Microcystin-Bound Protein Patterns in Different Cultures of Microcystis aeruginosa and Field Samples
Abstract
:1. Introduction
2. Results
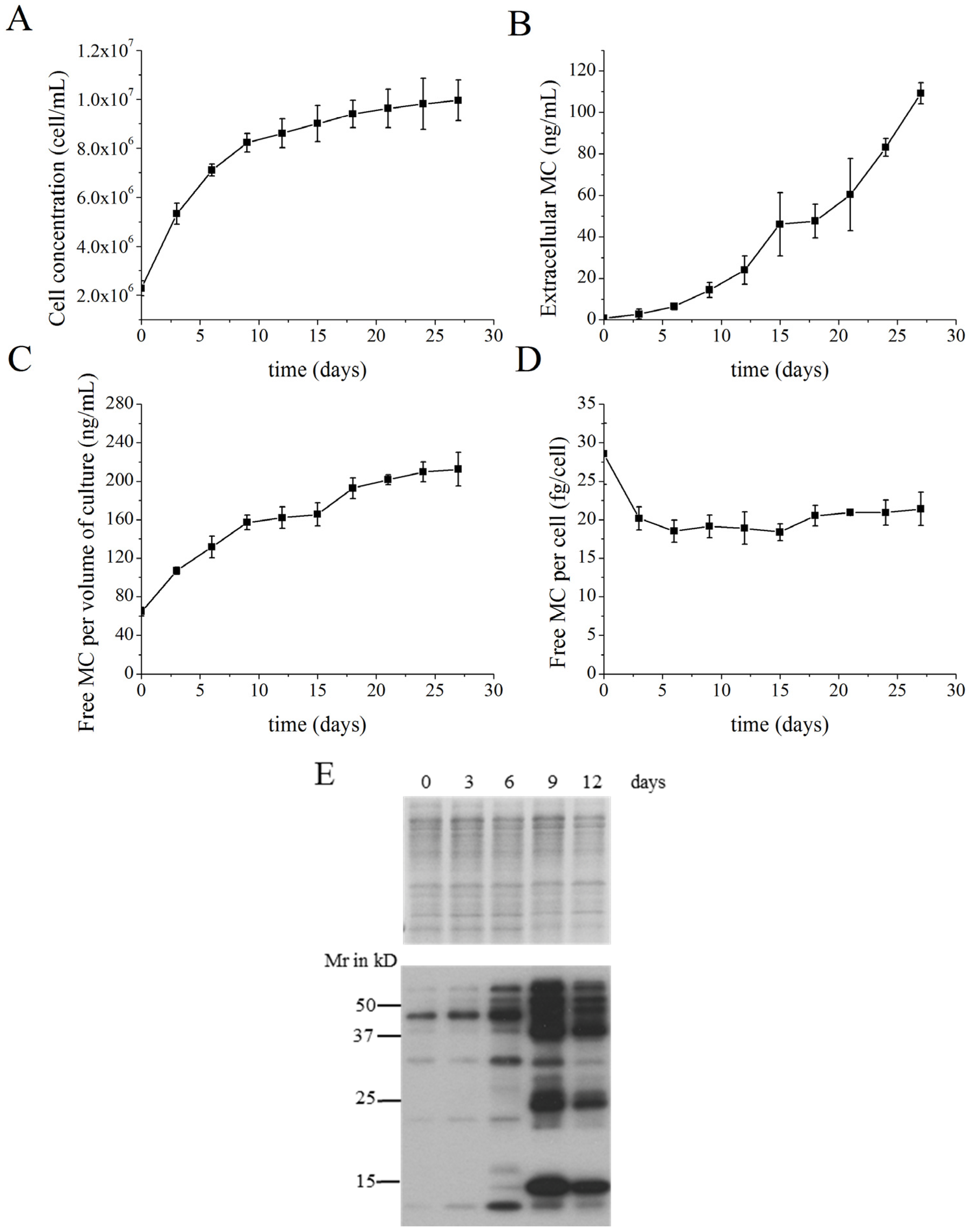
2.1. MC Production and Release in Batch Cultures
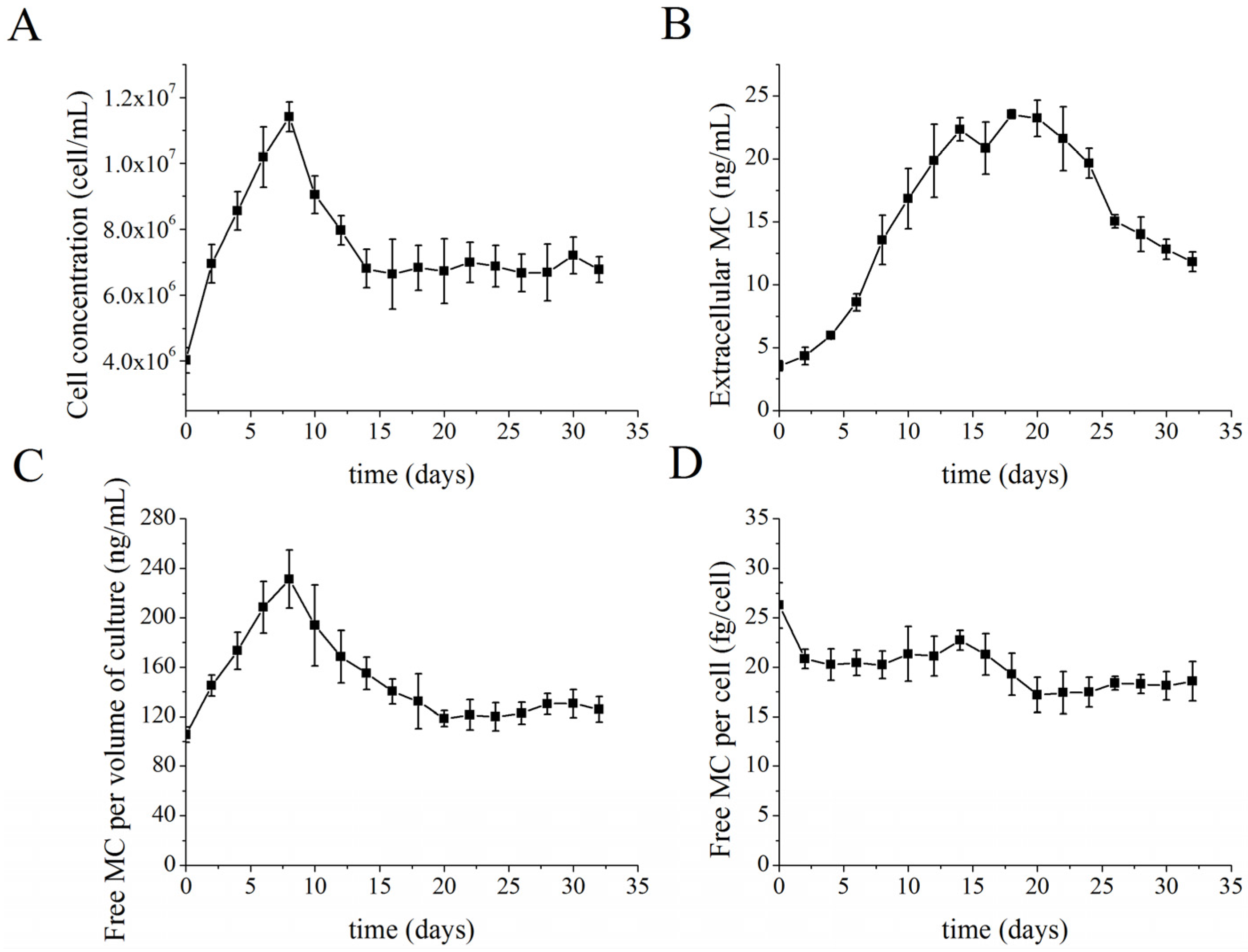
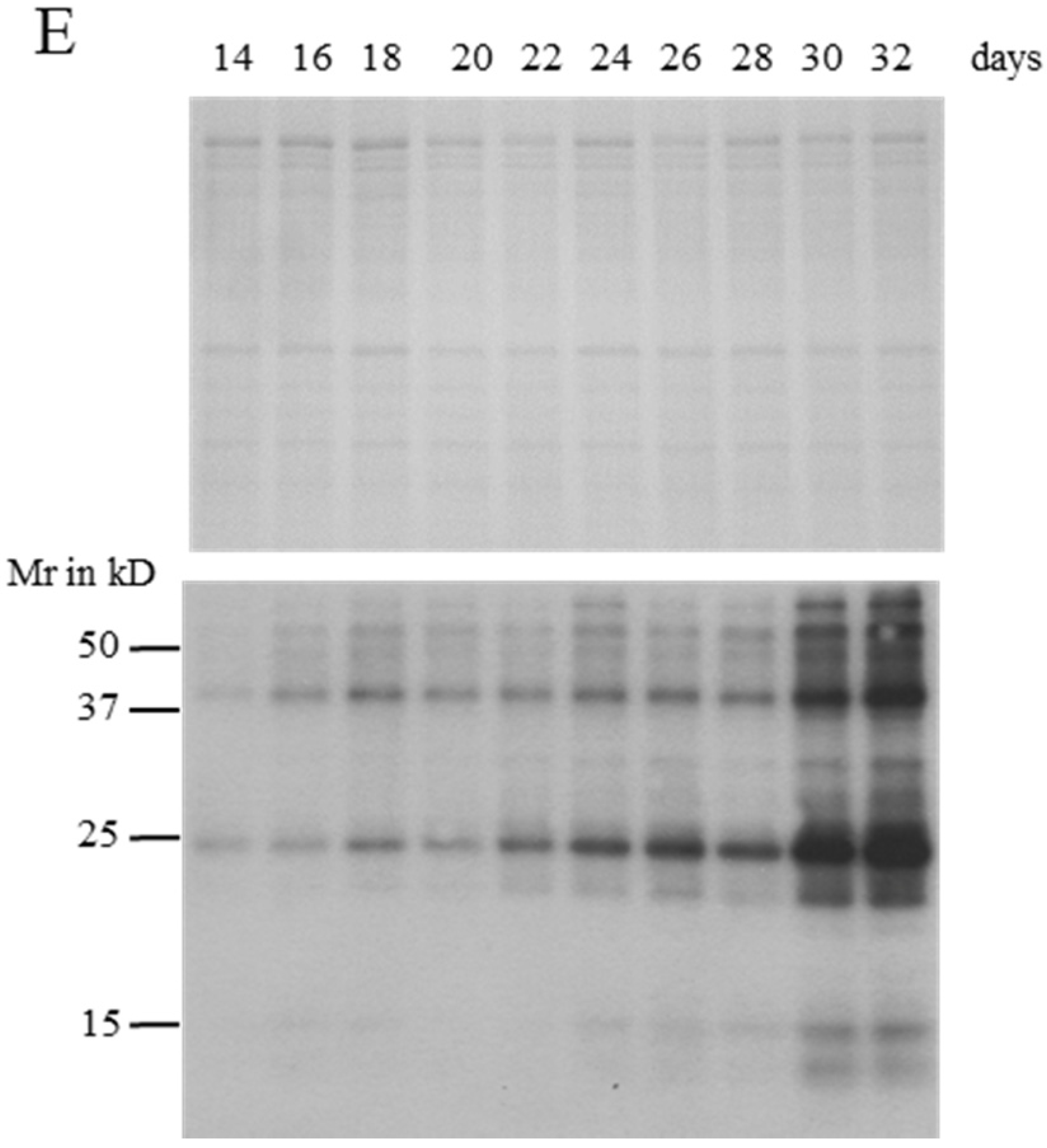
2.2. MC Production and Release in Semi-Continuous Culture
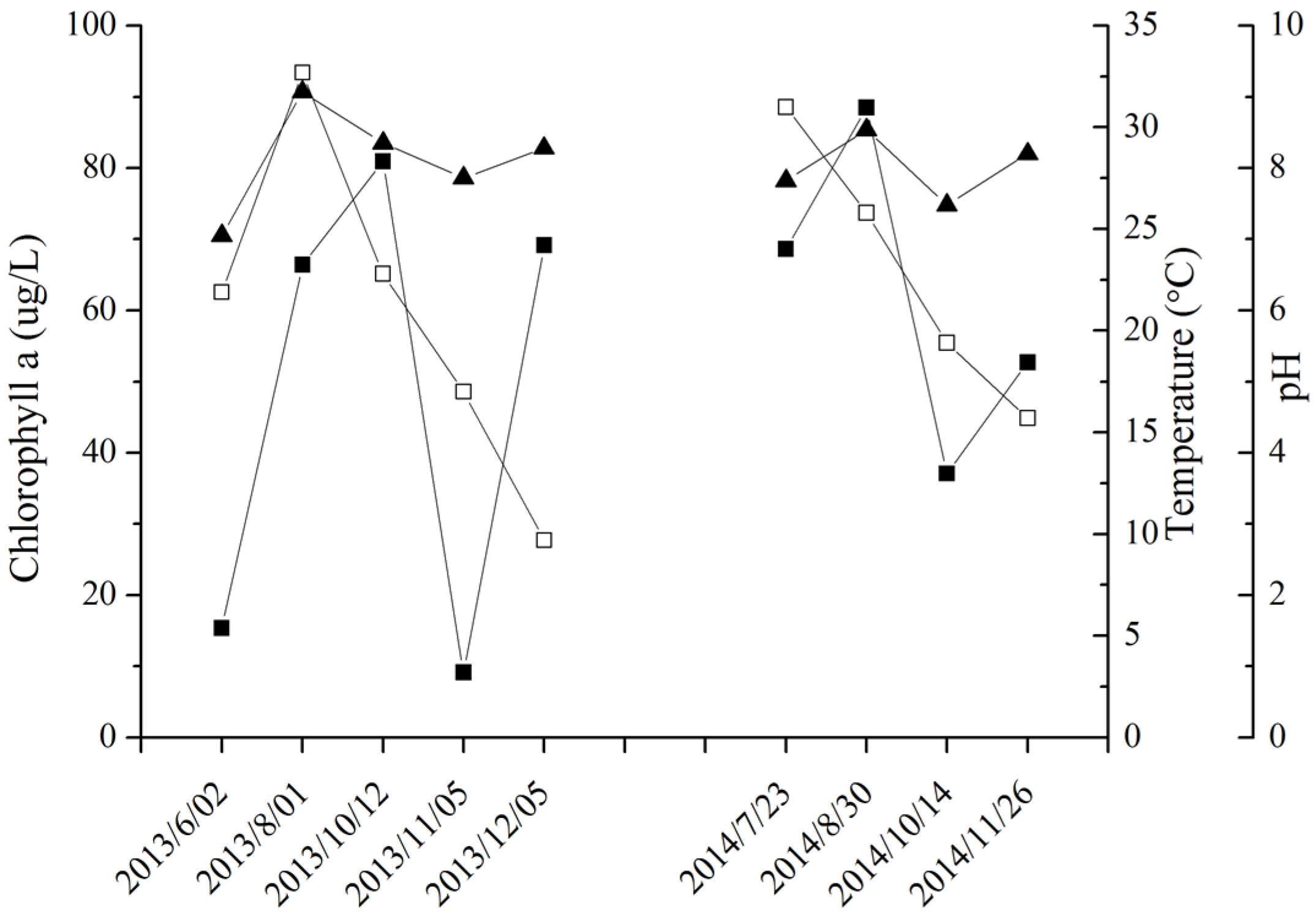
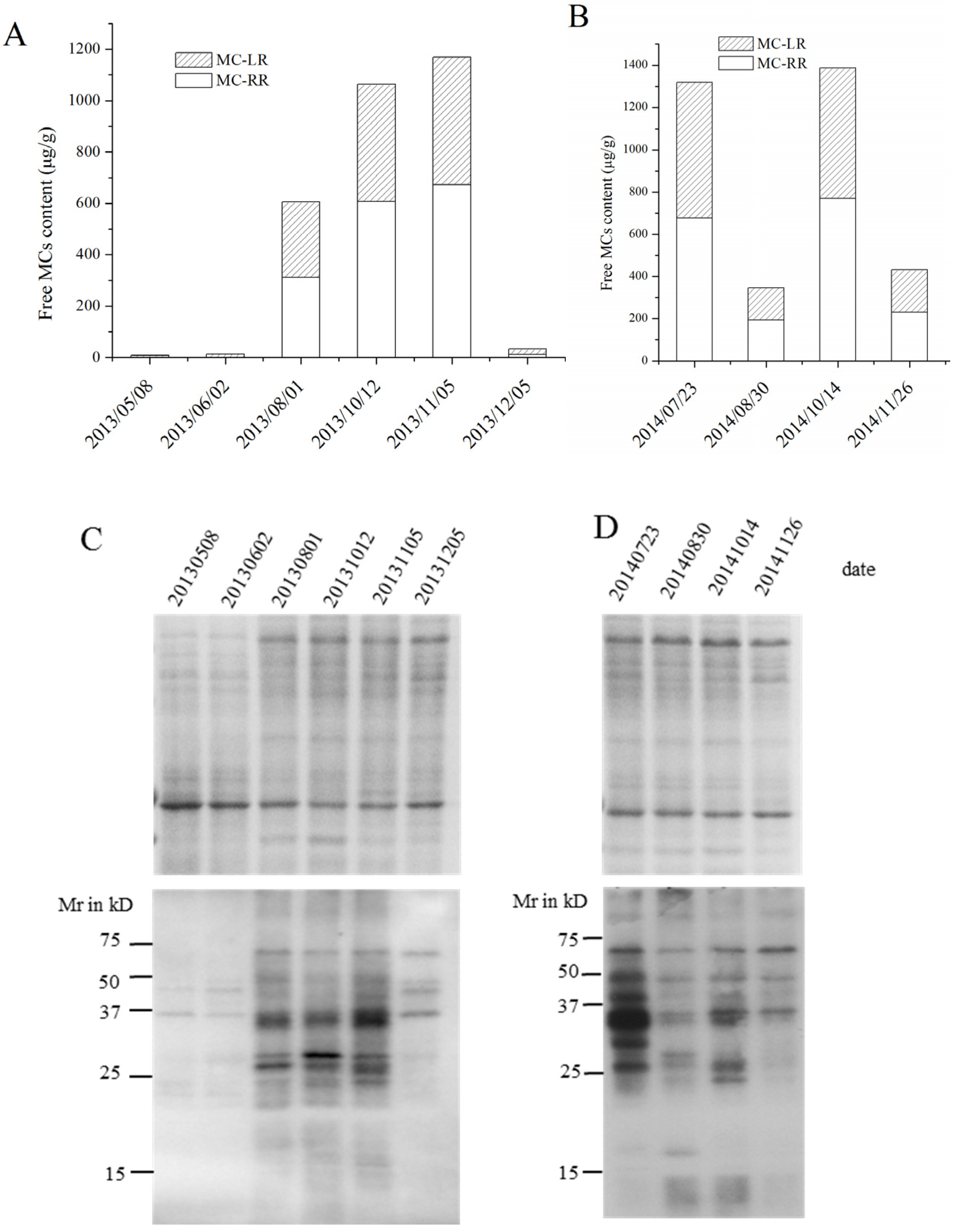
2.3. MC Production in Field Samples from Lake Taihu
2.4. Identification of MC-Bound Proteins
3. Discussion
4. Materials and Methods
4.1. Cultures and Sampling
4.2. Cell Counting
4.3. MC Analysis
4.4. Sample Preparation and Immunoblot Analysis
4.5. Two-Dimensional Electrophoresis and Immunoblotting
4.6. Mass Spectrometry (MS) Analysis and Protein Identification
4.7. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vencent, W.F. Cyanobacteria. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Elsevier: Oxford, UK, 2009; pp. 226–232. [Google Scholar]
- Carmichael, W.W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Ufelmann, H.; Kruger, T.; Luckas, B.; Shrenk, D. Human and rat hepatocyte toxicity and protein phosphatase 1 and 2A inhibitory activity of naturally occurring desmethyl-microcystins and nodularins. Toxicology 2012, 293, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, S.J.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and elimination of cyanobacterial toxins in drinking water treatment plants. Toxicol. Appl. Pharmacol. 2005, 203, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Von Döhren, H. Cyanobacterial peptides-nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [PubMed]
- Rantala, A.; Fewer, D.P.; Hisbergues, M.; Rouhiainen, L.; Vaitomaa, J.; Börner, T.; Sivonen, K. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Makower, A.K.; Schuurmans, J.M.; Groth, D.; Zilliges, Y.; Matthijs, H.C.P.; Dittmann, E. Transcriptomics-aided dissection of the intracellular and extracellular roles of microcystin in Microcystis aeruginosa PCC 7806. Appl. Environ. Microbiol. 2015, 81, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Xiao, Y.; Zhu, L.; Wu, Z.; Liu, J.; Hu, C.; Song, L. The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ. Microbiol. 2012, 14, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Jähnichen, S.; Ihle, T.; Petzoldt, T.; Benndorf, J. Impact of inorganic carbon availability on microcystin production by Microcystis aeruginosa PCC 7806. Appl. Environ. Microbiol. 2007, 73, 6994–7002. [Google Scholar] [CrossRef] [PubMed]
- Phelan, R.R.; Downing, T.G. A growth advantage for microcystin production by Microcystis PCC7806 under high light. J. Phycol. 2011, 47, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Alexova, R.; Fujii, M.; Birch, D.; Cheng, J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation. Environ. Microbiol. 2011, 13, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.; Steinhauser, D.; Dittmann, E. Metabolomic analysis indicates a pivotal role of the hepatotoxin microcystin in high light adaptation of Microcystis. Environ. Microbiol. 2015, 17, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Briand, E.; Yérémian, C.; Humbert, J.F.; Quiblier, C. Competition between microcystin- and non-microcystin-producing Planktothrix agardhii cyanobacteria strains under different environmental conditions. Environ. Microbiol. 2008, 10, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Song, L.; Sedmak, B. UVB radiation as a potential selective factor favoring microcystin producing bloom forming cyanobacteria. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Runnegar, M.; Berndt, N.; Kong, S.M.; Lee, E.Y.C.; Zhang, L.F. In vivo and in vitro binding of microcystin to protein phosphatase 1 and 2A. Biochem. Biophys. Res. Commun. 1995, 216, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, A.; Härmälä-Braskén, A.S.; Hellman, J.; Meriluoto, J.; Eriksson, J.E. Identification of ATP-synthase as a novel intracellular target for microcystin-LR. Chem. Biol. Interact. 2003, 142, 223–237. [Google Scholar] [CrossRef]
- Jüttner, F.; Lüthi, H. Topology and enhanced toxicity of bound microcystins in Microcystis PCC 7806. Toxicon 2008, 51, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Vela, L.; Sevilla, E.; Gonzalez, C.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Exploring the interaction of microcystin-LR with proteins and DNA. Toxicol. In Vitro 2008, 22, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.; Fastner, J.; Dittmann, E. Microcystin production revisited: Conjugate formation makes a major contribution. Environ. Microbiol. 2013, 15, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Utkilen, H.; Gjølme, N. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 1995, 61, 797–800. [Google Scholar] [PubMed]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Wood, S.A.; Dietrich, D.R.; Gary, S.C.; Hamilton, D.P. Increasing Microcystis cell density enhances microcystin synthesis: A mesocosm study. Inland Waters 2012, 2, 17–22. [Google Scholar] [CrossRef]
- Wiedner, C.; Visser, P.M.; Fastner, J.; Metcalf, J.S.; Codd, G.A.; Mur, L.R. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003, 69, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.A.; Červený, J.; Zavřel, T.; Nedbal, L. On the dynamics and constraints of batch culture growth of the cyanobacterium Cyanothece sp. ATCC 51142. J. Biotechnol. 2012, 162, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Hyenstrand, P. Fate of intracellular microcystins in the cyanobacterium Microcystis aeruginosa (Chroococcales, Cyanophyceae). Phycologia 2007, 46, 277–283. [Google Scholar] [CrossRef]
- Park, H.D.; Iwami, C.; Watanabe, M.F.; Harada, K.; Okino, T.; Hayashi, H. Temporal variabilities of the concentrations of intra- and extracellular microcystin and toxic Microcystis species in a hypertrophic lake, Lake Suwa, Japan (1991–1994). Environ. Toxicol. Water Qual. 1998, 13, 61–72. [Google Scholar] [CrossRef]
- Pearson, L.A.; Hisbergues, M.; Borner, T.; Dittmann, E.; Neilan, B.A. Inactivation of an ABC transporter gene, mcyH, results in loss of microcystin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Appl. Environ. Microbiol. 2004, 70, 6370–6378. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Shan, K.; Lin, L.; Shen, W.; Huang, L.; Gan, N.; Song, L. Multi-year assessment of toxic genotypes and microcystin concentration in northern Lake Taihu, China. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xue, Q.; Steinman, A.D.; Zhao, Y.; Xie, L. Spatiotemporal dynamics of microcystin variants and relationships with environmental parameters in Lake Taihu, China. Toxins 2015, 7, 3224–3244. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Yu, L.; Kong, F.; Zhang, M.; Yang, Z.; Shi, X.; Du, M. The dynamics of Microcystis genotypes and microcystin production and associations with environmental factors during blooms in Lake Chaohu, China. Toxins 2014, 6, 3238–3257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, L. Physiological comparison between colonial and unicellular forms of Microcystis aeruginosa Kütz (Cyanobacteria). Phycologia 2008, 47, 98–104. [Google Scholar] [CrossRef]
- Xiao, Y.; Gan, N.; Liu, J.; Zheng, L.; Song, L. Heterogeneity of buoyancy in response to light between two buoyant types of cyanobacterium Microcystis. Hydrobiologia 2012, 679, 297–311. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Miles, C.O.; Sandvik, M.; Nonga, H.E.; Ballot, A.; Wilkins, A.L.; Rise, F.; Jaabaek, A.H.; Loader, J.I. Conjugation of microcystins with thiols is reversible: Base-catalyzed deconjugation for chemical analysis. Chem. Res. Toxicol. 2016, 29, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Vasconcelos, V.M.; Antunes, A. Computational study of the covalent bonding of microcystins to cysteine residues—A reaction involved in the inhibition of the PPP family of protein phosphatases. FEBS J. 2013, 280, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Main, L.; Nicholson, B.K. Synthesis of 2′,6’-dihydroxychalcones by using tetrahydropyran-2-yl and trialkylsilyl protective groups: The crystal structure determination of 2′,6′-dihydroxy-2, 4,6-trimethoxychalcone. Aust. J. Chem. 1989, 42, 1103–1113. [Google Scholar] [CrossRef]
- Boncel, S.; Mączka, M.; Walczak, K.Z. Michael versus retro-Michael reaction in the regioselective synthesis of N-1 and N-3 uracil adducts. Tetrahedron 2010, 66, 8450–8457. [Google Scholar] [CrossRef]
- Stanic, A.; Uhlig, S.; Solhaug, A.; Rise, F.; Wilkins, A.L.; Miles, C.O. Nucleophilic addition of thiols to deoxynivalenol. J. Agric. Food Chem. 2015, 63, 7556–7566. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Xie, P.; He, J.; Guo, X.; Tuo, X.; Zhang, W.; Wu, L. Rapid conversion and reversible conjugation of glutathione detoxification of microcystins in bighead carp (Aristichthys nobilis). Aquat. Toxicol. 2014, 147, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The fate of microcystins in the environment and challenges for monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Liu, X.; Tan, J.; Li, D.; Yang, H. Diversity and dynamics of microcystin-producing cyanobacteria in China’s third largest lake, Lake Taihu. Harmful Algae 2009, 8, 637–644. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, A.Y.; Dai, Z.; Su, D.; Gaffrey, M.J.; Moore, R.J.; Jacobs, J.M.; Monroe, M.E.; Smith, R.D.; Koppenaal, D.W.; et al. Proteome-wide light/dark modulation of thiol oxidation in cyanobacteria revealed by quantitative site-specific redox proteomics. Mol. Cell Proteom. 2014, 13, 3270–3285. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, M.; Kieselbach, T. Disulphide proteomes and interactions with thioredoxin on the track towards understanding redox regulation in chloroplasts and cyanobacteria. J. Proteom. 2009, 72, 416–438. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Q.; Zhang, L.; Li, H.; Cui, Y.; Wu, Q. Identification of novel targets of cyanobacterial glutaredoxin. Arch. Biochem. Biophys. 2007, 458, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Chardonnet, S.; Sakr, S.; Cassier-Chauvat, C.; Le Marechal, P.; Chauvat, F.; Lemaire, S.D.; Decottignies, P. First proteomic study of S-glutathionylation in cyanobacteria. J. Proteome Res. 2014, 14, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Goloubinoff, P.; Gatenby, A.A.; Lorimer, G.H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature 1989, 337, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; van der Vies, S.M.; Glatz, A.; Török, Z.; Varvasovszki, V.; Horváth, I.; Vígh, L. The chaperonins of Synechocystis PCC6803 differ in heat inducibility and chaperone activity. Biochem. Biophys. Res. Commun. 2001, 289, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, M.E.; Florencio, F.J.; Lindahl, M. Selecting thioredoxins for disulphide proteomics: Target proteomes of three thioredoxins from the cyanobacterium Synechocystis sp. PCC 6803. Proteomics 2006, 6, S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Holtgrefe, S.; Gohlke, J.; Starmann, J. Regulation of plant cytosolic glyceraldehyde 3-phosphate dehydrogenase isoforms by thiol modifications. Physiol. Plant. 2008, 133, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Fukushima, Y.; Hara, S.; Hisabori, T. Redox control of the activity of phosphoglycerate kinase in Synechocystis sp. PCC6803. Plant. Cell Physiol. 2013, 54, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain Histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Nusch, E.A. Comparison of different methods for chlorophyll and phaeopigment determination. Archiv. Hydrobiol. Beih. Ergebn. Limnol. 1980, 14, 14–36. [Google Scholar]
- Kaplan, R.S.; Pedersen, P.L. Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black 10B. Anal. Biochem. 1985, 150, 97–104. [Google Scholar] [CrossRef]
- Harlow, E.D.; Lane, D.P. Antibodies: A Laboratory Manual, 1st ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, New York, NY, USA, 1988; pp. 636–640. [Google Scholar]
- Lei, L.M.; Wu, Y.S.; Gan, N.Q.; Song, L.R. An ELISA-like time-resolved fluorescence immunoassay for microcystin detection. Clin. Chim. Acta 2004, 348, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
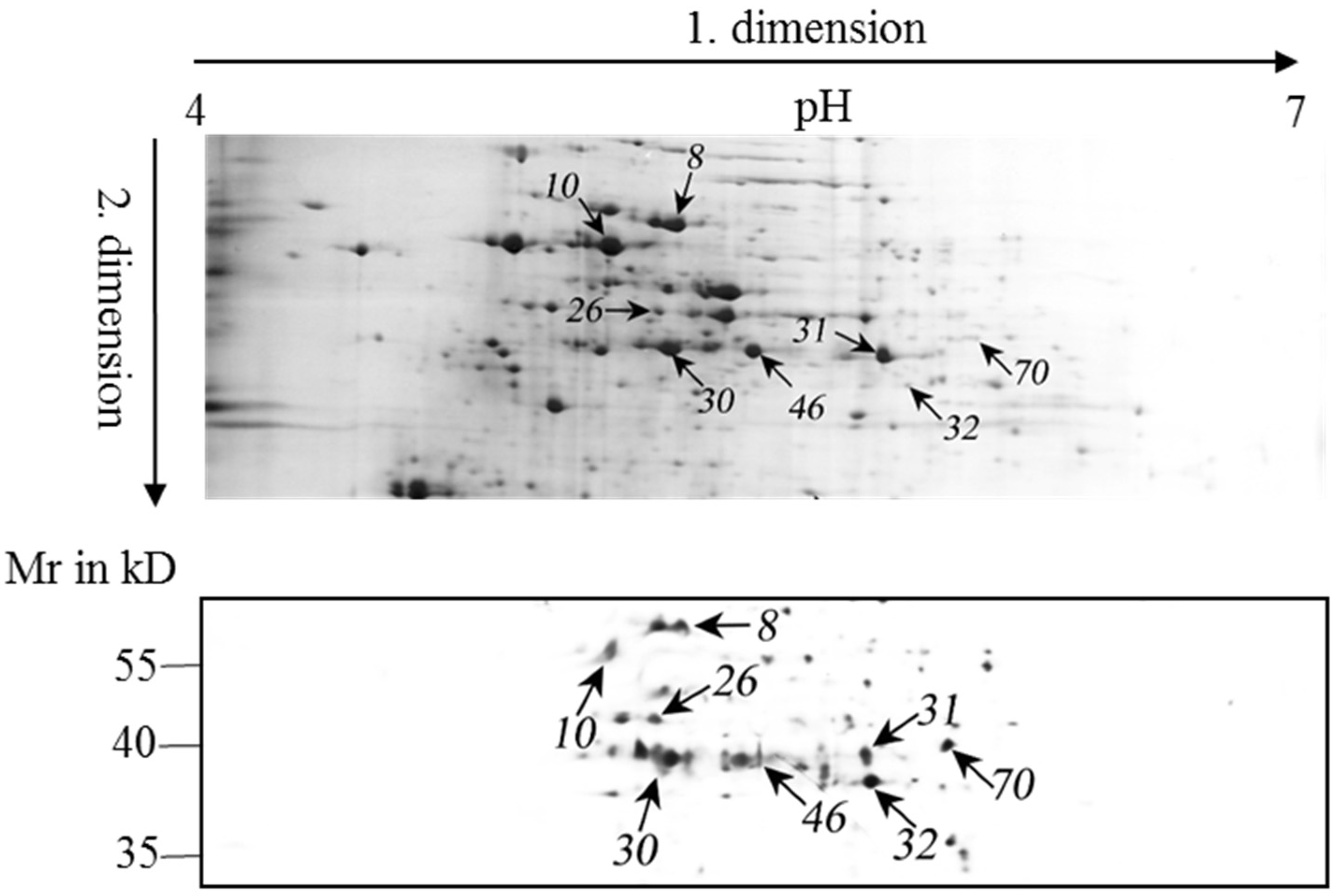
| Spot a | Accession Number b | Protein Name | Cys Residue c | Mass (Mr) d | pI Value e | Score f | Matched Peptides | Sequence Coverage | Functional Category |
|---|---|---|---|---|---|---|---|---|---|
| 46 | L8NVA2 | Phosphoribulokinase | 5 | 38,036 | 5.25 | 360 | 16 | 56% | Carbon metabolism |
| 30 | L8NKA9 | Phosphoglycerate kinase | 5 | 42,811 | 5.15 | 347 | 15 | 50% | Carbon metabolism |
| 31 | L8NQE4 | Fructose-bisphosphate aldolase, class II, Calvin cycle subtype | 4 | 39,156 | 5.4 | 368 | 15 | 45% | Carbon metabolism |
| 32 | L8NVZ5 | Glyceraldehyde-3-phosphate dehydrogenase | 5 | 37,128 | 5.76 | 190 | 8 | 29% | Carbon metabolism |
| 8 | L8NTF7 | 60 kDa chaperonin | 3 | 57,701 | 5.15 | 414 | 21 | 50% | Protein folding and assembling |
| 10 | L8NV94 | ATP synthase subunit alpha | 3 | 54,116 | 5.02 | 533 | 25 | 46% | ATP biosynthesis |
| 26 | L8NP76 | ThiF family protein | 6 | 42,979 | 5.13 | 379 | 11 | 45% | Thiamin synthesis |
| 70 | L8NUD8 | Acetyl-CoA acetyltransferases family protein | 3 | 41,396 | 5.71 | 311 | 12 | 32% | Transferase, for acetylation |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, N.; Hu, L.; Song, L.; Gan, N. Microcystin-Bound Protein Patterns in Different Cultures of Microcystis aeruginosa and Field Samples. Toxins 2016, 8, 293. https://doi.org/10.3390/toxins8100293
Wei N, Hu L, Song L, Gan N. Microcystin-Bound Protein Patterns in Different Cultures of Microcystis aeruginosa and Field Samples. Toxins. 2016; 8(10):293. https://doi.org/10.3390/toxins8100293
Chicago/Turabian StyleWei, Nian, Lili Hu, Lirong Song, and Nanqin Gan. 2016. "Microcystin-Bound Protein Patterns in Different Cultures of Microcystis aeruginosa and Field Samples" Toxins 8, no. 10: 293. https://doi.org/10.3390/toxins8100293






