Intraspecific Variation of Centruroides Edwardsii Venom from Two Regions of Colombia
Abstract
:1. Introduction
2. Results
2.1. Venom Enzymatic Activity

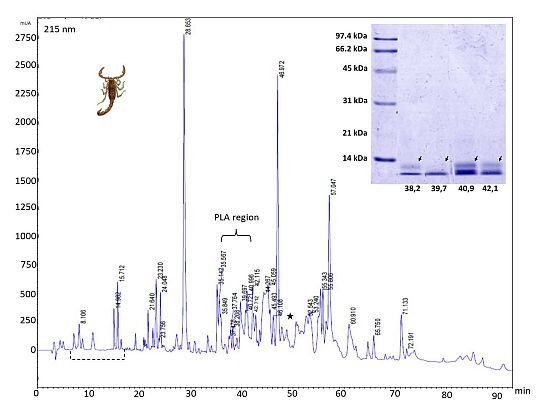
2.2. SDS-PAGE

2.3. Reverse-Phase Chromatography



2.4. Toxicological and Biological Activities
| Venom dose (mg/kg) | C. edwardsii | C. sculpturatus [27] | C. exilicauda [27] |
|---|---|---|---|
| 4.8 | Toxic | Lethal | Non toxic |
| 9.6 | Toxic | Lethal | Non toxic |
| 19.2 | Toxic | Not tested | Toxic |
| Venom concentration | Live larvae | Death larvae | Total | Mortality % |
|---|---|---|---|---|
| C. edwardsii from Antioquia | ||||
| 400 µg | 3 | 2 | 5 | 40 |
| C. edwardsii from Tolima | ||||
| 500 µg | 5 | 0 | 5 | 0 |
| 250 µg | 5 | 0 | 5 | 0 |
| 125 µg | 5 | 0 | 5 | 0 |
| 62.5 µg | 5 | 0 | 5 | 0 |
3. Discussion
4. Experimental Section
4.1. Venom Extraction
4.2. Indirect Hemolytic Activity
4.3. Coagulant Activity
4.4. Proteolytic Activity
4.5. Larvicidal Activity
4.6. Antimicrobial Activity
4.7. Electrophoretic Profile
4.8. Chromatographic Profile
4.9. Toxicity
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwartz, E.F.; Camargos, T.S.; Zamudio, F.Z.; Silva, L.P.; Bloch, C.; Caixeta, F.; Schwartz, C.A.; Possani, L.D. Mass spectrometry analysis, amino acid sequence and biological activity of venom components from the brazilian scorpion opisthacanthus cayaporum. Toxicon 2008, 51, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Velazquez, L.L.; Quintero-Hernandez, V.; Romero-Gutierrez, M.T.; Coronas, F.I.; Possani, L.D. Mass fingerprinting of the venom and transcriptome of venom gland of scorpion centruroides tecomanus. PLoS One 2013, 8, e66486. [Google Scholar] [CrossRef]
- Nastainczyk, W.; Meves, H.; Watt, D.D. A short-chain peptide toxin isolated from centruroides sculpturatus scorpion venom inhibits ether-a-go-go-related gene k(+) channels. Toxicon 2002, 40, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Gurrola, G.B.; Moreno-Hagelsieb, G.; Zamudio, F.Z.; Garcia, M.; Soberon, X.; Possani, L.D. The disulfide bridges of toxin 2 from the scorpion centruroides noxius hoffmann and its three-dimensional structure calculated using the coordinates of variant 3 from centruroides sculpturatus. FEBS Lett. 1994, 347, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Gurrola, G.B.; Rosati, B.; Rocchetti, M.; Pimienta, G.; Zaza, A.; Arcangeli, A.; Olivotto, M.; Possani, L.D.; Wanke, E. A toxin to nervous, cardiac, and endocrine erg k+ channels isolated from centruroides noxius scorpion venom. FASEB J. 1999, 13, 953–962. [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, Nineteenth Informational Supplement; Approved Standard M100-S19; National Committee for Clinical Standards: Wayne, PA, USA, 2009. [Google Scholar]
- Watt, D.D.; Simard, J.M. Neurotoxic proteins in scorpion venom. Toxin Rev. 1984, 3, 181–221. [Google Scholar] [CrossRef]
- Meves, H.; Rubly, N.; Watt, D.D. Voltage-dependent effect of a scorpion toxin on sodium current inactivation. Pflug. Archiv. 1984, 402, 24–33. [Google Scholar] [CrossRef]
- Meves, H.; Simard, J.M.; Watt, D.D. Biochemical and electrophysiological characteristics of toxins isolated from the venom of the scorpion centruroides sculpturatus. J. Phys. 1984, 79, 185–191. [Google Scholar]
- Coronas, F.I.; Balderas, C.; Lopez, L.P.; Possani, L.D.; Gurrola, G.B. Amino acid sequence determination and chemical synthesis of cllerg1 (gamma-ktx1.5), a K+ channel blocker peptide isolated from the scorpion centruroides limpidus limpidus. J. Braz. Chem. Soc. 2005, 16, 404–411. [Google Scholar] [CrossRef]
- Jouirou, B.; Mosbah, A.; Visan, V.; Grissmer, S.; M’Barek, S.; Fajloun, Z.; Van Rietschoten, J.; Devaux, C.; Rochat, H.; Lippens, G. Cobatoxin 1 from centruroides noxius scorpion venom: Chemical synthesis, three-dimensional structure in solution, pharmacology and docking on K+ channels. Biochem. J. 2004, 377, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Olamendi-Portugal, T.; Somodi, S.; Fernandez, J.A.; Zamudio, F.Z.; Becerril, B.; Varga, Z.; Panyi, G.; Gaspar, R.; Possani, L.D. Novel alpha-ktx peptides from the venom of the scorpion centruroides elegans selectively blockade kv1.3 over ikca1 k+ channels of t cells. Toxicon 2005, 46, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Ruiming, Z.; Yibao, M.; Yawen, H.; Zhiyong, D.; Yingliang, W.; Zhijian, C.; Wenxin, L. Comparative venom gland transcriptome analysis of the scorpion lychas mucronatus reveals intraspecific toxic gene diversity and new venomous components. BMC Genomics 2010, 11. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Omran, M.A.; Abdel-Nabi, I.M.; Ueda, H.; McVean, A. Intraspecific variation in the egyptian scorpion scorpio maurus palmatus venom collected from different biotopes. Toxicon 2009, 53, 349–359. [Google Scholar] [CrossRef]
- Cao, L.Y.; Dai, C.; Li, Z.J.; Fan, Z.; Song, Y.; Wu, Y.L.; Cao, Z.J.; Li, W.X. Antibacterial activity and mechanism of a scorpion venom peptide derivative in vitro and in vivo. PLoS One 2012, 7, e40135. [Google Scholar] [CrossRef]
- Cao, L.Y.; Li, Z.J.; Zhang, R.H.; Wu, Y.L.; Li, W.X.; Cao, Z.J. Stct2, a new antibacterial peptide characterized from the venom of the scorpion scorpiops tibetanus. Peptides 2012, 36, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Cociancich, S.; Goyffon, M.; Bontems, F.; Bulet, P.; Bouet, F.; Menez, A.; Hoffmann, J. Purification and characterization of a scorpion defensin, a 4kda antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem. Biophys. Res. Commun. 1993, 194, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Corzo, G.; Escoubas, P.; Villegas, E.; Barnham, K.J.; He, W.L.; Norton, R.S.; Nakajima, T. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion pandinus imperator. Biochem. J. 2001, 359, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Corzo, G.; Villegas, E.; Gomez-Lagunas, F.; Possani, L.D.; Belokoneva, O.S.; Nakajima, T. Oxyopinins, large amphipathic peptides isolated from the venom of the wolf spider oxyopes kitabensis with cytolytic properties and positive insecticidal cooperativity with spider neurotoxins. J. Biol. Chem. 2002, 277, 23627–23637. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; D’Suze, G.; Salazar, V.; Sevcik, C.; Shannon, J.D.; Sherman, N.E.; Fox, J.W. Antibacterial activity of six novel peptides from tityus discrepans scorpion venom. A fluorescent probe study of microbial membrane Na+ permeability changes. Toxicon 2009, 54, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sakai, A.; Matsushita, N.; Hanai, Y.; Nakagawa, Y.; Miyagawa, H. A novel amphipathic linear peptide with both insect toxicity and antimicrobial activity from the venom of the scorpion isometrus maculatus. Biosci. Biotechnol. Biochem. 2010, 74, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Carreto, S.; Quintero-Hernandez, V.; Jimenez-Vargas, J.M.; Corzo, G.; Possani, L.D.; Becerril, B.; Ortiz, E. Gene cloning and functional characterization of four novel antimicrobial-like peptides from scorpions of the family vaejovidae. Peptides 2012, 34, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Corzo, G.; Hahin, R. Scorpion venom peptides without disulfide bridges. IUBMB Life 2005, 57, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic alpha helical antimicrobial peptides. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- Escobar, E.; Flores, L.; Rivera, C. Antibacterial peptides from hadruroides mauryi and centruroides margaritatus venom. Rev. Peru. Boil. 2008, 15, 139–142. [Google Scholar]
- Garcia, F.; Villegas, E.; Espino-Solis, G.P.; Rodriguez, A.; Paniagua-Solis, J.F.; Sandoval-Lopez, G.; Possani, L.D.; Corzo, G. Antimicrobial peptides from arachnid venoms and their microbicidal activity in the presence of commercial antibiotics. J. Antibiot. 2013, 66, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Cruz, N.A.; Davila, S.; Licea, A.; Corona, M.; Zamudio, F.Z.; Garcia-Valdes, J.; Boyer, L.; Possani, L.D. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda wood and centruroides sculpturatus ewing. Biochimie 2004, 86, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, M.; Karbat, I.; Cohen, L.; Ilan, N.; Kahn, R.; Turkov, M.; Stankiewicz, M.; Stühmer, W.; Dong, K.; Gordon, D. The insecticidal potential of scorpion β-toxins. Toxicon 2007, 49, 473–489. [Google Scholar] [CrossRef] [PubMed]
- De Armas, L.F.; Sarmiento, D.L.; Florez, E. Composición del género centruroides marx, 1890 (scorpiones:Buthidae) en colombia, con al descripción de una nueva especie. Boletínde la Sociedad Entomológica Aragonesa 2012, 50, 105–114. [Google Scholar]
- Estrada-Gomez, S.; Munoz, L.J.V.; Castillo, J.C.Q. Extraction and partial characterization of venom from the colombian spider pamphobeteus aff. Nigricolor (aranae:Theraphosidae). Toxicon 2013, 76, 301–309. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, R.C.R.; Schwartz, E.F.; Possani, L.D. Mining on scorpion venom biodiversity. Toxicon 2010, 56, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.L., Jr.; Fletcher, M.D.; Weninger, K.; Anderson, T.E.; Martin, B.M. Vesicle-associated membrane protein (vamp) cleavage by a new metalloprotease from the brazilian scorpion tityus serrulatus. J. Biol. Chem. 2010, 285, 7405–7416. [Google Scholar] [CrossRef] [PubMed]
- Soudani, N.; Gharbi-Chihi, J.; Srairi-Abid, N.; Yazidi, C.M.; Planells, R.; Margotat, A.; Torresani, J.; El Ayeb, M. Isolation and molecular characterization of lvp1 lipolysis activating peptide from scorpion buthus occitanus tunetanus. Biochim. Biophys. Acta 2005, 1747, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Binford, G.J. An analysis of geographic and intersexual chemical variation in venoms of the spider tegenaria agrestis (agelenidae). Toxicon 2001, 39, 955–968. [Google Scholar] [CrossRef]
- De Sousa, L.; Borges, A.; Vasquez-Suarez, A.; Op den Camp, H.J.; Chadee-Burgos, R.I.; Romero-Bellorin, M.; Espinoza, J.; de Sousa-Insana, L.; Pino-Garcia, O. Differences in venom toxicity and antigenicity between females and males tityus nororientalis (buthidae) scorpions. J. Venom Res. 2010, 1, 61–70. [Google Scholar] [PubMed]
- Herzig, V.; Hodgson, W.C. Intersexual variations in the pharmacological properties of coremiocnemis tropix (araneae, theraphosidae) spider venom. Toxicon 2009, 53, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Espino-Solis, G.P.; Estrada, G.; Olamendi-Portugal, T.; Villegas, E.; Zamudio, F.; Cestele, S.; Possani, L.D.; Corzo, G. Isolation and molecular cloning of beta-neurotoxins from the venom of the scorpion centruroides suffusus suffusus. Toxicon 2011, 57, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, A.L.; del Rio-Portilla, F.; Picco, C.; Estrada, G.; Prestipino, G.; Possani, L.D.; Delepierre, M.; Corzo, G. Solution structure of native and recombinant expressed toxin cssii from the venom of the scorpion centruroides suffusus suffusus, and their effects on nav1.5 sodium channels. Biochim. Biophys. Acta 2012, 1824, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.F.; Garcia y Perez, L.G.; El Ayeb, M.; Kopeyan, C.; Bechis, G.; Jover, E.; Rochat, H. Purification and chemical and biological characterizations of seven toxins from the mexican scorpion, centruroides suffusus suffusus. J. Biol. Chem. 1987, 262, 4452–4459. [Google Scholar] [PubMed]
- Pintar, A.; Possani, L.D.; Delepierre, M. Solution structure of toxin 2 from centruroides noxius hoffmann, a beta-scorpion neurotoxin acting on sodium channels. J. Mol. Biol. 1999, 287, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Tapia, J.V.; Eliason, W.K.; Martin, B.M.; Lebreton, F.; Delepierre, M.; Possani, L.D.; Becerril, B. Cloning and characterization of the cdnas encoding Na+ channel-specific toxins 1 and 2 of the scorpion centruroides noxius hoffmann. Toxicon 1995, 33, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Avila, C.; Rojas, E.; Cerdas, L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in costa rica. Toxicon 1988, 26, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar] [PubMed]
- Wang, W.J.; Shih, C.H.; Huang, T.F. A novel p-i class metalloproteinase with broad substrate-cleaving activity, agkislysin, from agkistrodon acutus venom. Biochem. Biophys. Res. Commun. 2004, 324, 224–230. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. Available online: http://whqlibdoc.who.int/hq/2005/WHO_CDS_WHOPES_GCDPP_2005.13.pdf?ua=1 (accessed on 1 October 2013).
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Gutierrez, J.M.; Angulo, Y.; Sanz, L.; Juarez, P.; Calvete, J.J.; Lomonte, B. Isolation of an acidic phospholipase a2 from the venom of the snake bothrops asper of costa rica: Biochemical and toxicological characterization. Biochimie 2010, 92, 273–283. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Estrada-Gómez, S.; Cupitra, N.I.; Arango, W.M.; Muñoz, L.J.V. Intraspecific Variation of Centruroides Edwardsii Venom from Two Regions of Colombia. Toxins 2014, 6, 2082-2096. https://doi.org/10.3390/toxins6072082
Estrada-Gómez S, Cupitra NI, Arango WM, Muñoz LJV. Intraspecific Variation of Centruroides Edwardsii Venom from Two Regions of Colombia. Toxins. 2014; 6(7):2082-2096. https://doi.org/10.3390/toxins6072082
Chicago/Turabian StyleEstrada-Gómez, Sebastián, Nelson Ivan Cupitra, Walter Murillo Arango, and Leidy Johana Vargas Muñoz. 2014. "Intraspecific Variation of Centruroides Edwardsii Venom from Two Regions of Colombia" Toxins 6, no. 7: 2082-2096. https://doi.org/10.3390/toxins6072082