The Antagonistic Effect of Mycotoxins Deoxynivalenol and Zearalenone on Metabolic Profiling in Serum and Liver of Mice
Abstract
:1. Introduction
2. Results and Discussion
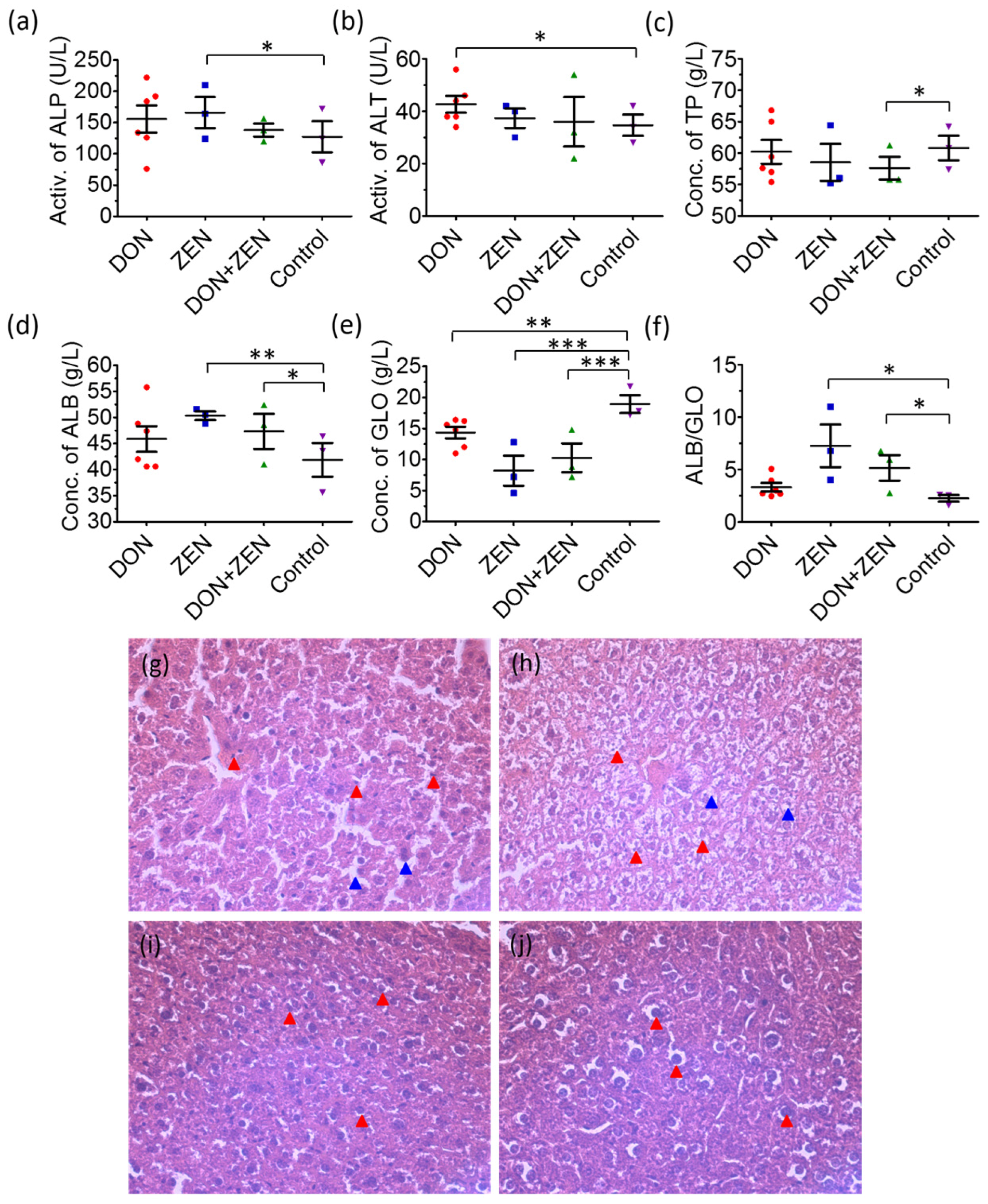
2.1. The Analysis of Blood Biochemical Indexes
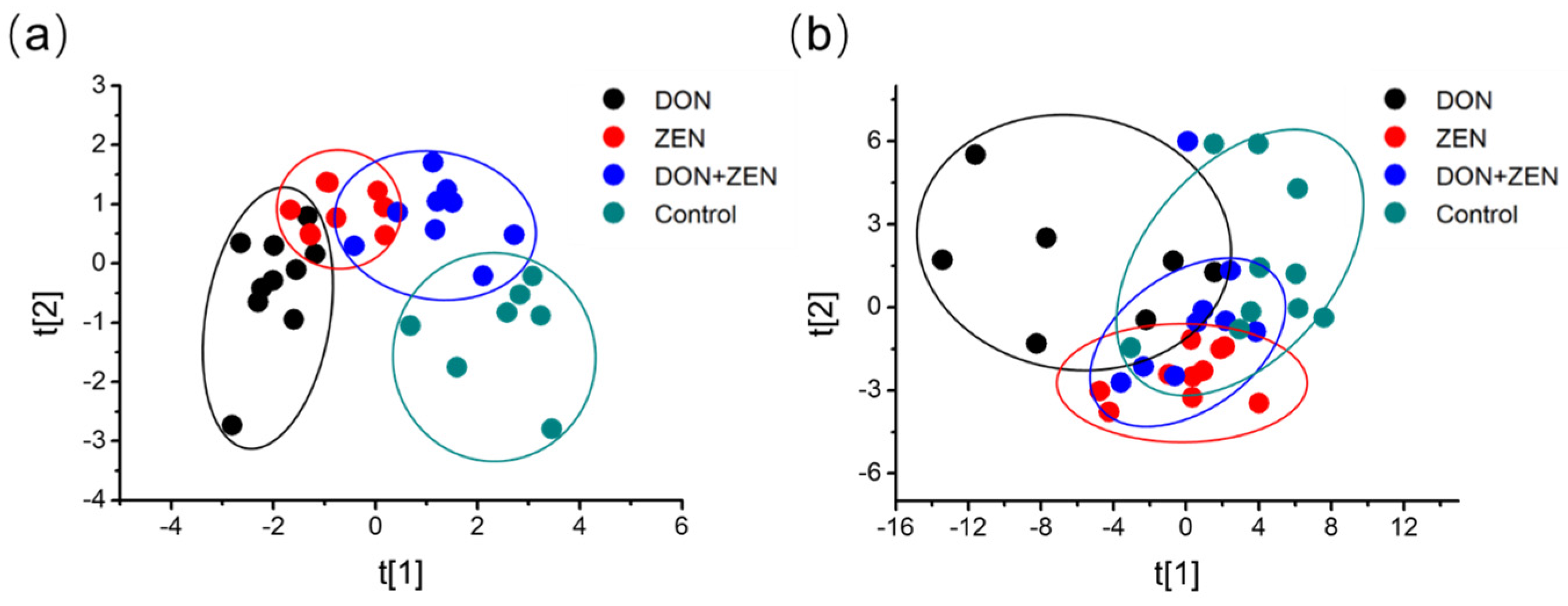
2.2. Multivariate Statistical Analysis
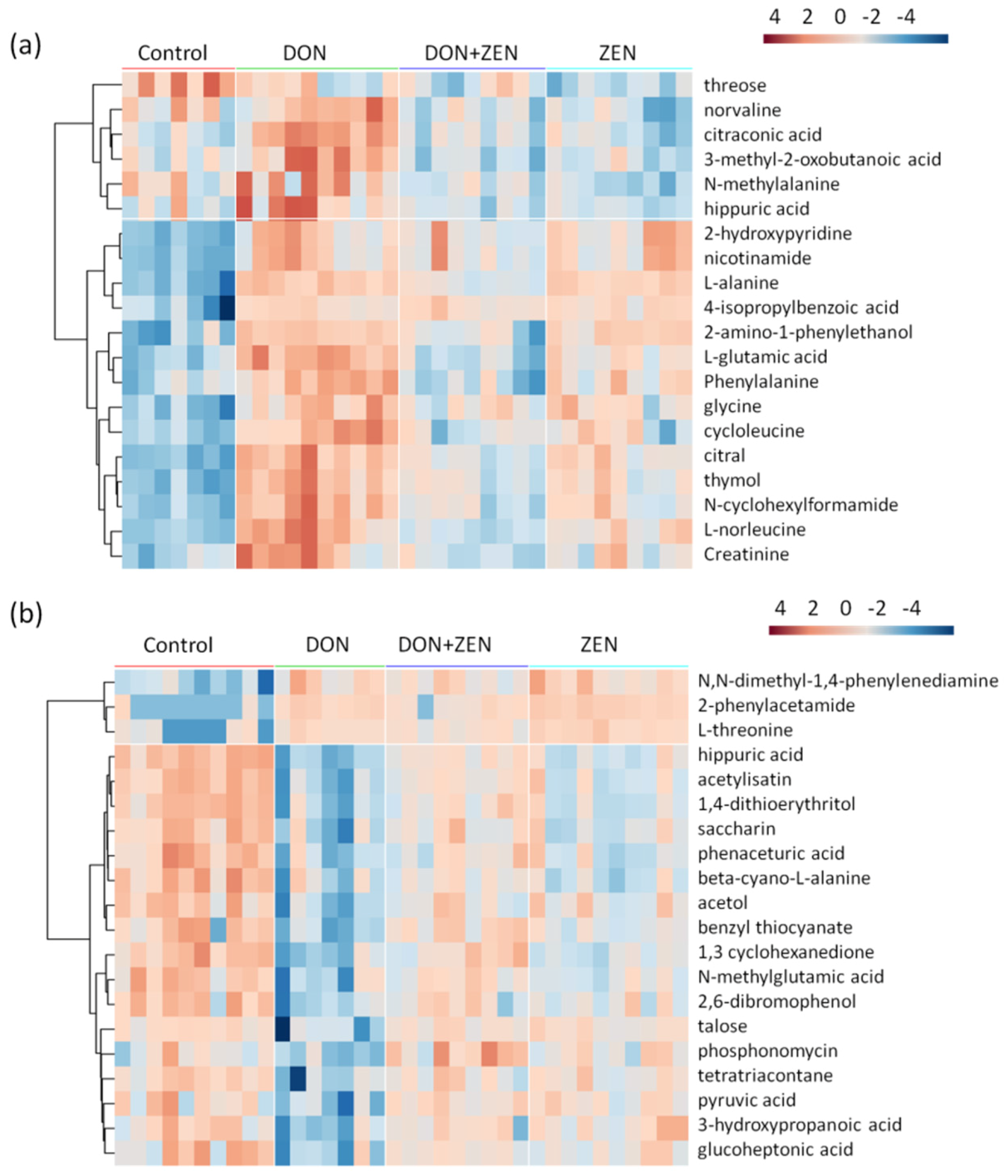
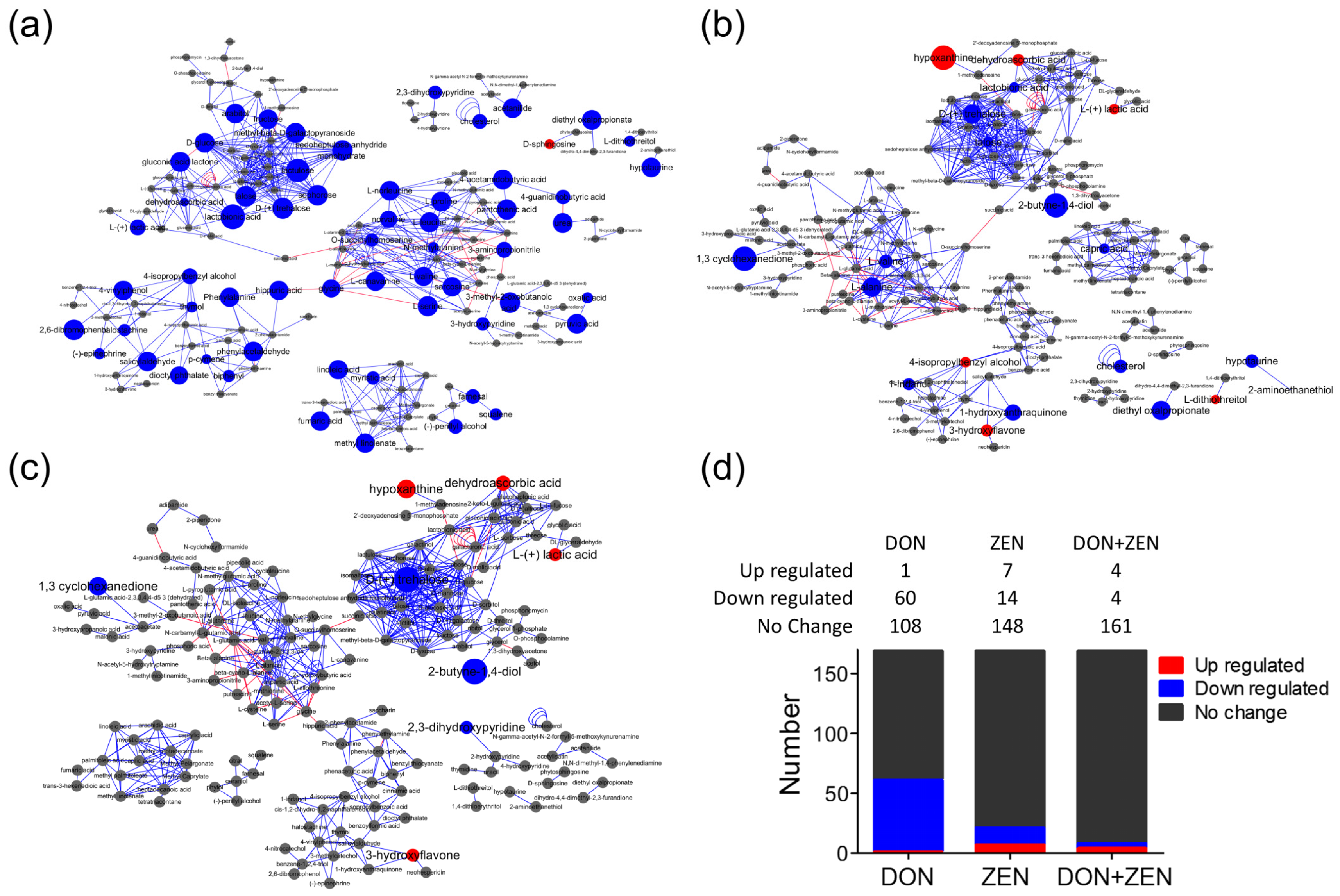
2.3. Metabolites Screening
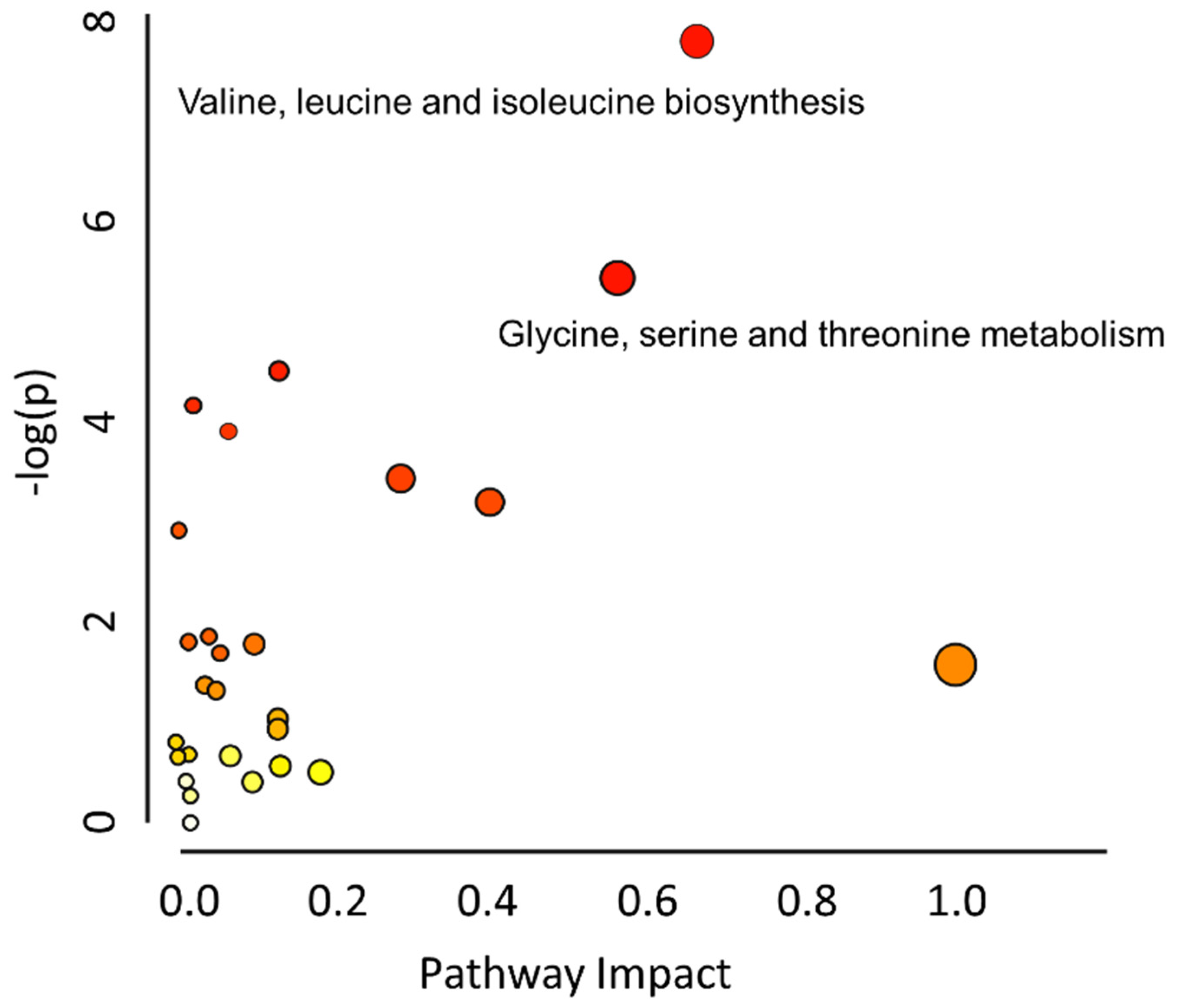
2.4. Metabolic Pathway Analysis
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Handling and Treatment
4.3. Metabolite Extraction
4.4. GC/MS Analysis
4.5. Metabolite Profiling Analysis
4.6. Multivariate Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lauren, D.; Smith, W. Stability of the Fusarium mycotoxins nivalenol, deoxynivalenol and zearalenone in ground maize under typical cooking environments. Food Addit. Contam. 2001, 18, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Krysinska-Traczyk, E.; Perkowski, J.; Dutkiewicz, J. Levels of fungi and mycotoxins in the samples of grain and grain dust collected from five various cereal crops in eastern poland. Ann. Agric. Environ. Med. 2007, 14, 159. [Google Scholar] [PubMed]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Klobasa, F.; Döll, S.; Ganter, M.; Flachowsky, G. Effects of graded levels of fusarium toxin contaminated wheat in diets for fattening pigs on growth performance, nutrient digestibility, deoxynivalenol balance and clinical serum characteristics. Arch. Anim. Nutr. 2004, 58, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Drochner, W.; Schollenberger, M.; Götz, S.; Lauber, U.; Tafaj, M.; Piepho, H.P. Subacute effects of moderate feed loads of isolated fusarium toxin deoxynivalenol on selected parameters of metabolism in weaned growing piglets. J. Anim. Physiol. Anim. Nutr. 2006, 90, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.-R.; Moon, Y.; Chung, Y. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Whitten, D.A.; Wilkerson, C.G.; Pestka, J.J. Dynamic changes in ribosome-associated proteome and phosphoproteome during deoxynivalenol-induced translation inhibition and ribotoxic stress. Toxicol. Sci. 2013, 138, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Whitten, D.A.; Wu, M.; Chan, C.; Wilkerson, C.G.; Pestka, J.J. Early phosphoproteomic changes in the mouse spleen during deoxynivalenol-induced ribotoxic stress. Toxicol. Sci. 2013, 135, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Porter, K.; Parameswaran, N.; Bae, H.K.; Pestka, J.J. Role of grp78/bip degradation and er stress in deoxynivalenol-induced interleukin-6 upregulation in the macrophage. Toxicol. Sci. 2009, 109, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Tatay, E.; Meca, G.; Font, G.; Ruiz, M.-J. Interactive effects of zearalenone and its metabolites on cytotoxicity and metabolization in ovarian cho-k1 cells. Toxicol. In Vitro 2014, 28, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Mitterbauer, R.; Weindorfer, H.; Safaie, N.; Krska, R.; Lemmens, M.; Ruckenbauer, P.; Kuchler, K.; Adam, G. A sensitive and inexpensive yeast bioassay for the mycotoxin zearalenone and other compounds with estrogenic activity. Appl. Environ. Microbiol. 2003, 69, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.E.; Ferrell, J.M.; Ostby, J.S. Alteration of behavioral sex differentiation by exposure to estrogenic compounds during a critical neonatal period: Effects of zearalenone, methoxychlor, and estradiol in hamsters. Toxicol. Appl. Pharmacol. 1985, 80, 127–136. [Google Scholar] [CrossRef]
- Katzenellenbogen, B.S.; Katzenellenbogen, J.A.; Mordecai, D. Zearalenones: Characterization of the estrogenic potencies and receptor interactions of a series of fungal β-resorcylic acid lactones. Endocrinology 1979, 105, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Gu, W.; Sun, C.; Sun, J.; Jiang, H.; Zhang, Y.; Sun, X. A novel recombinant cell fluorescence biosensor based on toxicity of pathway for rapid and simple evaluation of don and zen. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Q.; Zhang, Z.; Lin, P.; Lei, L.; Wang, A.; Jin, Y. Endoplasmic reticulum stress cooperates in zearalenone-induced cell death of raw 264.7 macrophages. Int. J. Mol. Sci. 2015, 16, 19780–19795. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Y.; Zheng, Z.-H.; Son, Y.-O.; Shi, X.; Jang, Y.-O.; Lee, J.-C. Mycotoxin zearalenone induces aif-and ros-mediated cell death through p53-and mapk-dependent signaling pathways in raw264. 7 macrophages. Toxicol. In Vitro 2011, 25, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yu, J.-Y.; Lim, S.-S.; Son, Y.-O.; Kim, D.-H.; Lee, S.-A.; Shi, X.; Lee, J.-C. Cellular mechanisms of the cytotoxic effects of the zearalenone metabolites α-zearalenol and β-zearalenol on raw264. 7 macrophages. Toxicol. In Vitro 2013, 27, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Dänicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins 2013, 5, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warth, B.; Sulyok, M.; Berthiller, F.; Schuhmacher, R.; Krska, R. New insights into the human metabolism of the fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013, 220, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ficheux, A.; Sibiril, Y.; Parent-Massin, D. Co-exposure of fusarium mycotoxins: In vitro myelotoxicity assessment on human hematopoietic progenitors. Toxicon 2012, 60, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins b1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Halama, A. Metabolomics in cell culture—A strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch. Biochem. Biophys. 2014, 564, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Barupal, D.K.; Haldiya, P.K.; Wohlgemuth, G.; Kind, T.; Kothari, S.L.; Pinkerton, K.E.; Fiehn, O. Metamapp: Mapping and visualizing metabolomic data by integrating information from biochemical pathways and chemical and mass spectral similarity. BMC Bioinf. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Quanbeck, S.M.M.; Brachova, L.; Campbell, A.A.; Guan, X.; Perera, A.; He, K.; Rhee, S.Y.; Bais, P.; Dickerson, J.; Dixon, P. Metabolomics as a hypothesis-generating functional genomics tool for the annotation of arabidopsis thaliana genes of “unknown function”. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Zenobi, R. Single-cell metabolomics: Analytical and biological perspectives. Science 2013, 342. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of iceland. Gastroenterology 2013, 144, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Giorgio, V.; Liccardo, D.; Bedogni, G.; Morino, G.; Alisi, A.; Cianfarani, S. Vitamin d levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur. J. Endocrinol. 2014, 170, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Wang, G.X.; Liu, J.L.; Fan, J.J.; Cui, S. Toxic effects of zearalenone and its derivatives α-zearalenol on male reproductive system in mice. Reprod. Toxicol. 2007, 24, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. Pubchem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009, 37, W623–W633. [Google Scholar] [CrossRef] [PubMed]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic regulation of t lymphocytes. Annu. Rev. Immunol. 2013, 31. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Sinclair, C.; Pulendran, B. Translating glycolytic metabolism to innate immunity in dendritic cells. Cell Metab. 2014, 19, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Yiannaki, E.; Markala, D.; Arampatzis, S.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte reaction affects glucose influx and enzymes involved in aerobic glycolysis and glutaminolysis in alloreactive t-cells. Hum. Immunol. 2013, 74, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.N.; Mijal, R.S.; Keen, J.N.; Findlay, J.B.; Wild, C.P. Proteomic analysis of the effects of the immunomodulatory mycotoxin deoxynivalenol. Proteomics 2011, 11, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, L.; Li, R.; Wang, L.-N.; Yang, Y.; Liu, N.-N.; Zhang, C.-M.; Wang, Y.; Liu, P.; Tu, B.-B. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: Plasma metabolomics analysis. BMC Med. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ye, Y.; Deng, L.; Ma, H.; Fan, X.; Zhang, Y.; Yan, H.; Deng, X.; Li, Y.; Ma, Y. Gene expression profiling analysis of deoxynivalenol-induced inhibition of mouse thymic epithelial cell proliferation. Environ. Toxicol. Pharmacol. 2013, 36, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Ly-Verdú, S.; Gröger, T.M.; Arteaga-Salas, J.M.; Brandmaier, S.; Kahle, M.; Neschen, S.; de Angelis, M.H.; Zimmermann, R. Combining metabolomic non-targeted GC× GC–tof–ms analysis and chemometric asca-based study of variances to assess dietary influence on type 2 diabetes development in a mouse model. Anal. Bioanal. Chem. 2015, 407, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kind, T. Metabolite profiling in blood plasma. Metabolomics 2007, 358, 3–17. [Google Scholar]
- Ji, J.; Sun, J.; Pi, F.; Zhang, S.; Sun, C.; Wang, X.; Zhang, Y.; Sun, X. GC-tof/ms-based metabolomics approach to study the cellular immunotoxicity of deoxynivalenol on murine macrophage ana-1 cells. Chem. Biol. Interact. 2016, 256, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, P.; Pi, F.; Sun, C.; Jiang, H.; Sun, J.; Wang, X.; Zhang, Y.; Sun, X. GC-tof/ms-based metabolomic strategy for combined toxicity effects of deoxynivalenol and zearalenone on murine macrophage ana-1 cells. Toxicon 2016, 120, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. Ms-dial: Data-independent ms/ms deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Zhu, P.; Cui, F.; Pi, F.; Zhang, Y.; Li, Y.; Wang, J.; Sun, X. The Antagonistic Effect of Mycotoxins Deoxynivalenol and Zearalenone on Metabolic Profiling in Serum and Liver of Mice. Toxins 2017, 9, 28. https://doi.org/10.3390/toxins9010028
Ji J, Zhu P, Cui F, Pi F, Zhang Y, Li Y, Wang J, Sun X. The Antagonistic Effect of Mycotoxins Deoxynivalenol and Zearalenone on Metabolic Profiling in Serum and Liver of Mice. Toxins. 2017; 9(1):28. https://doi.org/10.3390/toxins9010028
Chicago/Turabian StyleJi, Jian, Pei Zhu, Fangchao Cui, Fuwei Pi, Yinzhi Zhang, Yun Li, Jiasheng Wang, and Xiulan Sun. 2017. "The Antagonistic Effect of Mycotoxins Deoxynivalenol and Zearalenone on Metabolic Profiling in Serum and Liver of Mice" Toxins 9, no. 1: 28. https://doi.org/10.3390/toxins9010028





