A Survey of Aflatoxin-Producing Aspergillus sp. from Peanut Field Soils in Four Agroecological Zones of China
Abstract
:1. Introduction
2. Results
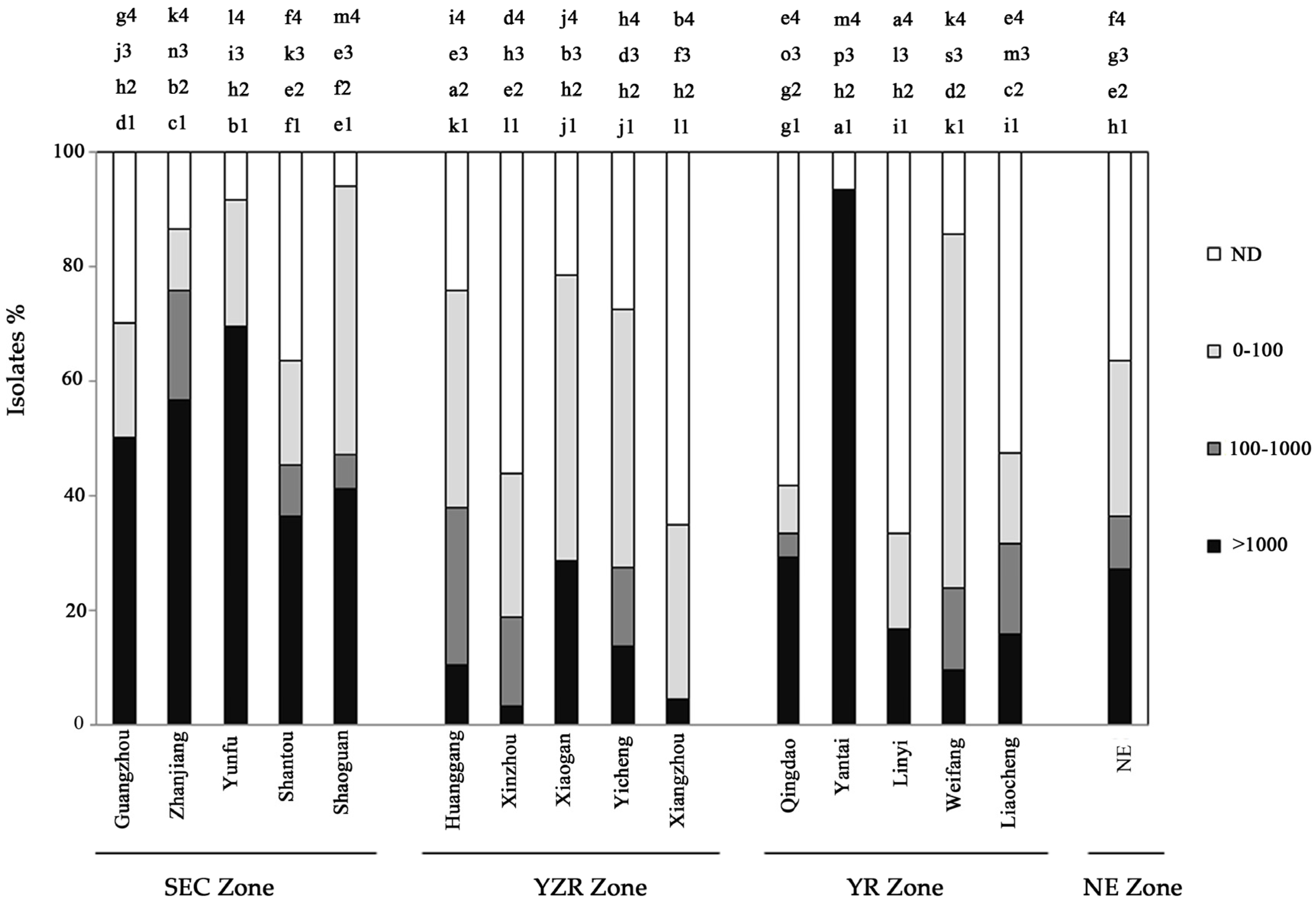
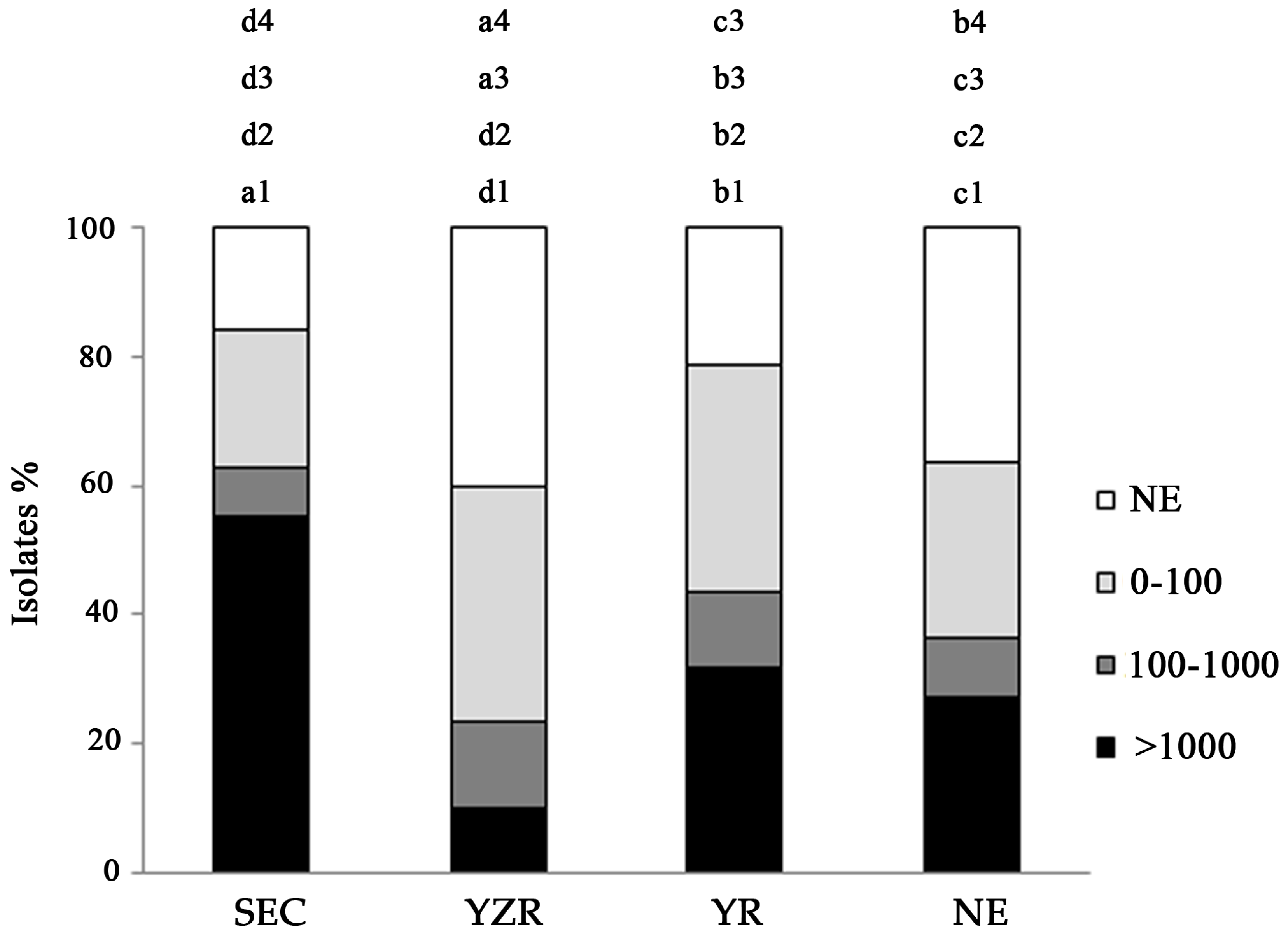
2.1. Distribution of Aflatoxin-Producing Aspergillus across China
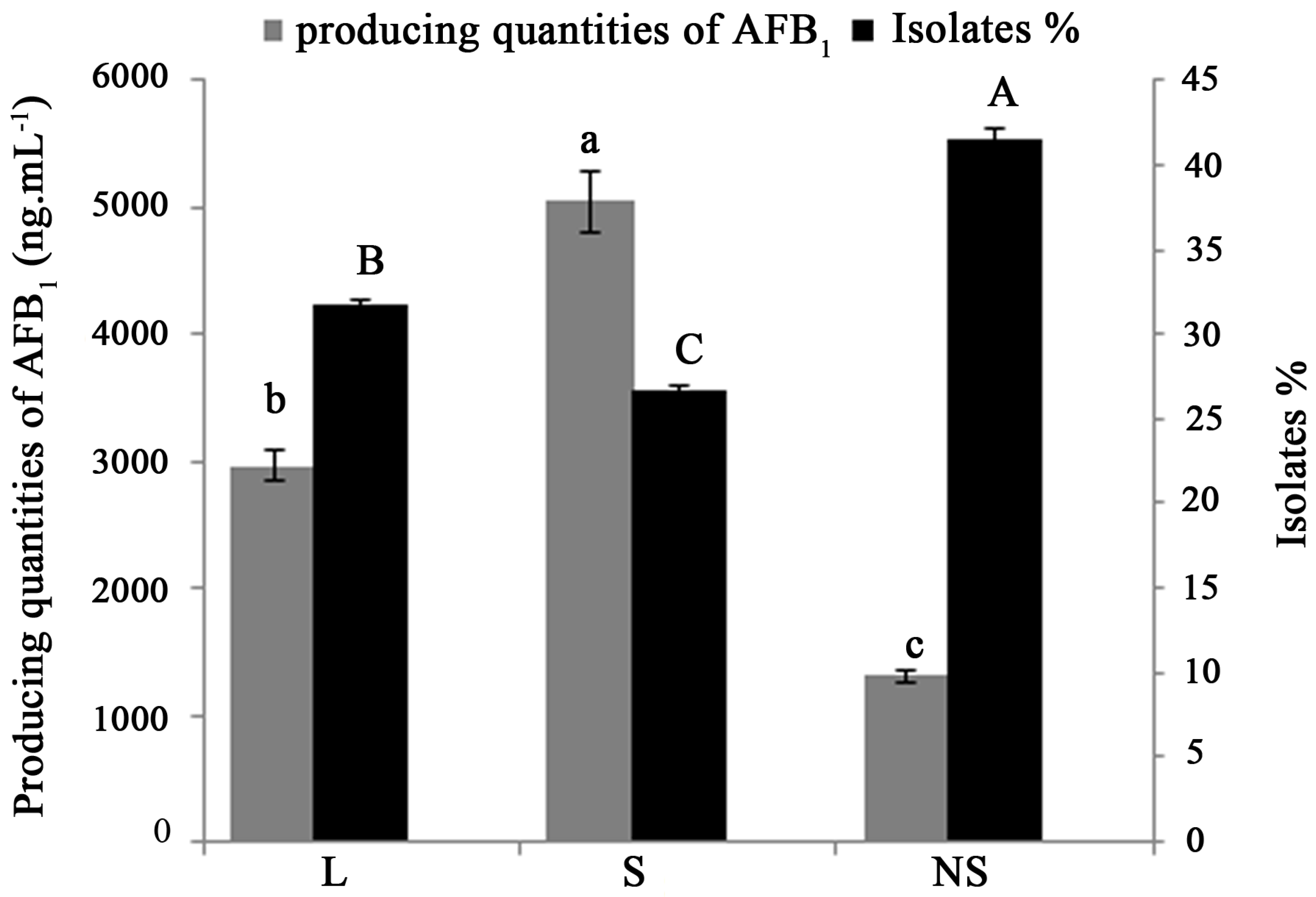
2.2. Distribution of Aflatoxin-Producing Aspergillus Chemotypes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Survey Sites
5.2. Survey Methods
5.3. Strain Isolation and Identification
5.4. Classification of Aflatoxin-Producing Aspergillus morphotypes
5.5. Mycotoxin Analyses
5.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Statistical Yearbook of China. Available online: http://www.stats.gov.cn/tjsj/ndsj/2016/indexch.htm/ (accessed on 20 September 2016).
- FAOSTAT: Crop. Available online: http://www.fao.org/faostat/zh/#data/QC (accessed on 20 September 2016).
- Yu, S.C.W. Peanut varieties and their genealogy in china; Shanghai scientific and Technical publishers: Shanghai, China, 2008. [Google Scholar]
- Barros, G.; Torres, A.; Chulze, S. Aspergillus flavus population isolated from soil of Argentina's peanut-growing region. Sclerotia production and toxigenic profile. J. Sci. Food Agric. 2005, 85, 2349–2353. [Google Scholar]
- Dorner, J.W. Managent and prevention of mycotoxins in peanuts. Food Addit. Contam.-Part A Chem. Anal. Control Exp. Risk Assess. 2008, 25, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Rodriguez, M.I.; Chulze, S.N. Genetic diversity within aspergillus flavus strains isolated from peanut-cropped soils in argentina. Soil Biol. Biochem. 2006, 38, 145–152. [Google Scholar]
- Mahgubi, A.E.; Puel, O.; Bailly, S.; Tadrist, S.; Querin, A.; Ouadia, A.; Oswald, I.P.; Bailly, J.D. Distribution and toxigenicity of aspergillus section flavi in spices marketed in Morocco. Food Control 2013, 32, 143–148. [Google Scholar] [CrossRef]
- Fountain, J.C.; Scully, B.T.; Chen, Z.Y.; Gold, S.E.; Glenn, A.E.; Abbas, H.K.; Lee, R.D.; Kemerait, R.C.; Guo, B. Effects of hydrogen peroxide on different toxigenic and atoxigenic isolates of aspergillus flavus. Toxins 2015, 7, 2985–2999. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Beltran, A.; Jaime, R.; Cotty, P.J. Aflatoxin-producing fungi in maize field soils from sea level to over 2000masl: A three year study in sonora, mexico. Fungal Biol. 2015, 119, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kana, J.R.; Harvey, J.; Wainaina, J.; Wanjuki, I.; Skilton, R.A.; Teguia, A. Assessment of aflatoxin contamination of maize, peanut meal and poultry feed mixtures from different agroecological zones in cameroon. Toxins 2013, 5, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, P.; Bai, Y.; Zhou, H. Aflatoxin b 1 in post-harvest peanuts and dietary risk in china. Food Control 2012, 23, 143–148. [Google Scholar] [CrossRef]
- Cotty, P.J. Aflatoxin-producing potential of communities of aspergillus section flavi from cotton producing areas in the united states. Mycol. Res. 1997, 101, 698–704. [Google Scholar] [CrossRef]
- Horn, B.W.; Dorner, J.W. Regional differences in production of aflatoxin b1 and cyclopiazonic acid by soil isolates of aspergillus flavus along a transect within the united states. Appl. Environ. Microbiol. 1999, 65, 1444–1449. [Google Scholar] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [PubMed]
- Tran-Dinh, N.; Kennedy, I.; Bui, T.; Carter, D. Survey of vietnamese peanuts, corn and soil for the presence of aspergillus flavus and aspergillus parasiticus. Mycopathologia 2009, 168, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on aspergillus section flavi isolated from maize in northern italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Sweany, R.R.; Damann, K.E., Jr; Kaller, M.D. Comparison of soil and corn kernel aspergillus flavus populations: Evidence for niche specialization. Phytopathology 2011, 101, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Amani, S.; Shams-Ghahfarokhi, M.; Banasaz, M.; Razzaghi-Abyaneh, M. Mycotoxin-producing ability and chemotype diversity of aspergillus section flavi from soils of peanut-growing regions in iran. Indian J. Microbiol. 2012, 52, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Atayde, D.D.; Reis, T.A.; Godoy, I.J.; Zorzete, P.; Reis, G.M.; Corrêa, B. Mycobiota and aflatoxins in a peanut variety grown in different regions in the state of são paulo, brazil. Crop Protect. 2012, 33, 7–12. [Google Scholar] [CrossRef]
- Nesci, A.; Montemarani, A.; Etcheverry, M. Assessment of mycoflora and infestation of insects, vector of aspergillus section flavi, in stored peanut from argentina. Mycotoxin Res. 2011, 27, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Jaimegarcia, R.; Cotty, P.J. Aspergillus flavus in soils and corncobs in south texas: Implications for management of aflatoxins in corn-cotton rotations. Plant Dis. 2007, 88, 1366–1371. [Google Scholar] [CrossRef]
- Scarpari, M.; Bello, C.; Pietricola, C.; Zaccaria, M.; Bertocchi, L.; Angelucci, A.; Ricciardi, M.R.; Scala, V.; Parroni, A.; Fabbri, A.A. Aflatoxin control in maize by trametes versicolor. Toxins 2014, 6, 3426–3437. [Google Scholar] [CrossRef] [PubMed]
- Astoreca, A.; Vaamonde, G.; Dalcero, A.; Marin, S.; Ramos, A. Abiotic factors and their interactions influence on the co-production of aflatoxin b 1 and cyclopiazonic acid by aspergillus flavus isolated from corn. Food Microbiol. 2014, 38, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Joffe, A.Z. Aflatoxin produced by 1,626 isolates of aspergillus flavus from groundnut kernels and soils in israel. Nature 1969, 221, 492. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.F.; Patriarca, A.; Locani, O.; Vaamonde, G. Natural co-occurrence of aflatoxin and cyclopiazonic acid in peanuts grown in argentina. Food Addit. Contam. 2001, 18, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Kobbeman, K.; Montalbano, B.G.; Cotty, P.J. Aflatoxin-producing aspergillus species from thailand. Int. J. Food Microbiol. 2007, 114, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.; Atehnkeng, J.; Sikora, R.A.; Bandyopadhyay, R.; Cotty, P.J. Distribution of aspergillus section flavi in soils of maize fields in three agroecological zones of nigeria. Soil Biol. Biochem. 2009, 41, 37–44. [Google Scholar] [CrossRef]
- Shearer, J.F.; Sweets, L.E.; Baker, N.K.; Tiffany, L.H. A study of aspergillus flavus/parasiticus in iowa crop fields: 1988–1990. Plant Dis. 1992, 76, 19–22. [Google Scholar] [CrossRef]
- Zorzete, P.; Reis, T.A.; Felício, J.D.; Baquião, A.C.; Makimoto, P.; Corrêa, B. Fungi, mycotoxins and phytoalexin in peanut varieties, during plant growth in the field. Food Chem. 2011, 129, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Orum, T.V.; Bigelow, D.M.; Nelson, M.R.; Howell, D.R.; Cotty, P.J. Spatial and temporal patterns of aspergillus flavus strain composition and propagule density in yuma county, arizona, soils. Plant Dis. 2007, 81, 911–916. [Google Scholar] [CrossRef]
- Antilla, L.; Cotty, P. The ars-acrpc partnership to control aflatoxin in arizona cotton: Current status. Mycopathologia 2002, 155, 64. [Google Scholar]
- Nesci, A.; Etcheverry, M. Aspergillus section flavi populations from field maize in argentina. Lett. Appl. Microbiol. 2002, 34, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.; Dorner, J. Soil populations of aspergillus species from section flavi along a transect through peanut-growing regions of the united states. Mycologia 1998, 767–776. [Google Scholar] [CrossRef]
- Garber, R.K.; Cotty, P.J. Formation of sclerotia and aflatoxins in developing cotton bolls infected by the s strain of aspergillus flavus and potential for biocontrol with an atoxigenic strain. Phytopathology 1997, 87, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Pildain, M.B.; Vaamonde, G.; Cabral, D. Analysis of population structure of aspergillus flavus from peanut based on vegetative compatibility, geographic origin, mycotoxin and sclerotia production. Int. J. Food Microbiol. 2004, 93, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Allameh, A.; Kazeroon-Shiri, A.; Ranjbar-Bahadori, S.; Mirzahoseini, H.; Rezaee, M.B. A survey on distribution of aspergillus section flavi in corn field soils in iran: Population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia 2006, 161, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Novas, M.V.; Cabral, D. Association of mycotoxin and sclerotia production with compatibility groups in aspergillus flavus from peanut in argentina. Plant Dis. 2002, 86, 215–219. [Google Scholar] [CrossRef]
- Okoth, S.; Nyongesa, B.; Ayugi, V.; Kang’Ethe, E.; Korhonen, H.; Joutsjoki, V. Toxigenic potential of aspergillus species occurring on maize kernels from two agro-ecological zones in kenya. Toxins 2012, 4, 991–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Wu, L.; Li, P.; Zhang, Z.; Zhou, H.; Bai, Y.; Chen, X.; Jiang, J. Risk assessment on dietary exposure to aflatoxin b₁ in post-harvest peanuts in the yangtze river ecological region. Toxins 2014, 7, 4157–4174. [Google Scholar] [CrossRef] [PubMed]
- Mylroie, J.E.; Ozkan, S.; Shivaji, R.; Windham, G.L.; Alpe, M.N.; Williams, W.P. Identification and quantification of a toxigenic and non-toxigenic aspergillus flavus strain in contaminated maize using quantitative real-time pcr. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lou, T.; Yan, L.; Michailides, T.J.; Ma, Z. Molecular characterization of toxigenic and atoxigenic aspergillus flavus isolates, collected from peanut fields in china. J. Appl. Microbiol. 2009, 107, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Weather History. Available online: http://lishi.tianqi.com/ (accessed on 25 September 2016).
- Cotty, D.P.J. Comparison of four media for the isolation of aspergillus flavus group fungi. Mycopathologia 1994, 125, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Santos, C.; Venâncio, A.; Lima, N. Species identification of aspergillus section flavi isolates from portuguese almonds using phenotypic, including maldi-tof icms, and molecular approaches. J. Appl. Microbiol. 2011, 111, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W.; Layton, R.C. Association of morphology and mycotoxin production with vegetative compatibility groups in aspergillus flavus, a. Parasiticus, and a. Tamarii. Mycologia 1996, 88, 574–587. [Google Scholar] [CrossRef]

| AZE b | District | %A.f c | %A.p d | Number of Isolates | cfu·g−1 Soil | Positive Rate (%) e | Soil pH | Soil Moisture (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | ||||||||
| SEC | Guangzhou | 100 | 0 | 20 | 0–399.6 | 27.8f | 36.7h | 6.28 | 21.08 |
| Zhanjiang | 94 | 6 | 32 | 0–1666.7 | 170e | 56.7e | 5.13 | 15.35 | |
| Yunfu | 94 | 6 | 33 | 0–5667.7 | 481.1c | 66.7d | 5.36 | 18.05 | |
| Shantou | 100 | 0 | 13 | 0–133.3 | 19.9f | 36.7h | 6.12 | 20.15 | |
| shaoguan | 100 | 0 | 17 | 0–2333.3 | 258.6d | 46.7f | 6.32 | 14.06 | |
| YZR | Huanggang | 100 | 0 | 29 | 33.3–6660 | 1920b | 100a | 6.86 | 19.20 |
| Xinzhou | 100 | 0 | 32 | 0–1000 | 174.3e | 83.3c | 5.66 | 17.53 | |
| Xiaogan | 100 | 0 | 14 | 0–666.7 | 55.5f | 33.3j | 4.95 | 7.30 | |
| Yicheng | 97 | 3 | 29 | 0–1000 | 297.5d | 96.7a | 6.79 | 11.29 | |
| Xiangzhou | 100 | 0 | 23 | 0–16,665 | 2749.3a | 90b | 6.55 | 12.57 | |
| YR | Qingdao | 92 | 8 | 24 | 0–300 | 52.2f | 30.0i | 4.78 | 7.84 |
| Yantai | 47 | 53 | 15 | 0–233.3 | 16.7f | 23.3j | 5.30 | 14.52 | |
| Linyi | 100 | 0 | 6 | 0–66.7 | 5.7f | 16.7k | 5.21 | 18.41 | |
| Weifang | 90 | 10 | 21 | 0–200 | 24.4f | 40.0gh | 5.90 | 5.90 | |
| Liaocheng | 100 | 0 | 19 | 0–166.7 | 33.7f | 43.3fg | 7.18 | 7.18 | |
| NE | Dalian | 100 | 0 | 1 | 0–33.3 | 1.1f | 3.3l | 5.50 | 13.90 |
| Jinzhou | 100 | 0 | 4 | 0–33.3 | 2.2f | 6.7l | 6.38 | 5.31 | |
| Fuxin | 0 | 100 | 1 | 0–66.7 | 3.3f | 6.7l | 5.53 | 5.11 | |
| Shenyang | 50 | 50 | 2 | 0–33.3 | 1.1f | 3.3l | 5.42 | 2.50 | |
| Tieling | 100 | 0 | 3 | 0–33.3 | 4.4f | 13.2k | 4.7 | 13.24 | |
| AZE b | District | Aflatoxin B1 (ng·mL−1) | ||||
|---|---|---|---|---|---|---|
| A. flavus | A. parasiticus | Average c | ||||
| Range | Mean | Range | Mean | |||
| SEC | Guangzhou | 0–25,300 | 8473a | - | - | 8473b |
| Zhanjiang | 0–25,812 | 5536d | 238–20,172 | 10,324b | 5788d | |
| Yunfu | 0–82,083 | 6493c | 70–1380 | 725c | 6173cd | |
| Shantou | 0–16,501 | 4178f | - | - | 4178e | |
| shaoguan | 0–26,293 | 3092h | - | - | 3092f | |
| Total | 5805A | - | 5465C | 5795A | ||
| YZR | Huanggang | 0–17,512 | 1220j | - | - | 1220gh |
| Xinzhou | 0–1089 | 73m | - | - | 73i | |
| Xiaogan | 0–28,970 | 7611b | - | - | 7611bc | |
| Yicheng | 0–34,870 | 1803i | 5.8 | 5.8c | 1741g | |
| Xiangzhou | 0–2662 | 147m | - | - | 147i | |
| Total | 1579C | - | 5.8D | 1566D | ||
| YR | Qingdao | 0–30,588 | 3493g | 4154–13,923 | 9039b | 3955ef |
| Yantai | 0–23,225 | 8343a | 7111–61,899 | 27,920a | 18784a | |
| Linyi | 0–2312 | 392l | - | - | 392hi | |
| Weifang | 0–60,331 | 3734f | 14.6–57.5 | 36c | 3381ef | |
| Liaocheng | 0–8640 | 710k | - | - | 710ghi | |
| Total | 3041B | - | 20,125A | 5453B | ||
| NE | NE | 0–3278 | 366 | 159–17,293 | 8726 | 1886 |
| Total | 0–3278 | 366D | 159–17,293 | 8726B | 1886C | |
| cfu·g−1 | %Positive Rate | % ˃ 1000 | %100–1000 | %0–100 | AFB1 | AFB1 Risk | %L | %S | %NS | TEM | %LONG | %LAT | pH | % Moisture | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cfu·g−1 | 1.00 | ||||||||||||||
| %Positive rate | 0.65 ** | 1.00 | |||||||||||||
| % ˃ 1000 | −0.34 | −0.26 | 1.00 | ||||||||||||
| %100–1000 | 0.13 | 0.37 | −0.40 | 1.00 | |||||||||||
| %0–100 | 0.17 | 0.10 | −0.51 | 0.21 | 1.00 | ||||||||||
| %L | 0.07 | 0.23 | −0.20 | 0.23 | 0.14 | 1.00 | |||||||||
| %S | −0.06 | −0.18 | 0.04 | −0.07 | −0.21 | −0.31 | 1.00 | ||||||||
| * NS | −0.03 | −0.11 | 0.17 | −0.17 | 0.00 | −0.78 ** | −0.35 | 1.00 | |||||||
| AFB1 | −0.31 | −0.32 | 0.87** | −0.41 | −0.36 | −0.25 | −0.31 | 0.45 | 1.00 | ||||||
| AFB1 risk | 0.39 | 0.50 * | 0.29 | 0.18 | 0.08 | 0.08 | 0.15 | −0.18 | 0.05 | 1.00 | |||||
| TEM | 0.33 | 0.61 * | −0.06 | 0.15 | 0.19 | 0.24 | −0.22 | −0.09 | −0.01 | 0.42 | 1.00 | ||||
| %LONG | −0.06 | −0.38 | −0.33 | −0.01 | 0.03 | −0.26 | −0.23 | 0.40 | −0.08 | −0.46 | −0.64 | 1.00 | |||
| %LAT | −0.36 | −0.71 ** | 0.03 | −0.14 | −0.21 | −0.30 | −0.10 | 0.36 | 0.18 | −0.48 | −0.76 ** | 0.78 ** | 1.00 | ||
| pH | 0.42 | 0.48 | −0.41 | 0.43 | 0.30 | 0.49 | −0.20 | −0.35 | −0.39 | 0.06 | 0.21 | −0.06 | −0.28 | 1.00 | |
| %moisture | 0.15 | 0.35 | 0.27 | −0.02 | −0.34 | 0.11 | 0.15 | −0.20 | 0.10 | 0.36 | 0.42 | −0.64 ** | −0.29 | 0.09 | 1.00 |
| AEZ | No. of Fields | CFU/g Soil | %Positive Rate | %Af b | %Ap b | No. of Tested Isolates | Aflatoxin B1c(ng·mL−1) |
|---|---|---|---|---|---|---|---|
| SEC | 5 | 191.5b | 48.7b | 96.6a | 3.4c | 121 | 5795a |
| YZR | 5 | 1039.3a | 80.7a | 99.1a | 0.9d | 127 | 1566c |
| YR | 5 | 26.5c | 22.7c | 85.9b | 14.1b | 85 | 5453d |
| NE | 5 | 2.4d | 6.6d | 81.8b | 18.2a | 11 | 1886b |
| AEZ a | District | Latitude (N) | Longitude (E) | Temp b | Agrotype | Landform | Alternative Crop |
|---|---|---|---|---|---|---|---|
| SEC | Guangzhou | 23.158029 | 113.273165 | 27.81 | Sandy loam | Paddy soil | Rice |
| Zhanjiang | 21.377219 | 110.25017 | 27.94 | Sandy loam | Arid hillside | Sugarcane | |
| Yunfu | 22.768595 | 111.570011 | 28.08 | Sandy loam | Arid hillside | Sweet potato | |
| Shantou | 23.285832 | 116.726481 | 26.62 | Sandy loam | Paddy soil | Rice | |
| Shaoguan | 24.682728 | 113.604549 | 26.88 | Sandy loam | Paddy soil | Rice | |
| YZR | Huanggang | 30.64299 | 114.872866 | 28.69 | Sandy loam | Plain | Cotton |
| Xinzhou | 30.841401 | 114.801259 | 28.65 | Sandy loam | Plain | Cotton | |
| Xiaogan | 31.562299 | 114.128097 | 28.40 | Sandy loam | Arid hillside | Sweet potato | |
| Yicheng | 31.719806 | 112.257788 | 26.74 | Sandy loam | Plain | Maize | |
| Xiangzhou | 32.087779 | 112.211772 | 27.69 | Clay loam | Mound | Maize | |
| YR | Qingdao | 35.79045 | 118.627918 | 25.02 | Sandy loam | Arid hillside | Maize |
| Yantai | 37.387331 | 121.60049 | 25.47 | Sandy loam | Arid hillside | Maize | |
| Linyi | 35.8725 | 120.04643 | 25.70 | Sandy loam | Arid hillside | Sweet potato | |
| Weifang | 36.706945 | 118.829914 | 26.44 | Sandy loam | Arid hillside | Maize | |
| Liaocheng | 36.866062 | 116.231478 | 26.38 | Sandy loam | Arid hillside | Maize | |
| NE | Dalian | 38.950245 | 121.565873 | 22 | Sandy loam | Arid hillside | Vegetable |
| Jinzhou | 41.117250 | 121.128323 | 21 | Sandy loam | Arid hillside | Maize | |
| Fuxin | 42.065175 | 121.757901 | 19.5 | Sandy loam | Arid hillside | Maize | |
| Shenyang | 42.74995 | 123.353519 | 20 | Sandy loam | Arid hillside | Maize | |
| Tieling | 42.785798 | 124.111098 | 20 | Sandy loam | Arid hillside | Maize |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Selvaraj, J.N.; Yang, Q.; Liu, Y. A Survey of Aflatoxin-Producing Aspergillus sp. from Peanut Field Soils in Four Agroecological Zones of China. Toxins 2017, 9, 40. https://doi.org/10.3390/toxins9010040
Zhang C, Selvaraj JN, Yang Q, Liu Y. A Survey of Aflatoxin-Producing Aspergillus sp. from Peanut Field Soils in Four Agroecological Zones of China. Toxins. 2017; 9(1):40. https://doi.org/10.3390/toxins9010040
Chicago/Turabian StyleZhang, Chushu, Jonathan Nimal Selvaraj, Qingli Yang, and Yang Liu. 2017. "A Survey of Aflatoxin-Producing Aspergillus sp. from Peanut Field Soils in Four Agroecological Zones of China" Toxins 9, no. 1: 40. https://doi.org/10.3390/toxins9010040






