A Single Dose of ViperfavTM May Be Inadequate for Vipera ammodytes Snake Bite: A Case Report and Pharmacokinetic Evaluation
Abstract
:1. Introduction
2. Case Description
2.1. Case Report
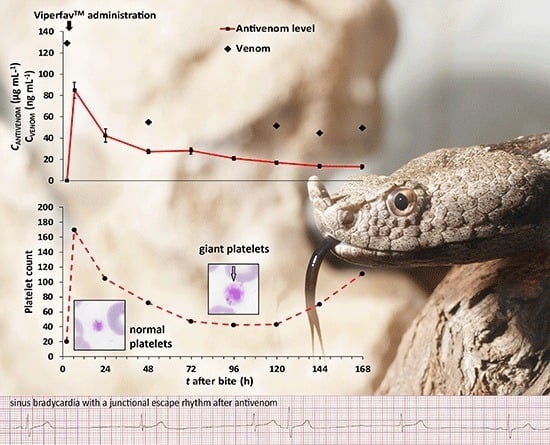
2.2. Detection of V. a. ammodytes Venom in Sera Samples
2.3. Pharmacokinetics of Antivenom Level Decrement
- when t ≤ tinf,c(t) = A × (1 − e−αt) + B × (1 − e−βt)
- when t > tinf,c(t) = A × (e−α(t − tinf) – e−αt) + B × (e−β(t − tinf) – e−βt)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Quantification of V. a. ammodytes Venom in Sera Samples
5.2. Quantification of F(ab’)2 Fragments in Sera Samples
5.3. Pharmacokinetic Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chippaux, J.P. Epidemiology of snakebites in Europe: A systematic review of the literature. Toxicon 2012, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Harry, P.; de Haro, L.; Asfar, P.; David, J.M. Evaluation de I’immunotherapie anti-viperine par fragments F(ab’)2 purifies (Viperfav™). Presse Med. 1999, 28, 1929–1934. [Google Scholar] [PubMed]
- De Haro, L.; Glaizal, M.; Tichadou, L.; Blanc-Brisset, I.; Hayek-Lanthois, M. Asp Viper (Vipera aspis) envenomation: Experience of the Marseille Poison Centre from 1996 to 2008. Toxins 2009, 1, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Boels, D.; Hamel, J.F.; Deguigne, M.B.; Harry, P. European viper envenomings: Assessment of Viperfav™ and other symptomatic treatments. Clin. Toxicol. 2012, 50, 189–196. [Google Scholar] [CrossRef] [PubMed]
- De Haro, L. Management of snakebites in France. Toxicon 2012, 60, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Jollivet, V.; Hamel, J.F.; de Haro, L.; Labadie, M.; Sapori, J.M.; Cordier, L.; Villa, A.; Nisse, P.; Puskarczyk, E.; Berthelon, L.; et al. European viper envenomation recorded by French poison control centers: A clinical assessment and management study. Toxicon 2015, 108, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Claudet, I.; Grouteau, E.; Cordier, L.; Franchitto, N.; Bréhin, C. Hyperglycemia is a risk factor for high-grade envenomations after European viper bites (Vipera spp.) in children. Clin. Toxicol. (Phila) 2016, 54, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Thein-Than; Kyi-Thein; Mg-Mg-Thwin. Plasma clearance time of Russell’s viper (Vipera russelli) antivenom in human snake bite victims. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 262–263. [Google Scholar] [CrossRef]
- Ho, M.; Silamut, K.; White, N.J.; Karbwang, J.; Looareesuwan, S.; Phillips, R.E.; Warell, D.A. Pharmacokinetics of three commercial antivenoms in patients envenomed by the Malayan pit viper, Calloselasma rhodostoma, in Thailand. Am. J. Trop. Med. Hyg. 1990, 42, 260–266. [Google Scholar] [PubMed]
- Meyer, W.P.; Habib, A.G.; Onayade, A.A.; Yakubu, A.; Smith, D.C.; Nasidi, A.; Daudu, I.J.; Warrell, D.A.; Theakston, R.D. First clinical experiences with a new ovine Fab Echis ocellatus snake bite antivenom in Nigeria: Randomized comparative trial with Institute Pasteur Serum (Ipser) Africa antivenom. Am. J. Trop. Med. Hyg. 1997, 56, 291–300. [Google Scholar] [PubMed]
- Nielsen, H.; Sørensen, H.; Faber, V.; Svehag, S.E. Circulating immune complexes, complement activation kinetics and serum sickness following treatment with heterologous anti-snake venom globulin. Scand. J. Immunol. 1978, 7, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ariaratnam, C.A.; Meyer, W.P.; Perera, G.; Eddelston, M.; Kuleratne, A.M.; Attapattu, W.; Sherife, R.; Richards, A.M.; Theakston, R.D.G.; Warrell, D.A. A new monospecific ovine Fab fragment antivenom for treatment of envenoming by the Sri Lankan Russell’s viper (Daboia russelli russelli): A preliminary dose-finding and pharmacokinetic study. Am. J. Trop. Med. Hyg. 1999, 61, 259–265. [Google Scholar] [PubMed]
- Seifert, S.A.; Boyer, L.V. Recurrence Phenomena after Immunoglobulin Therapy for Snake Envenomations: Part 1. Pharmacokinetics and Pharmacodynamics of Immunoglobulin Antivenoms and Related Antibodies. Ann. Emerg. Med. 2001, 37, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Maduwage, K.; Saiao, A.; Buckley, N.A.; Jayamanne, S.F.; Seyed, S.; Mohamed, F.; Chathuranga, U.; Mendes, A.; Abeysinghe, C.; et al. Population pharmacokinetics of an Indian F (ab’)2 snake antivenom in patients with Russell’s viper (Daboia russelii) bites. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Luksic, B.; Culic, V.; Stricevic, L.; Brizic, I.; Poljak, N.K.; Tadic, Z. Infant death after nose-horned viper (Vipera ammodytes ammodytes) bite in Croatia: A case report. Toxicon 2010, 56, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, A.M. Case report: Acute myocardial infarction complicating a viper bite. Am. J. Trop. Med. 2001, 64, 280–282. [Google Scholar]
- Frangides, C.Y.; Koulouras, V.; Kouni, S.N.; Tzortzatos, G.V.; Nikolaou, A.; Pneumaticos, J.; Pierrakeas, C.; Niarchos, C.; Kounis, N.G.; Koutsojannis, C.M. Snake venom poisoning in Greece. Experiences with 147 cases. Eur. J. Intern. Med. 2006, 17, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ludden, T.M.; Beal, S.L.; Sheiner, L.B. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J. Pharmacokinet. Biopharm. 1994, 22, 431–445. [Google Scholar] [CrossRef] [PubMed]

| Pharmacokinetic Parameters | Values |
|---|---|
| t1/2α | 6.56 h |
| t1/2β | 97.64 h |
| Vss | 213.7 mL·kg−1 |
| MRT | 130.25 h |
| AUC∞ | 6095 μg·h·mL−1 |
| AUMC | 793,893 μg·h2·mL−1 |
| CL | 1.64 (mL·h−1)·kg−1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurtović, T.; Brvar, M.; Grenc, D.; Lang Balija, M.; Križaj, I.; Halassy, B. A Single Dose of ViperfavTM May Be Inadequate for Vipera ammodytes Snake Bite: A Case Report and Pharmacokinetic Evaluation. Toxins 2016, 8, 244. https://doi.org/10.3390/toxins8080244
Kurtović T, Brvar M, Grenc D, Lang Balija M, Križaj I, Halassy B. A Single Dose of ViperfavTM May Be Inadequate for Vipera ammodytes Snake Bite: A Case Report and Pharmacokinetic Evaluation. Toxins. 2016; 8(8):244. https://doi.org/10.3390/toxins8080244
Chicago/Turabian StyleKurtović, Tihana, Miran Brvar, Damjan Grenc, Maja Lang Balija, Igor Križaj, and Beata Halassy. 2016. "A Single Dose of ViperfavTM May Be Inadequate for Vipera ammodytes Snake Bite: A Case Report and Pharmacokinetic Evaluation" Toxins 8, no. 8: 244. https://doi.org/10.3390/toxins8080244