Targeting Staphylococcus aureus Toxins: A Potential form of Anti-Virulence Therapy
Abstract
:1. Introduction
2. Toxins—the Major S. aureus Virulence Factor
2.1. Hemolysins (Alpha, Beta, Gamma, and Delta)
2.2. Leukotoxins
2.3. Staphylococcal Exfoliative Toxins (ETs)
2.4. Staphylococcal Enterotoxins (SEs) and Toxic-Shock Syndrome Toxin-1 (TSST-1)
3. Regulation of Toxin Production in S. aureus
3.1. The Two-Component Regulatory Systems—Agr and Sae
3.2. The sarA and sigB
4. From Antibiotics to Anti-Virulence Therapies
4.1. Targeting S. aureus Toxins: A Direct Approach
4.1.1. Targeting S. aureus Hemolysins
4.1.2. Targeting S. aureus Leukotoxins
4.1.3. Targeting Staphylococcal Enterotoxins
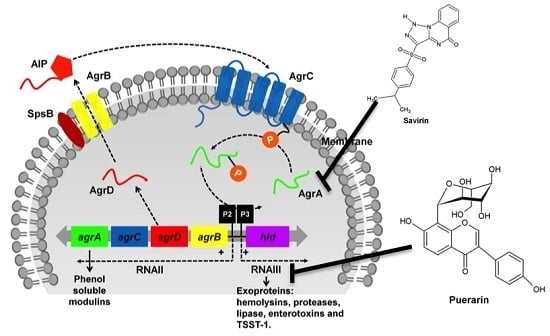
4.2. Targeting Pathways that Govern Toxin Production: An Indirect Approach
5. Caenorhabditis elegans as a Model for the Discovery of Novel Anti-Virulence Molecules
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antibiotic Resistance Threats in the United States. 2013. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/ (accessed on 11 November 2015).
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- McAdow, M.; DeDent, A.C.; Emolo, C.; Cheng, A.G.; Kreiswirth, B.N.; Missiakas, D.M.; Schneewind, O. Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infect. Immun. 2012, 80, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Jusko, M.; Potempa, J.; Kantyka, T.; Bielecka, E.; Miller, H.K.; Kalinska, M.; Dubin, G.; Garred, P.; Shaw, L.N.; Blom, A.M. Staphylococcal proteases aid in evasion of the human complement system. J. Innate Immun. 2014, 6, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, V.; Liang, X.; Horndahl, J.K.; Ganesh, V.K.; Smeds, E.; Foster, T.J.; Hook, M. Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp). J. Biol. Chem. 2011, 286, 29797–29805. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.M.; Miajlovic, H.; Foster, T.J. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Scali, F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, T.; Preissner, K.T.; Herrmann, M. The anti-inflammatory activities of Staphylococcus aureus. Trends Immunol. 2007, 28, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Bubeck Wardenburg, J. Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS Pathog. 2014, 10, e1003871. [Google Scholar] [CrossRef] [PubMed]
- Valeva, A.; Walev, I.; Pinkernell, M.; Walker, B.; Bayley, H.; Palmer, M.; Bhakdi, S. Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc. Natl. Acad. Sci. USA 1997, 94, 11607–11611. [Google Scholar] [CrossRef] [PubMed]
- Wilke, G.A.; Bubeck Wardenburg, J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Becker, R.E.; Sailer, A.; Turner, J.R.; Bubeck Wardenburg, J. Synergistic action of Staphylococcus aureus alpha-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell. Host Microbe 2015, 17, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Walev, I.; Weller, U.; Strauch, S.; Foster, T.; Bhakdi, S. Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus. Infect. Immun. 1996, 64, 2974–2979. [Google Scholar] [PubMed]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Girardot, R.; Piemont, Y.; Prevost, G.; Colin, D.A. Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding. Infect. Immun. 2009, 77, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.; Nariya, H.; Yokota, K.; Kamio, Y.; Gouaux, E. Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat. Struct. Biol. 1999, 6, 134–140. [Google Scholar] [PubMed]
- Verdon, J.; Girardin, N.; Lacombe, C.; Berjeaud, J.M.; Hechard, Y. Delta-hemolysin, an update on a membrane-interacting peptide. Peptides 2009, 30, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Surewaard, B.G.; de Haas, C.J.; Vervoort, F.; Rigby, K.M.; DeLeo, F.R.; Otto, M.; van Strijp, J.A.; Nijland, R. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell. Microbiol. 2013, 15, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Phenol-soluble modulins. Int. J. Med. Microbiol. 2014, 304, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.; Sharif, O.; Stich, K.; Doninger, B.; Biaggio, M.; Colinge, J.; Bilban, M.; Mesteri, I.; Hazemi, P.; Lemmens-Gruber, R.; et al. TLR 2 and CD14 mediate innate immunity and lung inflammation to staphylococcal Panton-Valentine leukocidin in vivo. J. Immunol. 2011, 186, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Inden, K.; Kaneko, J.; Miyazato, A.; Yamamoto, N.; Mouri, S.; Shibuya, Y.; Nakamura, K.; Aoyagi, T.; Hatta, M.; Kunishima, H.; et al. Toll-like receptor 4-dependent activation of myeloid dendritic cells by leukocidin of Staphylococcus aureus. Microbes Infect. 2009, 11, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, F., III; Kozhaya, L.; Rawlings, S.A.; Reyes-Robles, T.; DuMont, A.L.; Myszka, D.G.; Landau, N.R.; Unutmaz, D.; Torres, V.J. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 2013, 493, 51–55. [Google Scholar] [PubMed]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F., III; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.; van Kessel, K.P.; Vandenesch, F.; et al. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell. Host Microbe 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 2010, 64, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, M.; Wladyka, B.; Dubin, G. Exfoliative toxins of Staphylococcus aureus. Toxins 2010, 2, 1148–1165. [Google Scholar] [CrossRef] [PubMed]
- Eyre, R.W.; Stanley, J.R. Human autoantibodies against a desmosomal protein complex with a calcium-sensitive epitope are characteristic of pemphigus foliaceus patients. J. Exp. Med. 1987, 165, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Hanakawa, Y.; Schechter, N.M.; Lin, C.; Garza, L.; Li, H.; Yamaguchi, T.; Fudaba, Y.; Nishifuji, K.; Sugai, M.; Amagai, M.; et al. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. J. Clin. Invest. 2002, 110, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Monday, S.R.; Vath, G.M.; Ferens, W.A.; Deobald, C.; Rago, J.V.; Gahr, P.J.; Monie, D.D.; Iandolo, J.J.; Chapes, S.K.; Davis, W.C.; et al. Unique superantigen activity of staphylococcal exfoliative toxins. J. Immunol. 1999, 162, 4550–4559. [Google Scholar] [PubMed]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Chen, C.L.; Huang, W.C.; Cheng, Y.L.; Hsieh, C.Y.; Wang, C.Y.; Hong, M.Y. Different types of cell death induced by enterotoxins. Toxins 2010, 2, 2158–2176. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef]
- Betley, M.J.; Schlievert, P.M.; Bergdoll, M.S.; Bohach, G.A.; Iandolo, J.J.; Khan, S.A.; Pattee, P.A.; Reiser, R.R. Staphylococcal gene nomenclature. ASM News 1990, 56, 182. [Google Scholar]
- Berkley, S.F.; Hightower, A.W.; Broome, C.V.; Reingold, A.L. The relationship of tampon characteristics to menstrual toxic shock syndrome. JAMA 1987, 258, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Stach, C.S.; Herrera, A.; Schlievert, P.M. Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol. Res. 2014, 59, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S.M.; Williams, D.N.; Osterholm, M.T.; Tofte, R.W.; Posalaky, Z. Toxic shock syndrome: Clinical, laboratory, and pathologic findings in nine fatal cases. Ann. Intern. Med. 1982, 96, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Recsei, P.; Kreiswirth, B.; O’Reilly, M.; Schlievert, P.; Gruss, A.; Novick, R.P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 1986, 202, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Todd, D.A.; Velazquez, J.V.; Cech, N.B.; Sonenshein, A.L. Cody-mediated regulation of the Staphylococcus aureus Agr system integrates nutritional and population density signals. J. Bacteriol. 2014, 196, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Koomey, J.M.; Butler, C.A.; Projan, S.J.; Fischetti, V.A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 1992, 89, 6462–6466. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, A.T.; Cheung, A.L.; Nagel, R. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch. Microbiol. 1997, 168, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, M.E.; Khan, S.A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J. Biol. Chem. 1988, 263, 6276–6280. [Google Scholar] [PubMed]
- Bayles, K.W.; Iandolo, J.J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J. Bacteriol 1989, 171, 4799–4806. [Google Scholar] [PubMed]
- Regassa, L.B.; Couch, J.L.; Betley, M.J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect. Immun. 1991, 59, 955–962. [Google Scholar] [PubMed]
- Vojtov, N.; Ross, H.F.; Novick, R.P. Global repression of exotoxin synthesis by staphylococcal superantigens. Proc. Natl. Acad. Sci. USA 2002, 99, 10102–10107. [Google Scholar] [CrossRef] [PubMed]
- Mallonee, D.H.; Glatz, B.A.; Pattee, P.A. Chromosomal mapping of a gene affecting enterotoxin A production in Staphylococcus aureus. Appl. Environ. Microbiol. 1982, 43, 397–402. [Google Scholar] [PubMed]
- Kreiswirth, B.N.; Lofdahl, S.; Betley, M.J.; O’Reilly, M.; Schlievert, P.M.; Bergdoll, M.S.; Novick, R.P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 1983, 305, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, S.; Tegmark, K. Regulation of virulence determinants in Staphylococcus aureus. Int J. Med. Microbiol. 2001, 291, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Said-Salim, B.; Dunman, P.M.; McAleese, F.M.; Macapagal, D.; Murphy, E.; McNamara, P.J.; Arvidson, S.; Foster, T.J.; Projan, S.J.; Kreiswirth, B.N. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 2003, 185, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Smeltzer, M.S.; Hart, M.E.; Iandolo, J.J. Phenotypic characterization of xpr, a global regulator of extracellular virulence factors in Staphylococcus aureus. Infect. Immun. 1993, 61, 919–925. [Google Scholar] [PubMed]
- Dunman, P.M.; Murphy, E.; Haney, S.; Palacios, D.; Tucker-Kellogg, G.; Wu, S.; Brown, E.L.; Zagursky, R.J.; Shlaes, D.; Projan, S.J. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 2001, 183, 7341–7353. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P.; Projan, S.J.; Kornblum, J.; Ross, H.F.; Ji, G.; Kreiswirth, B.; Vandenesch, F.; Moghazeh, S. The agr P2 operon: An autocatalytic sensory transduction system in Staphylococcus aureus. Mol. Gen. Genet. 1995, 248, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Thoendel, M.; Horswill, A.R. Identification of Staphylococcus aureus AgrD residues required for autoinducing peptide biosynthesis. J. Biol. Chem. 2009, 284, 21828–21838. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Jarraud, S.; Ji, G.; Greenland, T.; Pedraza, A.; Etienne, J.; Novick, R.P.; Vandenesch, F. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol. Microbiol. 1998, 28, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P.; Ross, H.F.; Projan, S.J.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993, 12, 3967–3975. [Google Scholar] [PubMed]
- Koenig, R.L.; Ray, J.L.; Maleki, S.J.; Smeltzer, M.S.; Hurlburt, B.K. Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J. Bacteriol. 2004, 186, 7549–7555. [Google Scholar] [CrossRef] [PubMed]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.H.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell. 2008, 32, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Novick, R.P. Translation ofRNAIII, the Staphylococcus aureus agr regulatory RNA molecule, can be activated by a 3’-end deletion. FEMS Microbiol. Lett. 1995, 133, 155–161. [Google Scholar] [PubMed]
- Giraudo, A.T.; Calzolari, A.; Cataldi, A.A.; Bogni, C.; Nagel, R. The sae locus of Staphylococcus aureus encodes a two-component regulatory system. FEMS Microbiol. Lett. 1999, 177, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Voyich, J.M.; Vuong, C.; DeWald, M.; Nygaard, T.K.; Kocianova, S.; Griffith, S.; Jones, J.; Iverson, C.; Sturdevant, D.E.; Braughton, K.R.; et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 2009, 199, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Date, S.V.; Modrusan, Z.; Lawrence, M.; Morisaki, J.H.; Toy, K.; Shah, I.M.; Kim, J.; Park, S.; Xu, M.; Basuino, L.; et al. Global gene expression of methicillin-resistant Staphylococcus aureus USA300 during human and mouse infection. J. Infect. Dis. 2014, 209, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, A.T.; Raspanti, C.G.; Calzolari, A.; Nagel, R. Characterization of a Tn551-mutant of Staphylococcus aureus defective in the production of several exoproteins. Can. J. Microbiol. 1994, 40, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Hammer, N.D.; Campbell, J.P.; Benson, M.A.; Perrien, D.S.; Mrak, L.N.; Smeltzer, M.S.; Torres, V.J.; Skaar, E.P. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell. Host Microbe 2013, 13, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P.; Jiang, D. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 2003, 149, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Rogasch, K.; Ruhmling, V.; Pane-Farre, J.; Hoper, D.; Weinberg, C.; Fuchs, S.; Schmudde, M.; Broker, B.M.; Wolz, C.; Hecker, M.; et al. Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J. Bacteriol. 2006, 188, 7742–7758. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003, 48, 1429–1449. [Google Scholar] [CrossRef] [PubMed]
- Bronner, S.; Monteil, H.; Prevost, G. Regulation of virulence determinants in Staphylococcus aureus: Complexity and applications. FEMS Microbiol. Rev. 2004, 28, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Beenken, K.E.; Blevins, J.S.; Smeltzer, M.S. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 2003, 71, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane-Khadka, R.; Cantore, S.A.; Riordan, J.T.; Delgado, A.; Norman, A.E.; Duenas, S.; Zaman, S.; Horan, S.; Wilkinson, B.J.; Gustafson, J.E. SarA inactivation reduces vancomycin-intermediate and ciprofloxacin resistance expression by Staphylococcus aureus. Int. J. Antimicrob. Agents 2009, 34, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.G.; Heinrichs, J.H.; Cheung, A.L. The molecular architecture of the sar locus in Staphylococcus aureus. J. Bacteriol 1996, 178, 4563–4570. [Google Scholar] [PubMed]
- Chien, Y.; Manna, A.C.; Projan, S.J.; Cheung, A.L. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J. Biol. Chem. 1999, 274, 37169–37176. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Bayer, M.G.; Heinrichs, J.H. Sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J. Bacteriol. 1997, 179, 3963–3971. [Google Scholar] [PubMed]
- Reyes, D.; Andrey, D.O.; Monod, A.; Kelley, W.L.; Zhang, G.; Cheung, A.L. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J. Bacteriol. 2011, 193, 6020–6031. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Eberhardt, K.J.; Chung, E.; Yeaman, M.R.; Sullam, P.M.; Ramos, M.; Bayer, A.S. Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Invest. 1994, 94, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.S.; Elasri, M.O.; Allmendinger, S.D.; Beenken, K.E.; Skinner, R.A.; Thomas, J.R.; Smeltzer, M.S. Role of sarA in the pathogenesis of Staphylococcus aureus musculoskeletal infection. Infect. Immun. 2003, 71, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.N.; Beaver, M.; Beenken, K.; Smeltzer, M.; Horswill, A.R.; Kielian, T. Staphylococcus aureus sarA regulates inflammation and colonization during central nervous system biofilm formation. PLoS ONE 2013, 8, e84089. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; de Lencastre, H.; Tomasz, A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: Molecular cloning and DNA sequencing. J. Bacteriol. 1996, 178, 6036–6042. [Google Scholar] [PubMed]
- Zhang, S.; Reeves, A.; Woodbury, R.L.; Haldenwang, W.G. Coexpression patterns of sigma(B) regulators in Bacillus subtilis affect sigma(B) inducibility. J. Bacteriol. 2005, 187, 8520–8525. [Google Scholar] [CrossRef] [PubMed]
- Andrey, D.O.; Jousselin, A.; Villanueva, M.; Renzoni, A.; Monod, A.; Barras, C.; Rodriguez, N.; Kelley, W.L. Impact of the regulators sigB, rot, sarA and sarS on the toxic shock Tst promoter and TSST-1 expression in Staphylococcus aureus. PLoS ONE 2015, 10, e0135579. [Google Scholar] [CrossRef] [PubMed]
- Ziebandt, A.K.; Weber, H.; Rudolph, J.; Schmid, R.; Hoper, D.; Engelmann, S.; Hecker, M. Extracellular proteins of Staphylococcus aureus and the role of sarA and sigma B. Proteomics 2001, 1, 480–493. [Google Scholar] [CrossRef]
- Schmidt, K.A.; Donegan, N.P.; Kwan, W.A., Jr.; Cheung, A. Influences of sigma B and agr on expression of staphylococcal enterotoxin B (seb) in Staphylococcus aureus. Can. J. Microbiol. 2004, 50, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, I.M.; Arvidson, S.; Foster, S.; Tarkowski, A. Sigma factor B and RsbU are required for virulence in Staphylococcus aureus-induced arthritis and sepsis. Infect. Immun. 2004, 72, 6106–6111. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Bischoff, M.; Lattar, S.M.; Noto Llana, M.; Pfortner, H.; Niemann, S.; Geraci, J.; Van de Vyver, H.; Fraunholz, M.J.; Cheung, A.L.; et al. Sigma factor sigB is crucial to mediate Staphylococcus aureus adaptation during chronic infections. PLoS Pathog. 2015, 11, e1004870. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature 2011, 476, 393–394. [Google Scholar] [CrossRef] [PubMed]
- World Antibiotic Awareness Week. Available online: http://www.who.int/mediacentre/events/2015/world-antibiotic-awareness-week/event/en/ (accessed on 24 December 2015).
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, B.; Schuster, M. The sociomicrobiology of antivirulence drug resistance: A proof of concept. MBio 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C.; Popat, R.; Diggle, S.P.; Brown, S.P. Targeting virulence: Can we make evolution-proof drugs? Nat. Rev. Microbiol. 2014, 12, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ragle, B.E.; Bubeck Wardenburg, J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect. Immun. 2009, 77, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Hilliard, J.J.; Shi, Y.; Tkaczyk, C.; Cheng, L.I.; Yu, X.; Datta, V.; Ren, S.; Feng, H.; Zinsou, R.; et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, C.; Hua, L.; Varkey, R.; Shi, Y.; Dettinger, L.; Woods, R.; Barnes, A.; MacGill, R.S.; Wilson, S.; Chowdhury, P.; et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin. Vaccine Immunol. 2012, 19, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Foletti, D.; Strop, P.; Shaughnessy, L.; Hasa-Moreno, A.; Casas, M.G.; Russell, M.; Bee, C.; Wu, S.; Pham, A.; Zeng, Z.; et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus alpha-hemolysin. J. Mol. Biol. 2013, 425, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Pooja, J.; Ajit, S. Staphylococcus aureus β-hemolysin-neutralizing single-domain antibody isolated from phage display library of indian desert camel. Asian Pac. J. Trop Med. 2010, 3, 1–7. [Google Scholar]
- Rouha, H.; Badarau, A.; Visram, Z.C.; Battles, M.B.; Prinz, B.; Magyarics, Z.; Nagy, G.; Mirkina, I.; Stulik, L.; Zerbs, M.; et al. Five birds, one stone: Neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 2015, 7, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Karginov, V.A.; Nestorovich, E.M.; Schmidtmann, F.; Robinson, T.M.; Yohannes, A.; Fahmi, N.E.; Bezrukov, S.M.; Hecht, S.M. Inhibition of S. aureus alpha-hemolysin and B. anthracis lethal toxin by beta-cyclodextrin derivatives. Bioorg. Med. Chem. 2007, 15, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.C.; Caballero, A.R.; Balzli, C.L.; Tang, A.; O’Callaghan, R.J. Chemical inhibition of alpha-toxin, a key corneal virulence factor of Staphylococcus aureus. Invest. Ophthalmol. Vis. Sci. 2009, 50, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Ragle, B.E.; Karginov, V.A.; Bubeck Wardenburg, J. Prevention and treatment of Staphylococcus aureus pneumonia with a beta-cyclodextrin derivative. Antimicrob. Agents Chemother. 2010, 54, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qiu, J.; Zhang, Y.; Lu, C.; Dai, X.; Wang, J.; Li, H.; Wang, X.; Tan, W.; Luo, M.; et al. Oroxylin a inhibits hemolysis via hindering the self-assembly of alpha-hemolysin heptameric transmembrane pore. PLoS Comput. Biol. 2013, 9, e1002869. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, D.; Zhang, Y.; Dong, J.; Wang, J.; Niu, X. Molecular modeling reveals the novel inhibition mechanism and binding mode of three natural compounds to staphylococcal alpha-hemolysin. PLoS ONE 2013, 8, e80197. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.C.; Teixeira, L.R.; Pol-Fachin, L.; Rodrigues, C.G. Inhibition of the hemolytic activity caused by Staphylococcus aureus alpha-hemolysin through isatin-schiff copper(ii) complexes. FEMS Microbiol. Lett. 2016, 363. [Google Scholar]
- Wang, J.; Zhou, X.; Liu, S.; Li, G.; Shi, L.; Dong, J.; Li, W.; Deng, X.; Niu, X. Morin hydrate attenuates Staphylococcus aureus virulence by inhibiting the self-assembly of alpha-hemolysin. J. Appl. Microbiol. 2015, 118, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Rashidieh, B.; Etemadiafshar, S.; Memari, G.; Mirzaeichegeni, M.; Yazdi, S.; Farsimadan, F.; Alizadeh, S. A molecular modeling based screening for potential inhibitors to alpha hemolysin from Staphylococcus aureus. Bioinformation 2015, 11, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Saravanan, V.; Lakshmi, P.T.V.; Annamalai, A. Inhibition of pore formation by blocking the assembly of Staphylococcus aureus α-hemolysin through a novel peptide inhibitor: An in silico approach. Int. J. Pept. Res. Ther. 2014, 20, 575–583. [Google Scholar] [CrossRef]
- Dreymueller, D.; Uhlig, S.; Ludwig, A. Adam-family metalloproteinases in lung inflammation: Potential therapeutic targets. Am. J. Physiol Lung Cell. Mol. Physiol. 2015, 308, L325–343. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, G.R.; DeDent, A.C.; Becker, R.E.; Berube, B.J.; Gebhardt, M.J.; Cao, H.; Bubeck Wardenburg, J. Targeting Staphylococcus aureus alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J. Infect. Dis. 2014, 210, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Gauduchon, V.; Cozon, G.; Vandenesch, F.; Genestier, A.L.; Eyssade, N.; Peyrol, S.; Etienne, J.; Lina, G. Neutralization of Staphylococcus aureus Panton-Valentine leukocidin by intravenous immunoglobulin in vitro. J. Infect. Dis. 2004, 189, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Mairpady Shambat, S.; Chen, P.; Nguyen Hoang, A.T.; Bergsten, H.; Vandenesch, F.; Siemens, N.; Lina, G.; Monk, I.R.; Foster, T.J.; Arakere, G.; et al. Modelling staphylococcal pneumonia in a human 3D lung tissue model system delineates toxin-mediated pathology. Dis. Model. Mech. 2015, 8, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Rouzic, N.; Janvier, F.; Libert, N.; Javouhey, E.; Lina, G.; Nizou, J.Y.; Pasquier, P.; Stamm, D.; Brinquin, L.; Pelletier, C.; et al. Prompt and successful toxin-targeting treatment of three patients with necrotizing pneumonia due to Staphylococcus aureus strains carrying the Panton-Valentine leukocidin genes. J. Clin. Microbiol. 2010, 48, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Laventie, B.J.; Rademaker, H.J.; Saleh, M.; de Boer, E.; Janssens, R.; Bourcier, T.; Subilia, A.; Marcellin, L.; van Haperen, R.; Lebbink, J.H.; et al. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc. Natl. Acad. Sci. USA 2011, 108, 16404–16409. [Google Scholar] [CrossRef] [PubMed]
- Karauzum, H.; Adhikari, R.P.; Sarwar, J.; Devi, V.S.; Abaandou, L.; Haudenschild, C.; Mahmoudieh, M.; Boroun, A.R.; Vu, H.; Nguyen, T.; et al. Structurally designed attenuated subunit vaccines for S. aureus LukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS ONE 2013, 8, e65384. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.P.; Kort, T.; Shulenin, S.; Kanipakala, T.; Ganjbaksh, N.; Roghmann, M.C.; Holtsberg, F.W.; Aman, M.J. Antibodies to S. aureus LukS-PV attenuated subunit vaccine neutralize a broad spectrum of canonical and non-canonical bicomponent leukotoxin pairs. PLoS ONE 2015, 10, e0137874. [Google Scholar] [CrossRef] [PubMed]
- Vandenesch, F.; Naimi, T.; Enright, M.C.; Lina, G.; Nimmo, G.R.; Heffernan, H.; Liassine, N.; Bes, M.; Greenland, T.; Reverdy, M.E.; et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emergence. Emerg. Infect. Dis. 2003, 9, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Otto, M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008, 16, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Hermos, C.R.; Yoong, P.; Pier, G.B. High levels of antibody to Panton-Valentine leukocidin are not associated with resistance to Staphylococcus aureus-associated skin and soft-tissue infection. Clin. Infect. Dis. 2010, 51, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Cardot-Martin, E.; Casalegno, J.S.; Badiou, C.; Dauwalder, O.; Keller, D.; Prevost, G.; Rieg, S.; Kern, W.V.; Cuerq, C.; Etienne, J.; et al. Alpha-defensins partially protect human neutrophils against Panton-Valentine leukocidin produced by Staphylococcus aureus. Lett. Appl. Microbiol. 2015, 61, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal enterotoxins. Toxins 2010, 2, 2177–2197. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; Blackman, M.; Kushnir, E.; Kappler, J. The toxicity of staphylococcal enterotoxin B in mice is mediated by t cells. J. Exp. Med. 1990, 171, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, B.; Zhou, Y.; Kline, B.; Spidel, J.; Chan, Y.Y.; Albone, E.; Turchin, H.; Chao, Q.; Henry, M.; Balogach, J.; et al. Generation and characterization of high affinity human monoclonal antibodies that neutralize staphylococcal enterotoxin B. J. Immune Based Ther. Vaccines 2010, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.E.; Rajagopalan, G.; Shah-Mahoney, N.; Lawlor, R.G.; Tilahun, A.Y.; Xie, C.; Natarajan, K.; Margulies, D.H.; Ratner, D.I.; Osborne, B.A.; et al. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect. Immun. 2010, 78, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Varshney, A.K.; Franklin, M.C.; Goger, M.; Wang, X.; Fries, B.C. Mechanisms mediating enhanced neutralization efficacy of staphylococcal enterotoxin B by combinations of monoclonal antibodies. J. Biol. Chem. 2015, 290, 6715–6730. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.K.; Wang, X.; Scharff, M.D.; MacIntyre, J.; Zollner, R.S.; Kovalenko, O.V.; Martinez, L.R.; Byrne, F.R.; Fries, B.C. Staphylococcal enterotoxin B-specific monoclonal antibody 20B1 successfully treats diverse Staphylococcus aureus infections. J. Infect. Dis. 2013, 208, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.K.; Wang, X.; Cook, E.; Dutta, K.; Scharff, M.D.; Goger, M.J.; Fries, B.C. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J. Biol. Chem. 2011, 286, 9737–9747. [Google Scholar] [CrossRef] [PubMed]
- Buonpane, R.A.; Churchill, H.R.; Moza, B.; Sundberg, E.J.; Peterson, M.L.; Schlievert, P.M.; Kranz, D.M. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat. Med. 2007, 13, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Buonpane, R.A.; Moza, B.; Rahman, A.K.; Wang, N.; Schlievert, P.M.; McCormick, J.K.; Sundberg, E.J.; Kranz, D.M. Neutralization of multiple staphylococcal superantigens by a single-chain protein consisting of affinity-matured, variable domain repeats. J. Infect. Dis. 2008, 198, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Sulfasalazine attenuates staphylococcal enterotoxin B-induced immune responses. Toxins 2015, 7, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Collins, L.V.; Cullor, J.S.; Hume, E.B.; Medina-Acosta, E.; Vieira da Motta, O.; O’Callaghan, R.; Rossitto, P.V.; Shirtliff, M.E.; Serafim da Silveira, L.; et al. Prevention of diseases caused by Staphylococcus aureus using the peptide RIP. Peptides 2000, 21, 1301–1311. [Google Scholar] [CrossRef]
- Gov, Y.; Bitler, A.; Dell’Acqua, G.; Torres, J.V.; Balaban, N. RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus pathogenesis: Structure and function analysis. Peptides 2001, 22, 1609–1620. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, Y.; Li, M.; Yu, Q.; Xue, X.; Li, Z.; Da, F.; Hou, Z.; Luo, X. RIP-V improves murine survival in a sepsis model by down-regulating RNAIII expression and alpha-hemolysin release of methicillin-resistant Staphylococcus aureus. Pharmazie 2015, 70, 81–87. [Google Scholar] [PubMed]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Dell’Acqua, G.; Orlando, F.; D’Amato, G.; Mocchegiani, F.; Silvestri, C.; Del Prete, M.S.; Rocchi, M.; et al. RNAIII-inhibiting peptide improves efficacy of clinically used antibiotics in a murine model of staphylococcal sepsis. Peptides 2005, 26, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Cirioni, O.; Ghiselli, R.; Goteri, G.; Scalise, A.; Orlando, F.; Silvestri, C.; Riva, A.; Saba, V.; Madanahally, K.D.; et al. RNAIII-inhibiting peptide enhances healing of wounds infected with methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, H.; Wang, L.; Song, Z.; Shi, L.; Li, W.; Deng, X.; Wang, J. Isorhamnetin attenuates Staphylococcus aureus-induced lung cell injury by inhibiting alpha-hemolysin expression. J. Microbiol. Biotechnol. 2015. [Google Scholar]
- Wang, J.; Qiu, J.; Dong, J.; Li, H.; Luo, M.; Dai, X.; Zhang, Y.; Leng, B.; Niu, X.; Zhao, S.; et al. Chrysin protects mice from Staphylococcus aureus pneumonia. J. Appl. Microbiol. 2011, 111, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, W.H.; Zhou, X.; Liu, Y.H.; Li, Z.; Tang, Y.S.; Kou, X.; Wang, S.D.; Bao, M.; Qu, L.D.; et al. Puerarin protects against Staphylococcus aureus-induced injury of human alveolar epithelial aA49 cells via downregulating alpha-hemolysin secretion. Microb. Drug Resist. 2014, 20, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Niu, X.; Wang, J.; Xing, Y.; Leng, B.; Dong, J.; Li, H.; Luo, M.; Zhang, Y.; Dai, X.; et al. Capsaicin protects mice from community-associated methicillin-resistant Staphylococcus aureus pneumonia. PLoS ONE 2012, 7, e33032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.F.; Dong, J.; Wei, J.Y.; Wang, Y.N.; Dai, X.H.; Wang, X.; Luo, M.J.; Tan, W.; Deng, X.M.; et al. Inhibition of alpha-toxin production by subinhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia 2013, 86, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdian, V.; Pesho, M.; Truitt, B.; Bollinger, L.; Patel, P.; Nithianantham, S.; Yu, G.; Delaney, E.; Jankowsky, E.; Shoham, M. Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3645–3652. [Google Scholar] [CrossRef] [PubMed]
- Sully, E.K.; Malachowa, N.; Elmore, B.O.; Alexander, S.M.; Femling, J.K.; Gray, B.M.; DeLeo, F.R.; Otto, M.; Cheung, A.L.; Edwards, B.S.; et al. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 2014, 10, e1004174. [Google Scholar] [CrossRef] [PubMed]
- Daly, S.M.; Elmore, B.O.; Kavanaugh, J.S.; Triplett, K.D.; Figueroa, M.; Raja, H.A.; El-Elimat, T.; Crosby, H.A.; Femling, J.K.; Cech, N.B.; et al. Omega-hydroxyemodin limits Staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob. Agents Chemother. 2015, 59, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Mansson, M.; Nielsen, A.; Kjaerulff, L.; Gotfredsen, C.H.; Wietz, M.; Ingmer, H.; Gram, L.; Larsen, T.O. Inhibition of virulence gene expression in Staphylococcus aureus by novel depsipeptides from a marine photobacterium. Mar. Drugs 2011, 9, 2537–2552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, A.; Mansson, M.; Bojer, M.S.; Gram, L.; Larsen, T.O.; Novick, R.P.; Frees, D.; Frokiaer, H.; Ingmer, H. Solonamide B inhibits quorum sensing and reduces Staphylococcus aureus mediated killing of human neutrophils. PLoS ONE 2014, 9, e84992. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Mansson, M.; Wietz, M.; Varming, A.N.; Phipps, R.K.; Larsen, T.O.; Gram, L.; Ingmer, H. Nigribactin, a novel siderophore from Vibrio nigripulchritudo, modulates Staphylococcus aureus virulence gene expression. Mar. Drugs 2012, 10, 2584–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjaerulff, L.; Nielsen, A.; Mansson, M.; Gram, L.; Larsen, T.O.; Ingmer, H.; Gotfredsen, C.H. Identification of four new agr quorum sensing-interfering cyclodepsipeptides from a marine Photobacterium. Mar. Drugs 2013, 11, 5051–5062. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H.; Cho, M.H.; Lee, J. Flavone reduces the production of virulence factors, staphyloxanthin and alpha-hemolysin, in Staphylococcus aureus. Curr. Microbiol. 2012, 65, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Burton, N.; Cooper, R. Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus (MRSA) exposed to manuka honey in vitro demonstrated down-regulation of virulence markers. J. Antimicrob. Chemother. 2014, 69, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Ravikumar, R.; Santhosh, R.S.; Princy, S.A. SarA based novel therapeutic candidate against Staphylococcus aureus associated with vascular graft infections. Front. Microbiol. 2015, 6, 416. [Google Scholar] [CrossRef] [PubMed]
- Sifri, C.D.; Begun, J.; Ausubel, F.M.; Calderwood, S.B. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 2003, 71, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Conly, J.; McClure, J.A.; Elsayed, S.; Louie, T.; Zhang, K. Caenorhabditis elegans as a host model for community-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2010, 16, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Yehye, W.A.; Abd Rahman, N.; Tan, M.W.; Nathan, S. Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement. Altern. Med. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Moy, T.I.; Conery, A.L.; Larkins-Ford, J.; Wu, G.; Mazitschek, R.; Casadei, G.; Lewis, K.; Carpenter, A.E.; Ausubel, F.M. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 2009, 4, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E.; Urbach, J.M.; Ausubel, F.M. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 2010, 10, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Barczak, A.K.; Hung, D.T. Productive steps toward an antimicrobial targeting virulence. Curr. Opin. Microbiol. 2009, 12, 490–496. [Google Scholar] [CrossRef] [PubMed]




| Toxin Target(s) | Type | Name | Mode of Action | Phase of Development | References |
|---|---|---|---|---|---|
| α-hemolysin | Monoclonal antibody | MAbs 7B8 and 1A9 | Antagonizes toxin activity by inhibiting the formation of fully assembled α-hemolysin oligomer. | Testing in animal model (mice pneumonia model) | [90] |
| α-hemolysin | Monoclonal antibody | MAb 2A3.1 (and affinity-optimized 2A3 variant—LC10) | Neutralizes toxin and prevents toxin-mediated cell lysis via a blockade of α-toxin heptamer formation on erythrocyte membranes. | Testing in animal models (S. aureus dermonecrosis murine model and mouse model of S. aureus pneumonia) | [91,92] |
| α-hemolysin | Monoclonal antibody | MAb LTM14 | Prevents binding of toxin to the plasma membrane of susceptible host cells. | Testing in animal models (mice pneumonia, skin and bacteremia models) | [93] |
| α-hemolysin | Chemical compound | β-cyclodextrin derivatives | Blocks the transmembrane pores formed by the toxin and terminates ion conductance through the pores. | Testing in animal models (S. aureus pneumonia mice model) | [96,98] |
| α-hemolysin | Natural compound | Oroxylin A, Oroxin A and Oroxin B | Binds to the “stem” region of α-hemolysin and restricts the conformational transition of toxin from monomer to oligomer. | In vitro assays | [99,100] |
| α-hemolysin | Chemical compound | Isatin-Schiff copper (II) complexes | Prevents the formation of ion channels by obstructing the constriction region of the α-hemolysin channel. | In vitro assays | [101] |
| α-hemolysin | Natural compound | Morin hydrate | Inhibits self-assembly of the heptameric transmembrane pore of α-hemolysin. | Testing in animals (mice pneumonia model) | [102] |
| α-hemolysin | Chemical compound | ADAM10 inhibitor (GI254023X) | Inhibits the binding of α-hemolysin to its receptor (ADAM10). | Testing in animals (mice model of recurrent skin and soft-tissue infection) | [106] |
| β-hemolysin | Single-domain antibody | dAb/SAE Cl-7-5 | Neutralizes S. aureus Hlb activity. | In vitro assays | [94] |
| α-hemolysin and bi-component leukocidins | Monoclonal antibody | MAb Hla-F#5 | Cross-neutralizes α-hemolysin and leukocidins by recognizing the conserved conformational epitope. | Testing in animal models (murine models of S. aureus pneumonia and bacteremia/sepsis) | [95] |
| PVL and α-hemolysin | Polyclonal antibody | Human intravenous polyclonal immunoglobuin (IVIg)—Tegeline | Inhibits the lytic effect of PVL on polymorphonuclear cells and neutralizes α-hemolysin. | In vitro assays for PVL; in vivo peritonitis murine model for hemolysin | [107,108] |
| PVL and γ-hemolysin | Humanized heavy chain-only antibody | Bivalent and tetravalent anti-PVL mAbs | Blocks binding of PVL to target cells and inhibits pore formation on target cells by γ-hemolysin. | Testing in animal models (rabbit model of toxin-induced endophthalmitis) | [110] |
| PVL and other leukotoxins | Polyclonal antibody | Anti-LukS-mut9 | Cross-neutralizes the lytic activity of various leukotoxins on polymorphonuclear cells. | Testing in animal models (toxin-challenged mouse model) | [112] |
| PVL | Antimicrobial peptide | α-defensin HNP3 | Binds to both LukS-PV and LukF-PV and reduces PVL-induced necrosis in human neutrophils by interfering with pore formation. | In vitro assays | [116] |
| SEB | Monoclonal antibody | HuMAb-154 | Binds to SEB, neutralizes the toxin and inhibits SEB-induced production of proinflammatory cytokines. | Testing in animal models (mice model challenged by SEB) | [119] |
| SEB | Monoclonal antibody | MAb 20B1 | Binds and neutralizes SEB. | Testing in animal models (mice sepsis, superficial skin and deep-tissue infection models) | [121,122] |
| SEB | Protein | Soluble Vβ protein | As a receptor antagonist that offers high-affinity binding to SEB superantigens and neutralizes the toxicity of SEB. | Testing in animal model (rabbit model of SEB-induced disease) | [124] |
| SEB and TSST-1 | Protein | Broad spectrum Vβ protein | Binds to superantigens and neutralizes both SEB and TSST-1 activities. | In vitro assays | [125] |
| SEB | FDA-approved drug | Sulfasalazine | Reverses SEB-stimulated toxic effect by inhibiting the production of proinflammatory cytokines, T-cell proliferation and NFκB activation. | In vitro assays | [126] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Neoh, H.-m.; Nathan, S. Targeting Staphylococcus aureus Toxins: A Potential form of Anti-Virulence Therapy. Toxins 2016, 8, 72. https://doi.org/10.3390/toxins8030072
Kong C, Neoh H-m, Nathan S. Targeting Staphylococcus aureus Toxins: A Potential form of Anti-Virulence Therapy. Toxins. 2016; 8(3):72. https://doi.org/10.3390/toxins8030072
Chicago/Turabian StyleKong, Cin, Hui-min Neoh, and Sheila Nathan. 2016. "Targeting Staphylococcus aureus Toxins: A Potential form of Anti-Virulence Therapy" Toxins 8, no. 3: 72. https://doi.org/10.3390/toxins8030072