Mycotoxigenic Potentials of Fusarium Species in Various Culture Matrices Revealed by Mycotoxin Profiling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Characterization of Fusarium Species
2.2. Applicability of LC-MS/MS Method
2.3. Principal Component Analysis
2.4. Mycotoxin-Producing Capacities of Fusarium Species in Different Growth Media
2.4.1. Group I/Fusaric Acid Producers
2.4.2. Group II/Type A Trichothecene Producers
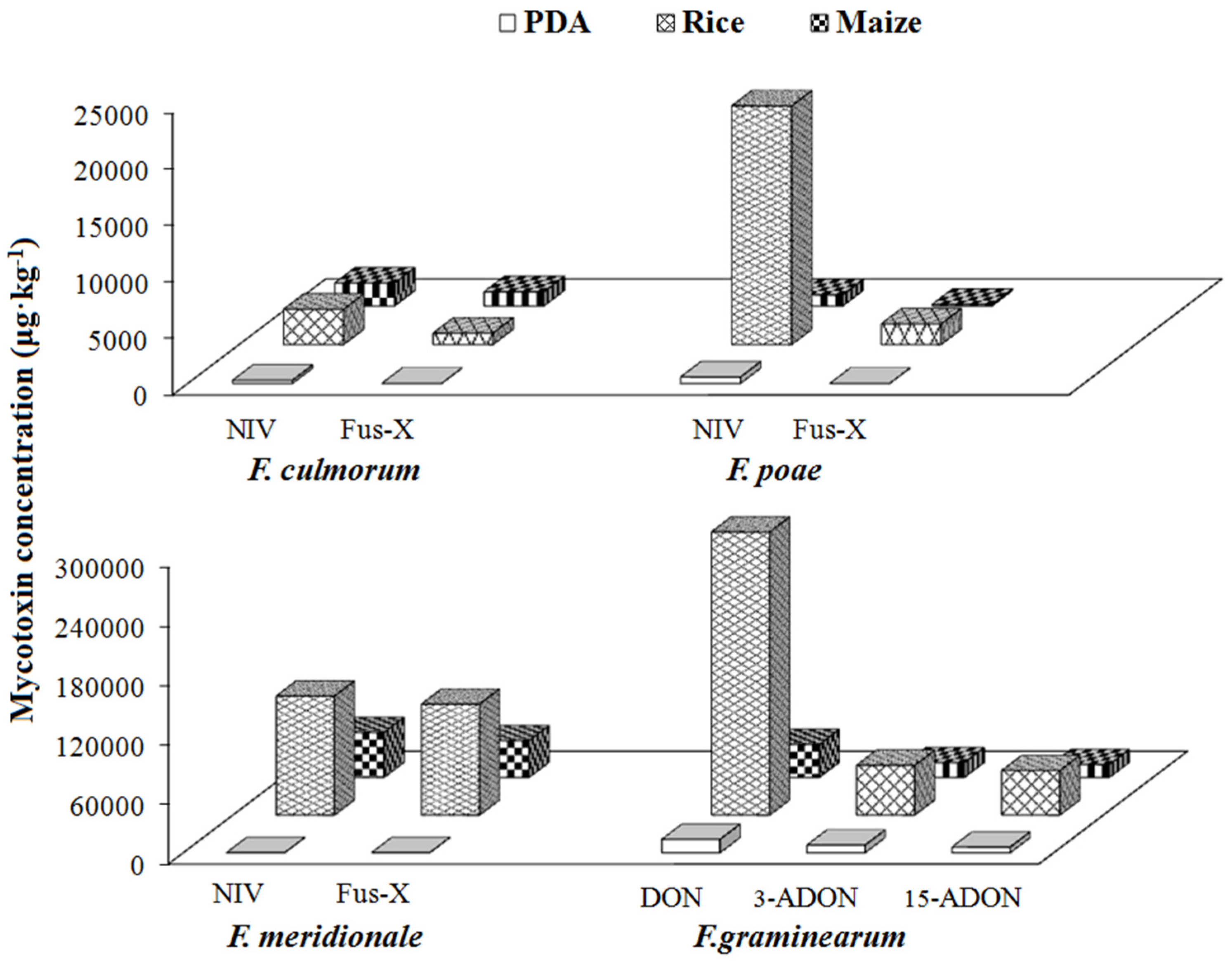
2.4.3. Group III/Type B Trichothecene Producers
3. Conclusions
4. Materials and Methods
4.1. Fungal Strains, Materials and Chemicals
4.2. Molecular Characterization of the Fusarium Strains
4.3. Preparation of Different Types of Growth Media
4.4. Inoculation of the Targeted Fungal Strains
4.5. Analysis of Multiple Mycotoxins
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duan, C.X.; Qin, Z.H.; Yang, Z.H.; Li, W.X.; Sun, S.L.; Zhu, Z.D.; Wang, X.M. Identification of pathogenic fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.P.; Enz, J.; Lukach, J.; Stover, R. Environmental conditions associated with Fusarium head blight epidemics of wheat and barley in the northern great plains, north america. Cereal Res. Commun. 1997, 25, 777–778. [Google Scholar]
- Kemp, G.H.J.; Pretorius, Z.A.; Wingfield, M.J. Fusarium glume spot of wheat: A newly recorded mite-associated disease in south africa. Plant Dis. 1996, 80, 48–51. [Google Scholar] [CrossRef]
- Gortz, A.; Oerke, E.C.; Steiner, U.; Waalwijk, C.; de Vries, I.; Dehne, H.W. Biodiversity of Fusarium species causing ear rot of maize in Germany. Cereal Res. Commun. 2008, 36, 617–622. [Google Scholar] [CrossRef]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.T.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisin production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D. Fumonisin b(1)-nonproducing strains of Fusarium verticillioides cause maize (zea mays) ear infection and ear rot. J. Agric. Food Chem. 2000, 48, 5773–5780. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Cartwright, R.D.; Xie, W.; Mirocha, C.J.; Richard, J.L.; Dvorak, T.J.; Sciumbato, G.L.; Shier, W.T. Mycotoxin production by Fusarium proliferatum isolates from rice with Fusarium sheath rot disease. Mycopathologia 1999, 147, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Clear, R.M.; O’Donnell, K.; McCormick, S.; Turkington, T.K.; Tekauz, A.; Gilbert, J.; Kistler, H.C.; Busman, M.; Ward, T.J. Diversity of Fusarium head blight populations and trichothecene toxin types reveals regional differences in pathogen composition and temporal dynamics. Fungal Genet. Biol. 2015, 82, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.L.; Liu, N.; Liao, Y.C.; Sun, C.P.; Wang, S.X.; Wu, A.B. Functional agents to biologically control deoxynivalenol contamination in cereal grains. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Molto, G.A.; Gonzalez, H.H.L.; Resnik, S.L.; Gonzalez, A.P. Production of trichothecenes and zearalenone by isolates of Fusarium spp. from argentinian maize. Food Addit. Contam. 1997, 14, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Mita, G.; Haidukowski, M.; Santino, A.; Mule, G.; Visconti, A. Assessment of trichothecene chemotypes of Fusarium culmorum occurring in Europe. Food Addit. Contam. 2005, 22, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, M.; Muller, H.M.; Rufle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tan, Y.L.; Liu, N.; Yan, Z.; Liao, Y.C.; Chen, J.; De Saeger, S.; Hua, Y.; Zhang, Q.; Wu, A.B. Detoxification of deoxynivalenol via glycosylation represents novel insights on antagonistic activities of trichoderma when confronted with Fusarium graminearum. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Sudakin, D.L. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. 2013, 53, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Commission regulation (EC) 1881/2006 of december 19th 2006 replacing regulation (EC) 466/2001 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Commun. 2006, L364, 5–24. [Google Scholar]
- The National Food Safety Standard of Maximum Levels of Mycotoxin in Foods (GB 2761–2011). Available online: http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Maximum%20Levels%20of%20Mycotoxins%20in%20Foods_Beijing_China%20-%20Peoples%20Republic%20of_12-29-2014.pdf (accessed on 24 December 2016).
- Logrieco, A.; Mule, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant. Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Bosch, U.; Mirocha, C.J. Toxin production by Fusarium species from sugar-beets and natural occurrence of zearalenone in beets and beet fibers. Appl. Environ. Microb. 1992, 58, 3233–3239. [Google Scholar]
- Christ, D.S.; Marlander, B.; Varrelmann, M. Characterization and mycotoxigenic potential of Fusarium species in freshly harvested and stored sugar beet in Europe. Phytopathology 2011, 101, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Li, H.P.; Dang, F.J.; Qu, B.; Xu, Y.B.; Zhao, C.S.; Liao, Y.C. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol. Res. 2007, 111, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.A.; Schwarz, P.B.; Gillespie, J.; Rivera-Varas, V.V.; Secor, G.A. Trichothecene mycotoxins associated with potato dry rot caused by Fusarium graminearum. Phytopathology 2010, 100, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Samar, M.; Molto, G.; Resnik, S.; Pacin, A. Trichothecenes and zearalenone production by Fusarium species isolated from argentinean black beans. Mycotoxin Res. 2002, 18, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Llorens, A.; Mateo, R.; Hinojo, M.J.; Valle-Algarra, F.M.; Jimenez, M. Influence of environmental factors on the biosynthesis of type B trichothecenes by isolates of Fusarium spp. from Spanish crops. Int. J. Food Microbiol. 2004, 94, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.L.; Martins, H.M. Influence of water activity, temperature and incubation time on the simultaneous production of deoxynivalenol and zearalenone in corn (zea mays) by Fusarium graminearum. Food Chem. 2002, 79, 315–318. [Google Scholar] [CrossRef]
- Kokkonen, M.; Jestoi, M.; Laitila, A. Mycotoxin production of Fusarium langsethiae and Fusarium sporotrichioides on cereal-based substrates. Mycotoxin Res. 2012, 28, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Heutte, N.; Sage, L.; Pottier, D.; Bouchart, V.; Lebailly, P.; Garon, D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007, 45, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Brzonkalik, K.; Herrling, T.; Syldatk, C.; Neumann, A. The influence of different nitrogen and carbon sources on mycotoxin production in Alternaria alternata. Int. J. Food Microbiol. 2011, 147, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Jimenez, M. Trichothecenes and fumonisins produced in autoclaved tiger nuts by strains of Fusarium sporotrichioides and Fusarium moniliforme. Food Microbiol. 2000, 17, 167–176. [Google Scholar] [CrossRef]
- Azira, T.N.; Man, Y.B.C.; Hafidz, R.N.R.M.; Aina, M.A.; Amin, I. Use of principal component analysis for differentiation of gelatine sources based on polypeptide molecular weights. Food Chem. 2014, 151, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Marina, A.M.; Man, Y.B.C.; Amin, I. Use of the saw sensor electronic nose for detecting the adulteration of virgin coconut oil with rbd palm kernel olein. J. Am. Oil Chem. Soc. 2010, 87, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Wulff, E.G.; Sorensen, J.L.; Lubeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusarium spp. Associated with rice bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Stepien, L.; Koczyk, G.; Waskiewicz, A. Fum cluster divergence in fumonisins-producing Fusarium species. Fungal Biol. 2011, 115, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Kitajima, M.; Nagum, R.; Tsukiboshi, T.; Uegaki, R.; Nakajima, T.; Kushiro, M.; Nakagawa, H.; Shimizu, M.; Kageyama, K.; et al. A single nucleotide polymorphism in the translation elongation factor 1 alpha gene correlates with the ability to produce fumonisin in Japanese Fusarium fujikuroi. Fungal Biol. 2014, 118, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Hinojo, M.J.; Medina, A.; Valle-Algarra, F.M.; Gimeno-Adelantado, J.V.; Jimenez, M.; Mateo, R. Fumonisin production in rice cultures of Fusarium verticillioides under different incubation conditions using an optimized analytical method. Food Microbiol. 2006, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ung-Soo, L.; Myong-Yur, L.; Kwang-Sop, S.; Yun-Sik, M.; Chae-Min, C.; Ueno, Y. Production of fumonisin B1 and B2 by Fusarium moniliforme isolated from Korean corn kerneis for feed. Mycotoxin Res. 1994, 10, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Fadl-Allah, E.; Stack, M.; Goth, R.; Bean, G. Production of fumonisins B1, B2 and B3 by Fusarium proliferatum isolated from rye grains. Mycotoxin Res. 1997, 13, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Plattner, R.D.; Desjardins, A.E.; Klittich, C.J.R. Fumonisin B1 production by strains from different mating populations of Gibberella fujikuroi (Fusarium section liseola). Phytopathology 1992, 82, 341–345. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Rheeder, J.P.; Lamprecht, S.C.; Zeller, K.A.; Leslie, J.F. Fusarium andiyazi sp nov., a new species from sorghum. Mycologia 2001, 93, 1203–1210. [Google Scholar] [CrossRef]
- Abramson, D.; Clear, R.M.; Smith, D.M. Trichothecene production by Fusarium spp isolated from manitoba grain. Can. J. Plant Pathol. 1993, 15, 147–152. [Google Scholar] [CrossRef]
- Hestbjerg, H.; Nielsen, K.F.; Thrane, U.; Elmholt, S. Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar: An ecological interpretation. J. Agric. Food Chem. 2002, 50, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Ward, T.J.; O’Donnell, M.; Proctor, R.H.; Burkin, A.A.; Kononenko, G.P.; Gavrilova, O.P.; Aoki, T.; McCormick, S.P.; Gagkaeva, T.Y. Fusarium sibiricum sp. nov, a novel type a trichothecene-producing Fusarium from Northern Asia closely related to F-sporotrichioides and F-langsethiae. Int. J. Food Microbiol. 2011, 147, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.; Rubio, C.; Gene, J.; Cano, J.; Gil, J.; Benito, R.; Moranderia, M.J.; Miguez, E. Case of keratitis caused by an uncommon Fusarium species. J. Clin. Microbiol. 2003, 41, 5823–5826. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Ocamb, C.M. First report of production of fumonisin B-1 by Fusarium polyphialidicum collected from seeds of Pinus strobus. Plant Dis. 1995, 79, 642. [Google Scholar] [CrossRef]
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Scoz, L.B.; Astolfi, P.; Reartes, D.S.; Schmale, D.G.; Moraes, M.G.; Del Ponte, E.M. Trichothecene mycotoxin genotypes of Fusarium graminearum sensu stricto and Fusarium meridionale in wheat from southern Brazil. Plant Pathol. 2009, 58, 344–351. [Google Scholar] [CrossRef]
- Sydenham, E.W.; Marasas, W.F.O.; Thiel, P.G.; Shephard, G.S.; Nieuwenhuis, J.J. Production of mycotoxins by selected Fusarium-graminearum and F-crookwellense isolates. Food Addit. Contam. 1991, 8, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bakan, B.; Pinson, L.; Cahagnier, B.; Melcion, D.; Semon, E.; Richard-Molard, D. Toxigenic potential of Fusarium culmorum strains isolated from French wheat. Food Addit. Contam. 2001, 18, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Burlakoti, R.R.; Ali, S.; Secor, G.A.; Neate, S.M.; McMullen, M.P.; Adhikari, T.B. Comparative mycotoxin profiles of gibberella zeae populations from barley, wheat, potatoes, and sugar beets. Appl. Environ. Microbiol. 2008, 74, 6513–6520. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Li, H.P.; Qu, B.; Zhang, J.B.; Huang, T.; Chen, F.F.; Liao, Y.C. Development of a generic pcr detection of 3-acetyldeoxynivalenol-, 15-acetyldeoxynivalenol- and nivalenol-chemotypes of Fusarium graminearum clade. Int. J. Mol. Sci. 2008, 9, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- BLAST program. Avaliable online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 5 August 2016).
- Van Poucke, K.; Monbaliu, S.; Munaut, F.; Heungens, K.; De Saeger, S.; Van Hove, F. Genetic diversity and mycotoxin production of Fusarium lactis species complex isolates from sweet pepper. Int. J. Food Microbiol. 2012, 153, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Busko, M.; Chelkowski, J.; Popiel, D.; Perkowski, J. Solid substrate bioassay to evaluate impact of trichoderma on trichothecene mycotoxin production by Fusarium species. J. Sci. Food Agric. 2008, 88, 536–541. [Google Scholar] [CrossRef]
- Medina, A.; Magan, N. Temperature and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries. Food Microbiol. 2011, 28, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Feng, Z.H.; Shi, W.; Zhao, Z.H.; Wu, Y.J.; Wu, A.B. A quick, easy, cheap, effective, rugged, and safe sample pretreatment and liquid chromatography with tandem mass spectrometry method for the simultaneous quantification of 33 mycotoxins in lentinula edodes. J. Sep. Sci. 2014, 37, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
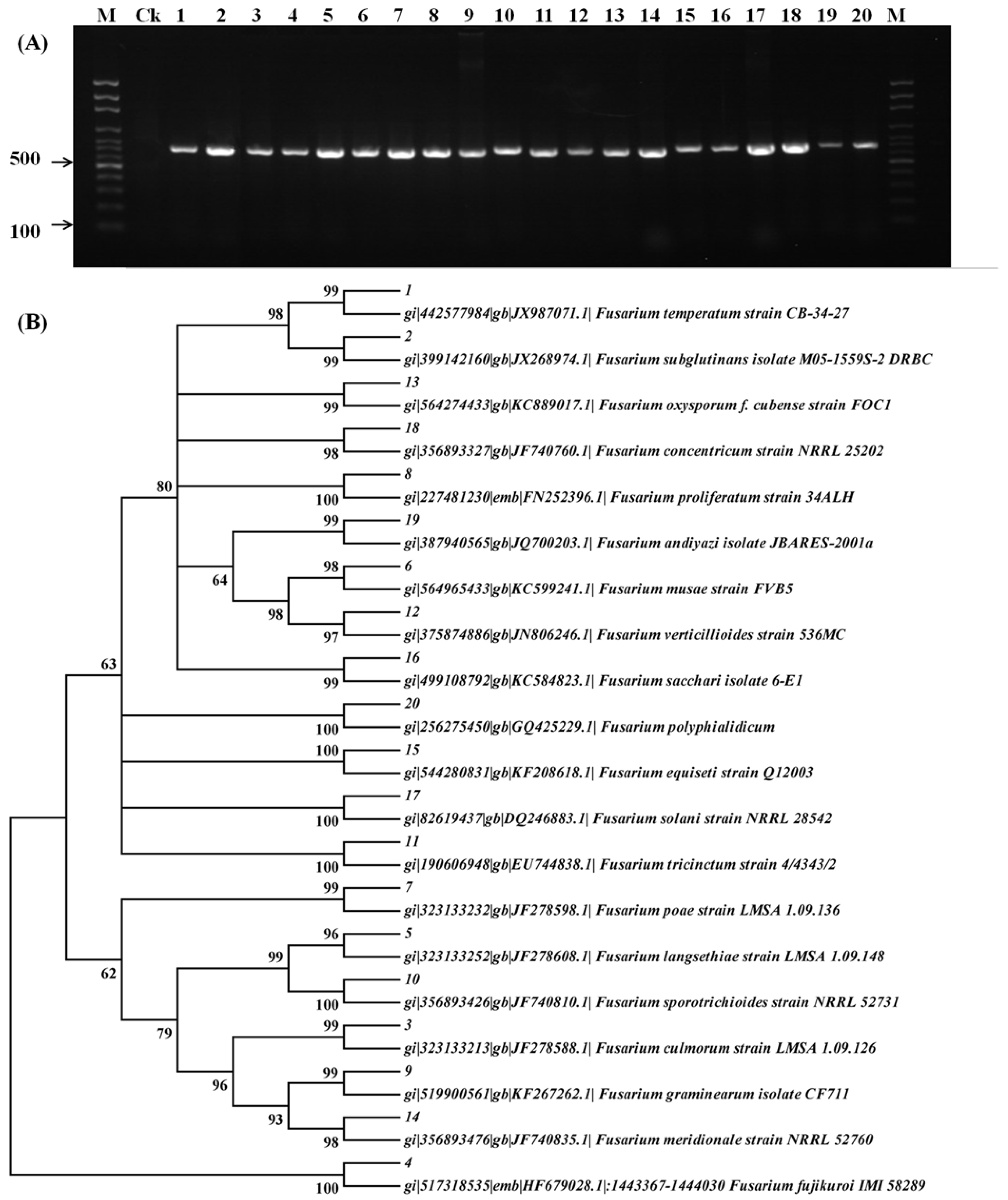
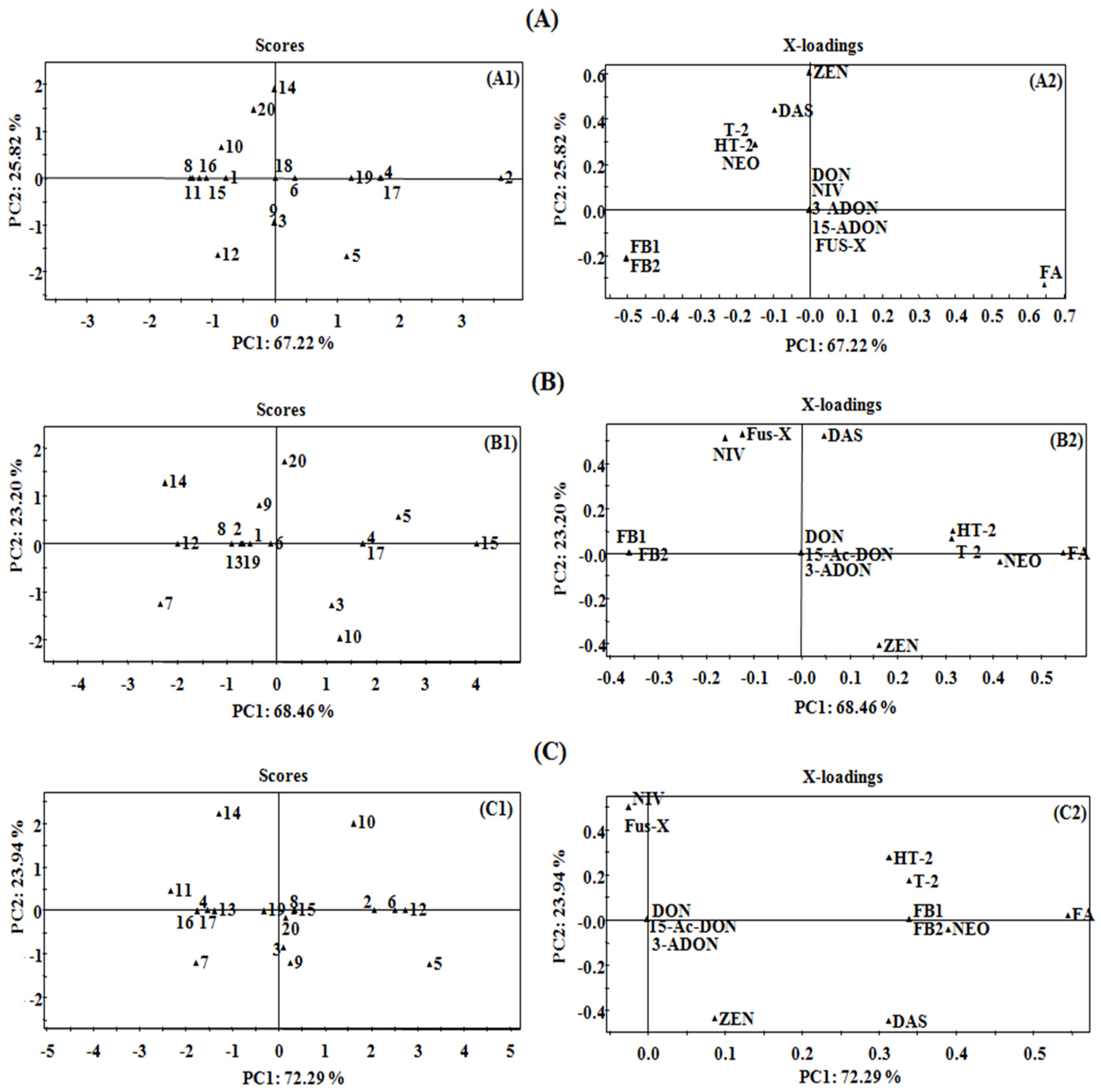

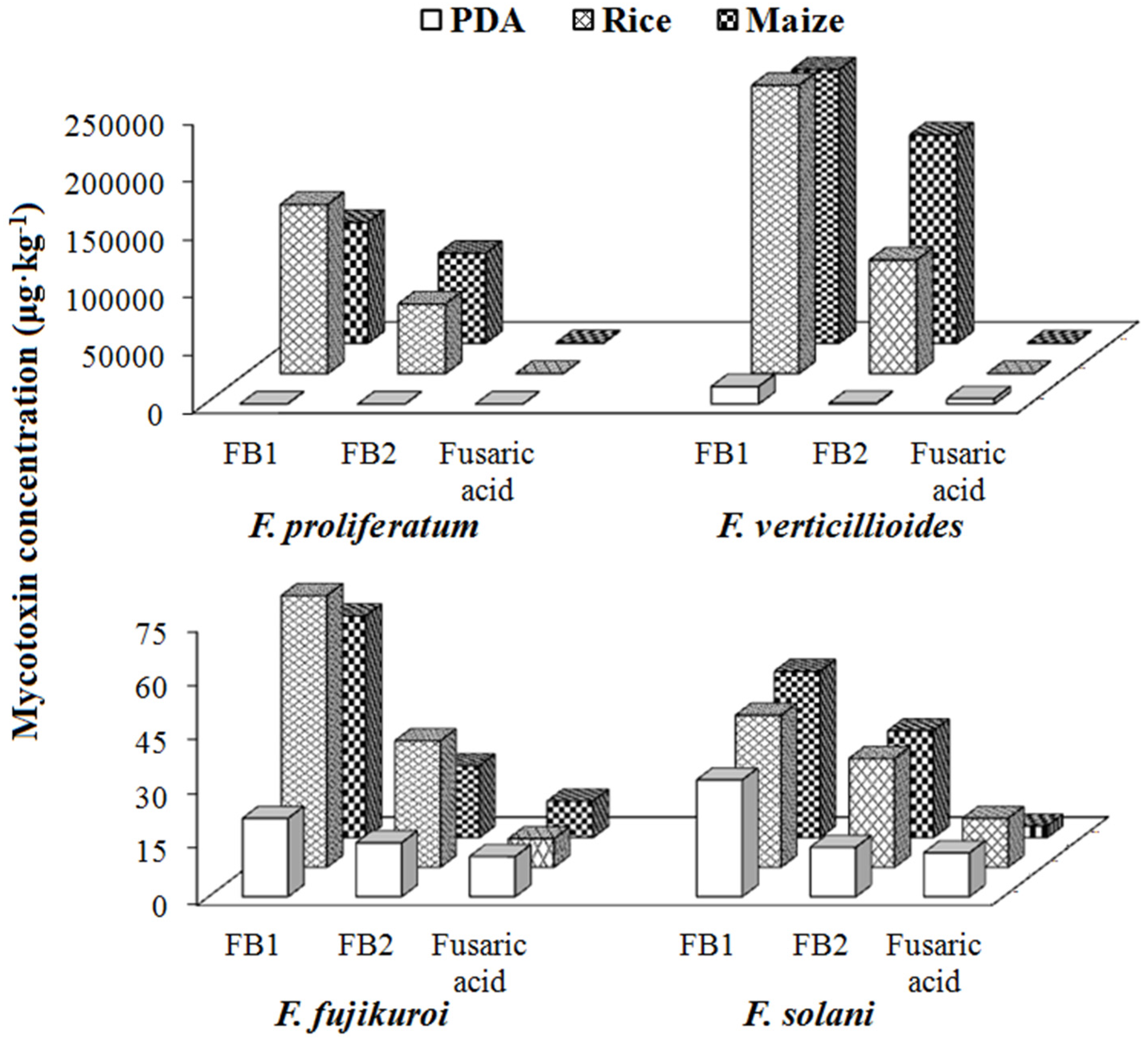
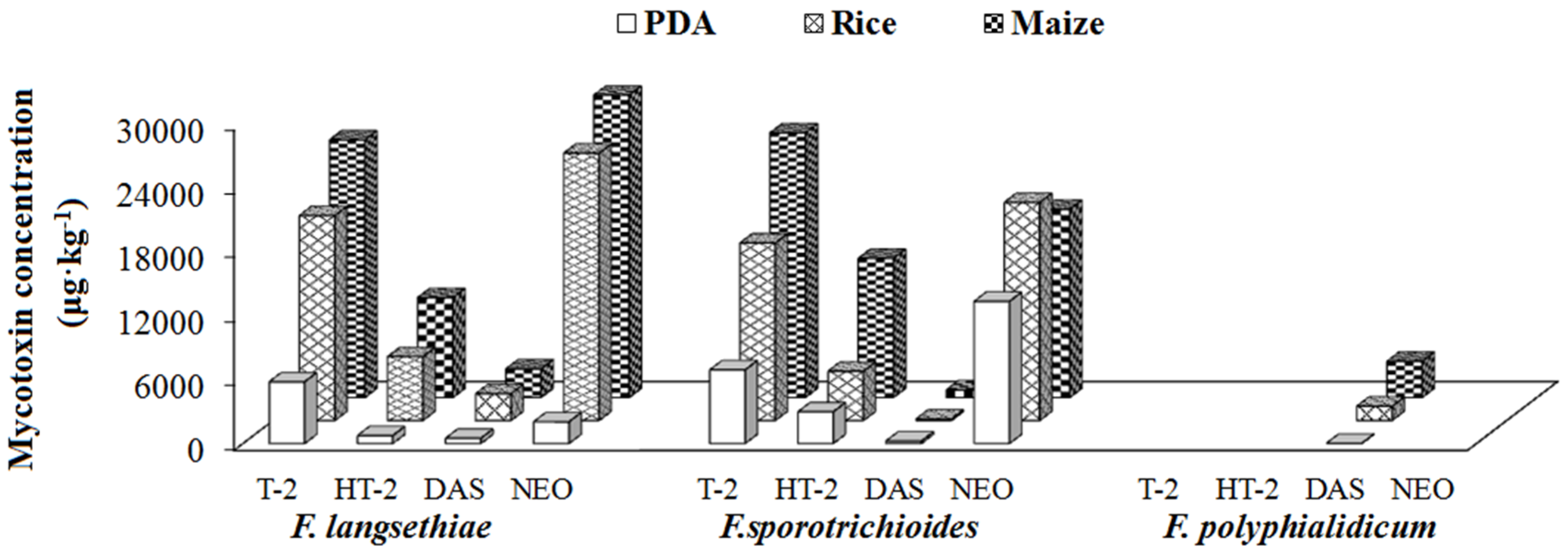
| Strain No. | Code | Fusarium Species | Origin | Host |
|---|---|---|---|---|
| 1 | MUCL 1 52463 | Fusarium temperatum | Belgium | Maize |
| 2 | MUCL 43485 | Fusarium subglutinans | United States | Maize |
| 3 | MUCL 42823 | Fusarium culmorum | Belgium | Wheat |
| 4 | MUCL 51036 | Fusarium fujikuroi | Philippines | Rice |
| 5 | MUCL 34988 | Fusarium langsethiae | - | Wheat |
| 6 | MUCL 52574 | Fusarium musae | Honduras | Banana |
| 7 | MUCL 53395 | Fusarium poae | Belgium | Maize |
| 8 | MUCL 43483 | Fusarium proliferatum | - | - |
| 9 | F-1 | Fusarium graminearum | China | Wheat |
| 10 | MUCL 53602 | Fusarium sporotrichioides | Belgium | Maize |
| 11 | MUCL 42821 | Fusarium tricinctum | Belgium | Wheat |
| 12 | MUCL 43478 | Fusarium verticillioides | United States | Maize |
| 13 | B40 = F50/1-i1-B | Fusarium oxysporum | China | Barley |
| 14 | MC1_30 | Fusarium meridionale | China | Maize |
| 15 | M-12-0203-A1 | Fusarium equiseti | China | Maize |
| 16 | M-12-0501-J1 | Fusarium sacchari | China | Maize |
| 17 | M-12-0601-D12 | Fusarium solani | China | Maize |
| 18 | Q29 | Fusarium concentricum | China | Green pepper |
| 19 | W21 | Fusarium andiyazi | China | Maize |
| 20 | XB4-1 | Fusarium polyphialidicum | China | Barley |
| Group | Strain No. | Fusarium Species | Major Mycotoxins Produced | Other Mycotoxins Produced | |
|---|---|---|---|---|---|
| Group I (Fusaric acid) | Subgroup I (Fumonisins and fusaric acid) | 8 | F. proliferatum | FB1, FB2, Fusaric acid | - |
| 12 | F. verticillioides | FB1, FB2, Fusaric acid | - | ||
| 4 | F. fujikuroi | FB1, FB2, Fusaric acid | - | ||
| 17 | F. solani | FB1, FB2, Fusaric acid | - | ||
| Subgroup II (Fusaric acid only) | 1 | F. temperatum | Fusaric acid | - | |
| 2 | F. subglutinans | Fusaric acid | - | ||
| 6 | F. musae | Fusaric acid | - | ||
| 11 | F. tricinctum | Fusaric acid | - | ||
| 13 | F. oxysporum | Fusaric acid | - | ||
| 15 | F. equiseti | Fusaric acid | - | ||
| 16 | F. sacchari | Fusaric acid | - | ||
| 18 | F. concentricum | Fusaric acid | - | ||
| 19 | F. andiyazi | Fusaric acid | - | ||
| Group II (Type A trichothecenes) | 5 | F. langsethiae | T-2, HT-2, NEO, DAS | - | |
| 10 | F. sporotrichioides | T-2, HT-2, NEO, DAS | - | ||
| 20 | F. polyphialidicum | DAS | - | ||
| Group III (Type B trichothecenes) | 3 | F. culmorum | NIV, Fus-X | T-2, HT-2, NEO, ZEN | |
| 7 | F. poae | NIV, Fus-X | T-2, HT-2, NEO, DAS | ||
| 14 | F. meridionale | NIV, Fus-X | NEO, ZEN | ||
| 9 | F. graminearum | DON, 15-ADON, 3-ADON | ZEN | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; De Saeger, S.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic Potentials of Fusarium Species in Various Culture Matrices Revealed by Mycotoxin Profiling. Toxins 2017, 9, 6. https://doi.org/10.3390/toxins9010006
Shi W, Tan Y, Wang S, Gardiner DM, De Saeger S, Liao Y, Wang C, Fan Y, Wang Z, Wu A. Mycotoxigenic Potentials of Fusarium Species in Various Culture Matrices Revealed by Mycotoxin Profiling. Toxins. 2017; 9(1):6. https://doi.org/10.3390/toxins9010006
Chicago/Turabian StyleShi, Wen, Yanglan Tan, Shuangxia Wang, Donald M. Gardiner, Sarah De Saeger, Yucai Liao, Cheng Wang, Yingying Fan, Zhouping Wang, and Aibo Wu. 2017. "Mycotoxigenic Potentials of Fusarium Species in Various Culture Matrices Revealed by Mycotoxin Profiling" Toxins 9, no. 1: 6. https://doi.org/10.3390/toxins9010006






