
| complex II | mepronil | 23 | |
| cyflumetofen | >300 | ||
| complex III | fluacrypyrim | 0.07 | |
| acequinocyl | 1.7 | ||
| bifenazate | 20 | ||
| Synthetic fungicide | complex II | boscalid | <0.01 |
| complex III | Pyribencarb | 0.43 | |
| cyazofamid | 0.70 | ||
| pyraclostrobin | 0.06 | ||
| kresoxim-methyl | 0.06 | ||
| azoxystrobin | 0.40 | ||
| trifloxystrobin | 0.90 | ||
| picoxystrobin | 8.6 | ||
| metominostrobin | 9.9 |
2.2. Inhibitory Activities of Synthetic Pesticides with Respiration Inhibitory Activity on Aflatoxin Production

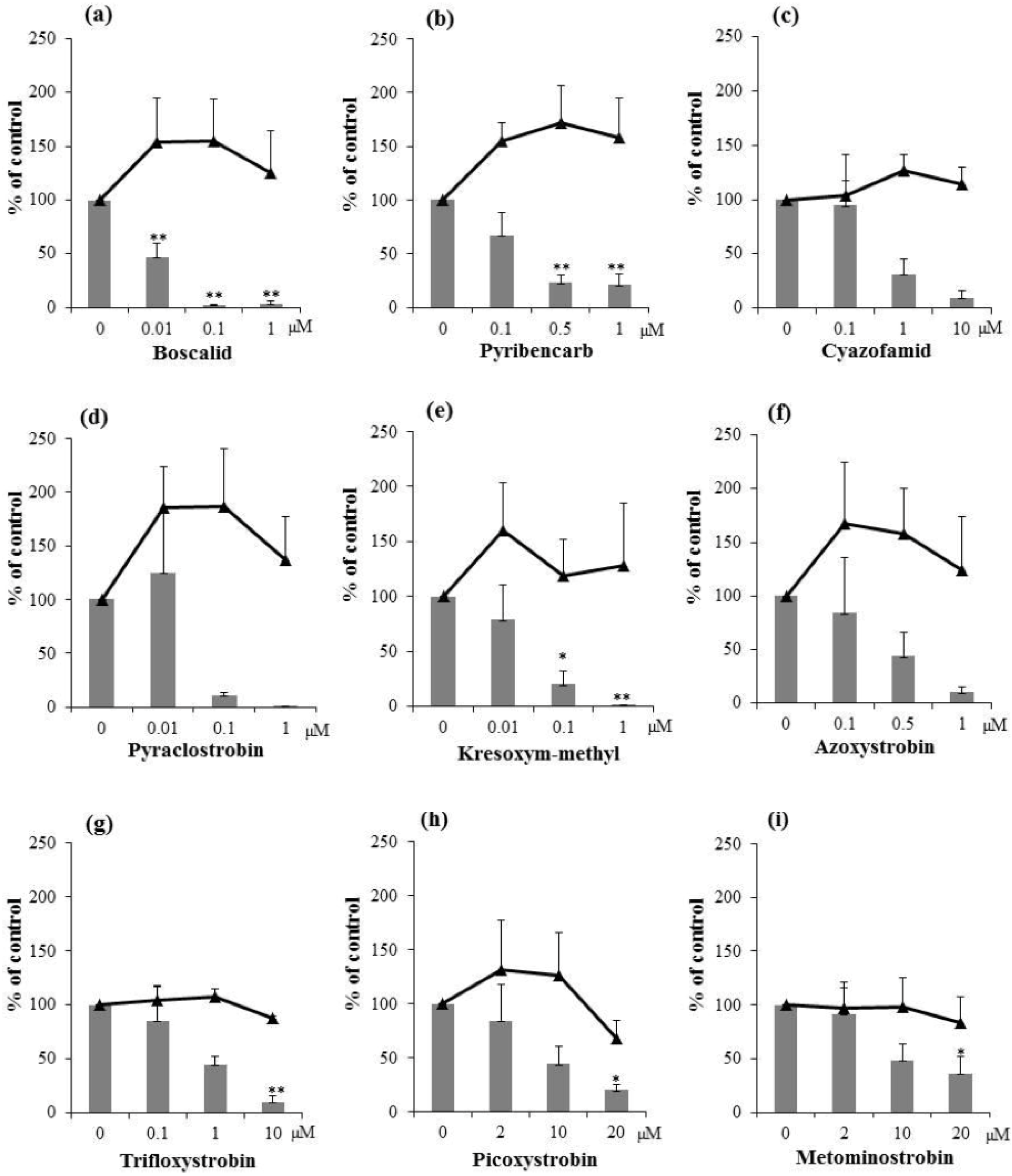
3. Experimental Section
3.1. Strains, Chemicals, and Culture Conditions
3.2. Analysis of Aflatoxin
3.3. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–616. [Google Scholar] [CrossRef]
- Richard, J.L.; Payne, G.A. Mycotoxins: Risks in Plant, Animal and Human Systems; Council for Agricultural Science and Technology Press: Ames, IA, USA, 2003. [Google Scholar]
- Snelders, E.; Huis in’t Velt, R.A.G.; Rijs, A.J.M.M.; Kema, G.H.J.; Melchers, W.J.G.; Verweij, P.E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 2009, 75, 4053–4057. [Google Scholar] [CrossRef]
- Abbas, H.K.; Wilkinson, J.R.; Zablotowicz, R.M.; Accinelli, C.; Abel, C.A.; Bruns, H.A.; Weaver, M.A. Ecology of Aspergillus flavus, regulation of aflatoxin production, and management strategies to reduce aflatoxin contamination of corn. Toxin Rev. 2009, 28, 142–153. [Google Scholar] [CrossRef]
- Dutton, M.F.; Anderson, M.S. Inhibition of aflatoxin biosynthesis by organophosphorous compounds. J. Food Prot. 1980, 43, 381–384. [Google Scholar]
- Sakuda, S. Mycotoxin production inhibitors from natural products. Mycotoxins 2010, 60, 79–86. [Google Scholar] [CrossRef]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef]
- Jermnak, U.; Yoshinari, T.; Sugiyama, Y.; Tsuyuki, R.; Nagasawa, H.; Sakuda, S. Isolation of methyl syringate as a specific aflatoxin production inhibitor from the essential oil of Betula alba and aflatoxin production inhibitory activities of its related compunds. Int. J. Food Microbiol. 2012, 153, 339–344. [Google Scholar] [CrossRef]
- Ōmura, S.; Tomoda, H.; Kimura, K.; Zhen, D.-Z.; Kunagai, H.; Igarashi, K.; Imamura, N.; Takahashi, Y.; Tanaka, Y.; Iwai, Y. Atpenins, new antifungal antibiotics produced by Penicillium sp. J. Antibiot. 1988, 41, 1769–1773. [Google Scholar] [CrossRef]
- Ishibashi, K. Studies on antibiotics from Helminthosporium sp. fungi. VII. Siccanin, a new antifungal antibiotic produced by Helminthosporium siccans. J. Antibiot. 1961, A15, 161–167. [Google Scholar]
- Mogi, T.; Kawakami, T.; Arai, H.; Igarashi, Y.; Matsushita, K.; Mori, M.; Shiomi, K.; Ōmura, S.; Harada, S.; Kita, K. Siccanin rediscovered as a species-selective succinate dehydrogenase inhibitor. J. Biochem. 2009, 146, 383–387. [Google Scholar] [CrossRef]
- Miyadera, H.; Shiomi, K.; Ui, H.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Miyoshi, H.; Osanai, A.; Kita, K.; Ōmura, S. Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase). Proc. Natl. Acad. Sci. USA 2003, 100, 473–477. [Google Scholar] [CrossRef]
- Lummen, P. Complex I inhibitors as insecticides and acaricides. Biochem. Biophys. Acta 1998, 1364, 287–296. [Google Scholar]
- Ikemi, N. Development of a new acaricide, cyflumetofen. J. Pestic. Chem. 2012, 37, 275–282. [Google Scholar]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dedydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Balba, H. Review of strobilurin fungicide chemicals. J. Environ. Sci. Health Part B: Pestic. Food Contam. Agric. Wastes 2007, 42, 441–451. [Google Scholar] [CrossRef]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J. Chemosensitization of aflatoxigenic fingi to antimycin A and strobilurin using salicylaldehyde, a volatile natural compound targeting cellular antioxidation system. Mycopathologia 2011, 171, 291–298. [Google Scholar] [CrossRef]
- Yoshinari, T.; Noda, Y.; Yoda, K.; Sezaki, H.; Nagasawa, H.; Sakuda, S. Inhibitory activity of blasticidin A, a strong aflatoxin production inhibitor, on protein synthesis of yeast: Selective inhibition of aflatoxin production by protein synthesis inhibitors. J. Antibiot. 2010, 63, 309–314. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sakuda, S.; Prabowo, D.F.; Takagi, K.; Shiomi, K.; Mori, M.; Ōmura, S.; Nagasawa, H. Inhibitory Effects of Respiration Inhibitors on Aflatoxin Production. Toxins 2014, 6, 1193-1200. https://doi.org/10.3390/toxins6041193
Sakuda S, Prabowo DF, Takagi K, Shiomi K, Mori M, Ōmura S, Nagasawa H. Inhibitory Effects of Respiration Inhibitors on Aflatoxin Production. Toxins. 2014; 6(4):1193-1200. https://doi.org/10.3390/toxins6041193
Chicago/Turabian StyleSakuda, Shohei, Diyan Febri Prabowo, Keiko Takagi, Kazuro Shiomi, Mihoko Mori, Satoshi Ōmura, and Hiromichi Nagasawa. 2014. "Inhibitory Effects of Respiration Inhibitors on Aflatoxin Production" Toxins 6, no. 4: 1193-1200. https://doi.org/10.3390/toxins6041193




