Improvement of the Pharmacological Properties of Maize RIP by Cysteine-Specific PEGylation
Abstract
:1. Introduction
2. Results
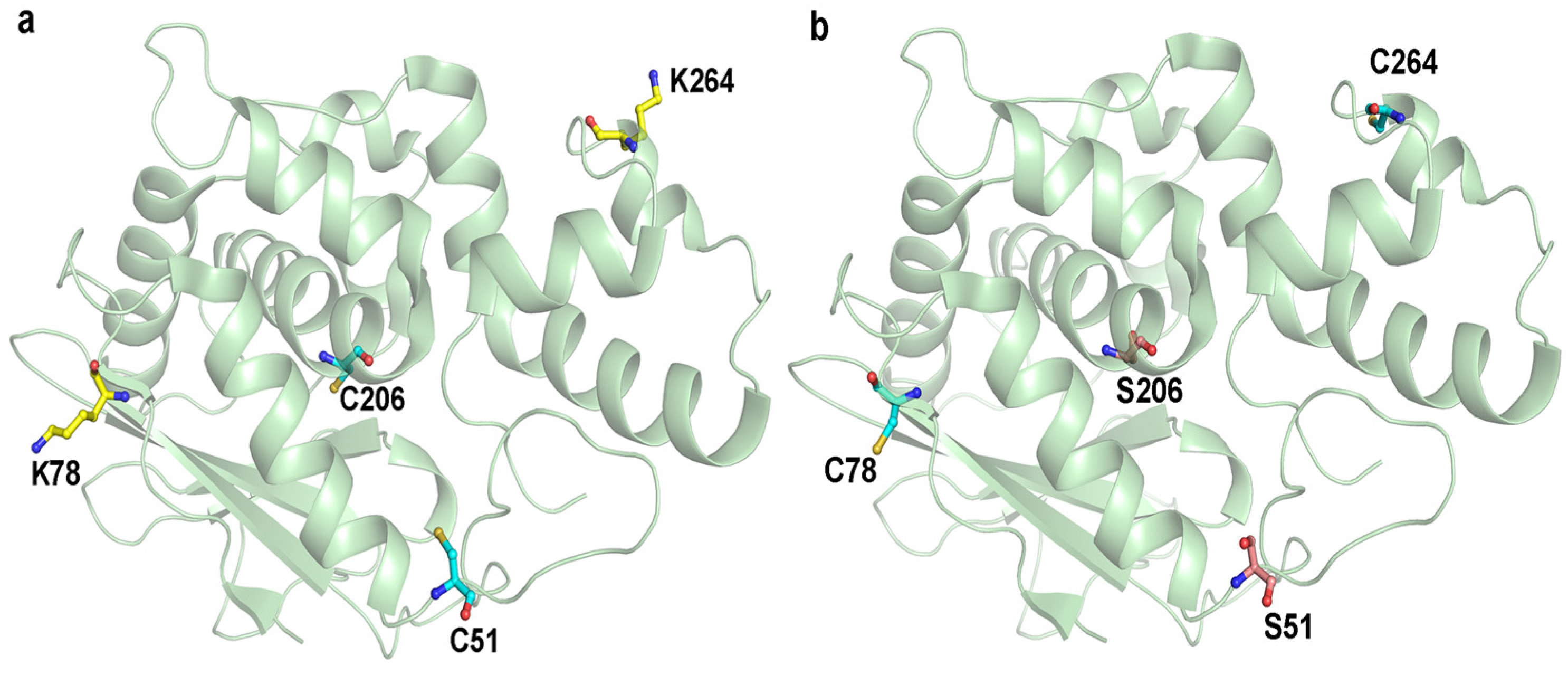
2.1. Selection of PEGylation Sites
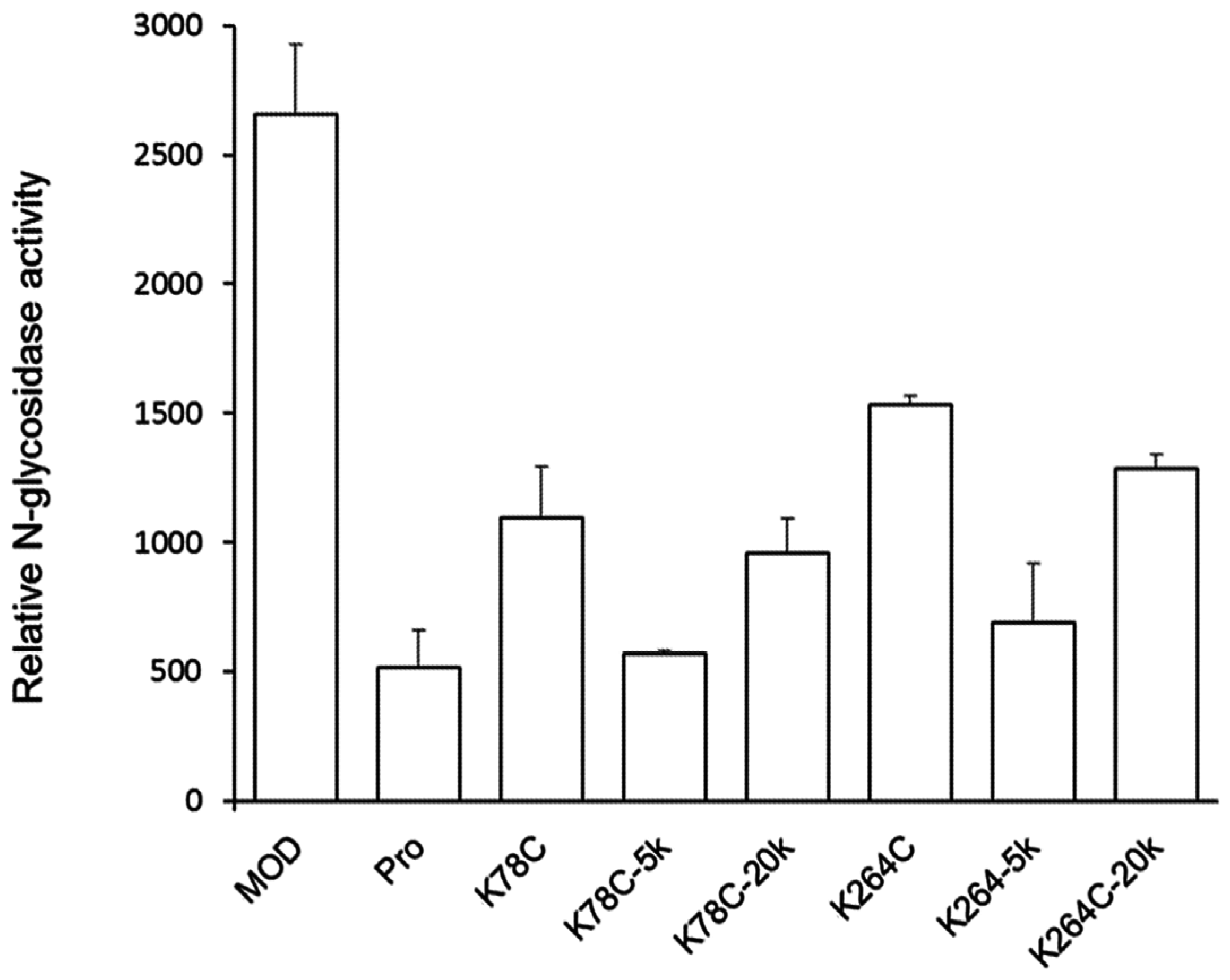
2.2. PEGylated Maize RIP Variants only Lost Half of the Ribosome-Inactivating Activity
2.3. PEG20k-Conjugated Maize RIP Variants Significantly Prolonged Circulating Half-Life in Rats
2.4. PEG20k-Modified Maize RIP Variants Elicit Weak Immune Responses in Mice
3. Discussion
4. Materials and Methods
4.1. Computer Modeling for PEGylation Sites
4.2. Cloning, Expression and Purification of Maize RIP Variants
4.3. Preparation of PEGylated Variants
4.4. Evaluation of Sarcin-Ricin Loop Depurination Activity
4.5. Pharmacokinetics Study
4.6. Immunogenicity Assay
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Endo, Y.; Tsurugi, K. The RNA N-glycosidase activity of ricin a-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J. Biol. Chem. 1988, 263, 8735–8739. [Google Scholar] [PubMed]
- Hartley, M.R.; Legname, G.; Osborn, R.; Chen, Z.; Lord, J.M. Single-chain ribosome inactivating proteins from plants depurinate Escherichia coli 23S ribosomal RNA. FEBS Lett. 1991, 290, 65–68. [Google Scholar] [CrossRef]
- Shaw, P.C.; Lee, K.M.; Wong, K.B. Recent advances in trichosanthin, a ribosome-inactivating protein with multiple pharmacological properties. Toxicon 2005, 45, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Domashevskiy, A.V.; Goss, D.J. Pokeweed antiviral protein, a ribosome inactivating protein: Activity, inhibition and prospects. Toxins (Basel) 2015, 7, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S. The history of ricin, abrin and related toxins. Toxicon 2004, 44, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Hey, T.D.; Hartley, M.; Walsh, T.A. Maize ribosome-inactivating protein (B-32). Homologs in related species, effects on maize ribosomes, and modulation of activity by Pro-peptide deletions. Plant Physiol. 1995, 107, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.N.; Wong, Y.T.; An, Y.J.; Cha, S.S.; Sze, K.H.; Au, S.W.; Wong, K.B.; Shaw, P.C. Structure-function study of maize ribosome-inactivating protein: Implications for the internal inactivation region and the sole glutamate in the active site. Nucleic Acids Res. 2007, 35, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Wang, R.R.; Mak, A.N.; Wong, K.B.; Zheng, Y.T.; Shaw, P.C. A switch-on mechanism to activate maize ribosome-inactivating protein for targeting HIV-infected cells. Nucleic Acids Res. 2010, 38, 6803–6812. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; Au, K.Y.; Zheng, H.Y.; Gao, L.M.; Zhang, X.; Luo, R.H.; Law, S.K.; Mak, A.N.; Wong, K.B.; Zhang, M.X.; et al. The recombinant maize ribosome-inactivating protein transiently reduces viral load in SHIV89.6 infected Chinese rhesus macaques. Toxins (Basel) 2015, 7, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Bellomy, K.; O’Neill, K.; Messinger, Y.; Johnson, T.; Chen, C.L. Toxicity, biological activity, and pharmacokinetics of TXU (anti-CD7)-pokeweed antiviral protein in chimpanzees and adult patients infected with human immunodeficiency virus. J. Pharmacol. Exp. Ther. 1999, 291, 1301–1307. [Google Scholar] [PubMed]
- Puri, M.; Kaur, I.; Perugini, M.A.; Gupta, R.C. Ribosome-inactivating proteins: Current status and biomedical applications. Drug Discov. Today 2012, 17, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Jevsevar, S.; Kunstelj, M.; Porekar, V.G. PEGylation of therapeutic proteins. Biotechnol. J. 2010, 5, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Byers, V.S.; Levin, A.S.; Malvino, A.; Waites, L.; Robins, R.A.; Baldwin, R.W. A phase II study of effect of addition of trichosanthin to zidovudine in patients with HIV disease and failing antiretroviral agents. AIDS Res. Hum. Retrovir. 1994, 10, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [PubMed]
- Kontermann, R.E. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs 2009, 23, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Hershfield, M.S.; Fernandez-Mejia, C.; Polmar, S.H.; Scudiery, D.; Berger, M.; Sorensen, R.U. Adenosine deaminase deficiency with late onset of recurrent infections: Response to treatment with polyethylene glycol-modified adenosine deaminase. J. Pediatr. 1988, 113, 312–317. [Google Scholar] [CrossRef]
- Wong, K.L.; Li, H.; Wong, K.K.; Jiang, T.; Shaw, P.C. Location and reduction of Icarapin antigenicity by site specific coupling to polyethylene glycol. Protein Pept. Lett. 2012, 19, 238–243. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; Shaw, P.C.; Xu, L.H.; Tam, S.C. Site-directed polyethylene glycol modification of trichosanthin: Effects on its biological activities, pharmacokinetics, and antigenicity. Life Sci. 1999, 64, 1163–1175. [Google Scholar] [CrossRef]
- He, X.H.; Shaw, P.C.; Tam, S.C. Reducing the immunogenicity and improving the in vivo activity of trichosanthin by site-directed pegylation. Life Sci. 1999, 65, 355–368. [Google Scholar] [CrossRef]
- ElliPro serve. Avaliable online: http://tools.iedb.org/ellipro/ (accessed on 10 September 2014).
- Kaur, I.; Gupta, R.C.; Puri, M. Ribosome inactivating proteins from plants inhibiting viruses. Virol. Sin. 2011, 26, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F. Ribosome-inactivating proteins: From toxins to useful proteins. Toxicon 2013, 67, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Till, M.A.; Zolla-Pazner, S.; Gorny, M.K.; Patton, J.S.; Uhr, J.W.; Vitetta, E.S. Human immunodeficiency virus-infected T cells and monocytes are killed by monoclonal human anti-gp41 antibodies coupled to ricin A chain. Proc. Natl. Acad. Sci. USA 1989, 86, 1987–1991. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Kung, H.F.; Huang, P.L.; Bourinbaiar, A.S.; Morell, J.L.; Brown, J.H.; Huang, P.L.; Tsai, W.P.; Chen, A.Y.; Huang, H.I.; et al. Human immunodeficiency virus type 1 (HIV-1) inhibition, DNA-binding, RNA-binding, and ribosome inactivation activities in the N-terminal segments of the plant anti-HIV protein GAP31. Proc. Natl. Acad. Sci. USA 1994, 91, 12208–12212. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Kung, H.F.; Huang, P.L.; Huang, P.L.; Li, B.Q.; Huang, P.; Huang, H.I.; Chen, H.C. A new class of anti-HIV agents: GAP31, DAPS 30 and 32. FEBS Lett. 1991, 291, 139–144. [Google Scholar] [CrossRef]
- Huang, P.L.; Sun, Y.; Chen, H.C.; Kung, H.F.; Lee-Huang, S. Proteolytic fragments of anti-HIV and anti-tumor proteins MAP30 and GAP31 are biologically active. Biochem. Biophys. Res. Commun. 1999, 262, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.L.; Feng, D.; Wu, J.; Sui, S.F. Trichosanthin inhibits integration of human immunodeficiency virus type 1 through depurinating the long-terminal repeats. Mol. Biol. Rep. 2010, 37, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Hao, S.J.; Liu, Y.D.; Hu, T.; Zhang, G.F.; Zhang, X.; Qi, Q.S.; Ma, G.H.; Su, Z.G. PEGylation markedly enhances the in vivo potency of recombinant human non-glycosylated erythropoietin: A comparison with glycosylated erythropoietin. J. Control. Release 2010, 145, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, S.; Li, J.; Meng, Y.; Zhao, X. Preparation of an antitumor and antivirus agent: Chemical modification of alpha-MMC and MAP30 from Momordica Charantia L. with covalent conjugation of polyethyelene glycol. Int. J. Nanomed. 2012, 7, 3133–3142. [Google Scholar]
- Ponomarenko, J.; Bui, H.H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. Ellipro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mak, A.N.; Shaw, P.C.; Sze, K.H. Solution structure of an active mutant of maize ribosome-inactivating protein (MOD) and its interaction with the ribosomal stalk protein P2. J. Mol. Biol. 2010, 395, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Melchior, W.B., Jr.; Tolleson, W.H. A functional quantitative polymerase chain reaction assay for ricin, Shiga toxin, and related ribosome-inactivating proteins. Anal. Biochem. 2010, 396, 204–211. [Google Scholar] [CrossRef] [PubMed]
| Treatment | AUC0-t (mg min/mL) | T1/2 (min) | Fold Increased Compared to Non-PEGylated Form |
|---|---|---|---|
| His-TAT-MOD | 0.46 | 8.0 | - |
| MOD-K78C | 0.59 | 9.4 | - |
| MOD-K78C-5k | 0.81 | 9.8 | 1.04 |
| MOD-K78C-20k | 2.65 | 47.6 | 5.06 |
| MOD-K264C | 0.68 | 10.1 | - |
| MOD-K264C-5k | 0.93 | 10.1 | 1 |
| MOD-K264C-20k | 2.57 | 171.1 | 16.94 |
| Primer | Sequence (5′-3′) |
|---|---|
| MOD-C15S-F | GTGATCAAACACTCTACCGACC |
| MOD-C15S-R | GGTCGGTAGAGTGTTTGATCAC |
| MOD-C206S-F | GTGGTCATGGTGTCTGAGGGGCTG |
| MOD-C206S-R | CAGCCCCTCAGACACCATGACCAC |
| MOD-K78C-F | ACAGAGCTCTGTACTAGGACC |
| MOD-K78C-R | GGTCCTAGTACAGAGCTCTGT |
| MOD-K264C-F | GACATGCAGTGTCTTGGCATC |
| MOD-K264C-R | GATGCCAAGACACTGCATGTC |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Au, K.-Y.; Shi, W.-W.; Qian, S.; Zuo, Z.; Shaw, P.-C. Improvement of the Pharmacological Properties of Maize RIP by Cysteine-Specific PEGylation. Toxins 2016, 8, 298. https://doi.org/10.3390/toxins8100298
Au K-Y, Shi W-W, Qian S, Zuo Z, Shaw P-C. Improvement of the Pharmacological Properties of Maize RIP by Cysteine-Specific PEGylation. Toxins. 2016; 8(10):298. https://doi.org/10.3390/toxins8100298
Chicago/Turabian StyleAu, Ka-Yee, Wei-Wei Shi, Shuai Qian, Zhong Zuo, and Pang-Chui Shaw. 2016. "Improvement of the Pharmacological Properties of Maize RIP by Cysteine-Specific PEGylation" Toxins 8, no. 10: 298. https://doi.org/10.3390/toxins8100298





