An N-Terminal Fragment of Yeast Ribosomal Protein L3 Inhibits the Cytotoxicity of Pokeweed Antiviral Protein in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids and Yeast Growth Conditions
2.2. Yeast Cell Viability Assay
2.3. Cell Fractionation and Immunoblot Analysis of Protein Expression
2.4. rRNA Depurination Assay
2.5. Real-Time PCR Analysis
2.6. Purification of PAP Protein from PAP or PAP/L3Δ99 Yeast Cells
2.7. Surface Plasmon Resonance (SPR) Analysis
2.8. Co-Immunoprecipitation
3. Results
3.1. Cytotoxicity of PAP is Reduced in Yeast Cells Co-Transformed with PAP and L3Δ99

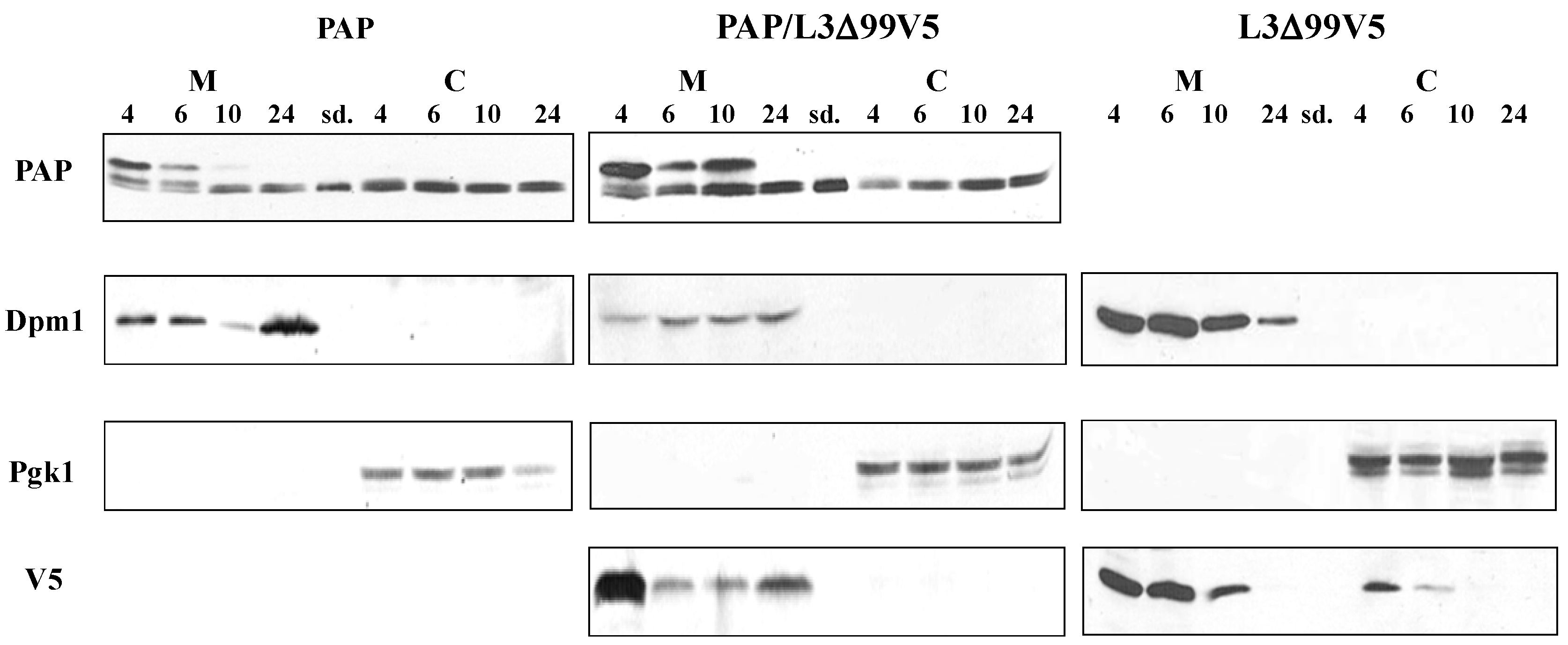
3.2. PAP Protein Expression Level is Elevated, but Ribosome Depurination is Reduced in Yeast Co-Expressing PAP and L3Δ99

3.3. L3Δ21 Attenuates the Cytotoxicity of PAP


3.4. PAP mRNA and Protein Level is Elevated, L3Δ mRNA and Protein Level is Reduced in Yeast Co-Transformed with PAP and L3Δ

3.5. PAP Interacts with L3Δ Peptide



4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Endo, Y.; Tsurugi, K. The RNA n-glycosidase activity of ricin a-chain. The characteristics of the enzymatic activity of ricin a-chain with ribosomes and with rRNA. J. Biol. Chem. 1988, 263, 8735–8739. [Google Scholar]
- Hudak, K.A.; Wang, P.; Tumer, N.E. A novel mechanism for inhibition of translation by pokeweed antiviral protein: Depurination of the capped RNA template. RNA 2000, 6, 369–380. [Google Scholar] [CrossRef]
- Montanaro, L.; Sperti, S.; Mattioli, A.; Testoni, G.; Stirpe, F. Inhibition by ricin of protein synthesis in vitro. Inhibition of the binding of elongation factor 2 and of adenosine diphosphate-ribosylated elongation factor 2 to ribosomes. Biochem. J. 1975, 146, 127–131. [Google Scholar]
- Osborn, R.W.; Hartley, M.R. Dual effects of the ricin a chain on protein synthesis in rabbit reticulocyte lysate. Inhibition of initiation and translocation. Eur. J. Biochem. 1990, 193, 401–407. [Google Scholar] [CrossRef]
- Hudak, K.A.; Dinman, J.D.; Tumer, N.E. Pokeweed antiviral protein accesses ribosomes by binding to L3. J. Biol. Chem. 1999, 274, 3859–3864. [Google Scholar] [CrossRef]
- Di, R.; Tumer, N.E. Expression of a truncated form of ribosomal protein l3 confers resistance to pokeweed antiviral protein and the fusarium mycotoxin deoxynivalenol. Mol. Plant Microbe Interact. 2005, 18, 762–770. [Google Scholar] [CrossRef]
- Kornitzer, D. Monitoring protein degradation. Methods Enzymol. 2002, 351, 639–647. [Google Scholar] [CrossRef]
- Parikh, B.A.; Coetzer, C.; Tumer, N.E. Pokeweed antiviral protein regulates the stability of its own mRNA by a mechanism that requires depurination but can be separated from depurination of the alpha-sarcin/ricin loop of rRNA. J. Biol. Chem. 2002, 277, 41428–41437. [Google Scholar] [CrossRef]
- Parikh, B.A.; Baykal, U.; Di, R.; Tumer, N.E. Evidence for retro-translocation of pokeweed antiviral protein from endoplasmic reticulum into cytosol and separation of its activity on ribosomes from its activity on capped RNA. Biochemistry 2005, 44, 2478–2490. [Google Scholar] [CrossRef]
- Rajamohan, F.; Mao, C.; Uckun, F.M. Binding interactions between the active center cleft of recombinant pokeweed antiviral protein and the alpha-sarcin/ricin stem loop of ribosomal RNA. J. Biol. Chem. 2001, 276, 24075–24081. [Google Scholar] [CrossRef]
- Otto, J.J.; Lee, S.W. Immunoprecipitation methods. Methods Cell Biol. 1993, 37, 119–127. [Google Scholar] [CrossRef]
- Harley, S.M.; Beevers, H. Ricin inhibition of in vitro protein synthesis by plant ribosomes. Proc. Natl. Acad. Sci. USA 1982, 79, 5935–5938. [Google Scholar] [CrossRef]
- Chiou, J.C.; Li, X.P.; Remacha, M.; Ballesta, J.P.; Tumer, N.E. The ribosomal stalk is required for ribosome binding, depurination of the rRNA and cytotoxicity of ricin a chain in saccharomyces cerevisiae. Mol. Microbiol. 2008, 70, 1441–1452. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.P.; Tumer, N.E. N-glycosylation does not affect the catalytic activity of ricin a chain but stimulates cytotoxicity by promoting its transport out of the endoplasmic reticulum. Traffic 2012, 13, 1508–1521. [Google Scholar] [CrossRef]
- Hudak, K.A.; Bauman, J.D.; Tumer, N.E. Pokeweed antiviral protein binds to the cap structure of eukaryotic mRNA and depurinates the mRNA downstream of the cap. RNA 2002, 8, 1148–1159. [Google Scholar] [CrossRef]
- Vivanco, J.M.; Tumer, N.E. Translation inhibition of capped and uncapped viral RNAs mediated by ribosome-inactivating proteins. Phytopathology 2003, 93, 588–595. [Google Scholar] [CrossRef]
- Tourlakis, M.E.; Karran, R.A.; Desouza, L.; Siu, K.W.; Hudak, K.A. Homodimerization of pokeweed antiviral protein as a mechanism to limit depurination of pokeweed ribosomes. Mol. Plant Pathol. 2010, 11, 757–767. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Di, R.; Tumer, N.E. An N-Terminal Fragment of Yeast Ribosomal Protein L3 Inhibits the Cytotoxicity of Pokeweed Antiviral Protein in Saccharomyces cerevisiae. Toxins 2014, 6, 1349-1361. https://doi.org/10.3390/toxins6041349
Di R, Tumer NE. An N-Terminal Fragment of Yeast Ribosomal Protein L3 Inhibits the Cytotoxicity of Pokeweed Antiviral Protein in Saccharomyces cerevisiae. Toxins. 2014; 6(4):1349-1361. https://doi.org/10.3390/toxins6041349
Chicago/Turabian StyleDi, Rong, and Nilgun E. Tumer. 2014. "An N-Terminal Fragment of Yeast Ribosomal Protein L3 Inhibits the Cytotoxicity of Pokeweed Antiviral Protein in Saccharomyces cerevisiae" Toxins 6, no. 4: 1349-1361. https://doi.org/10.3390/toxins6041349



