Safety Assessment of Lactobacillus helveticus KLDS1.8701 Based on Whole Genome Sequencing and Oral Toxicity Studies
Abstract
:1. Introduction
2. Results
2.1. Taxonomic Identification and Mobilomes
2.2. Antibiotic Resistance and Related Determinants
2.3. Putatively Adverse Metabolites and Associated Genes
2.4. Putative Virulence Factors
2.5. Acute Oral Toxicity Study
2.6. Subacute Oral Toxicity Study
2.6.1. Clinical Observations
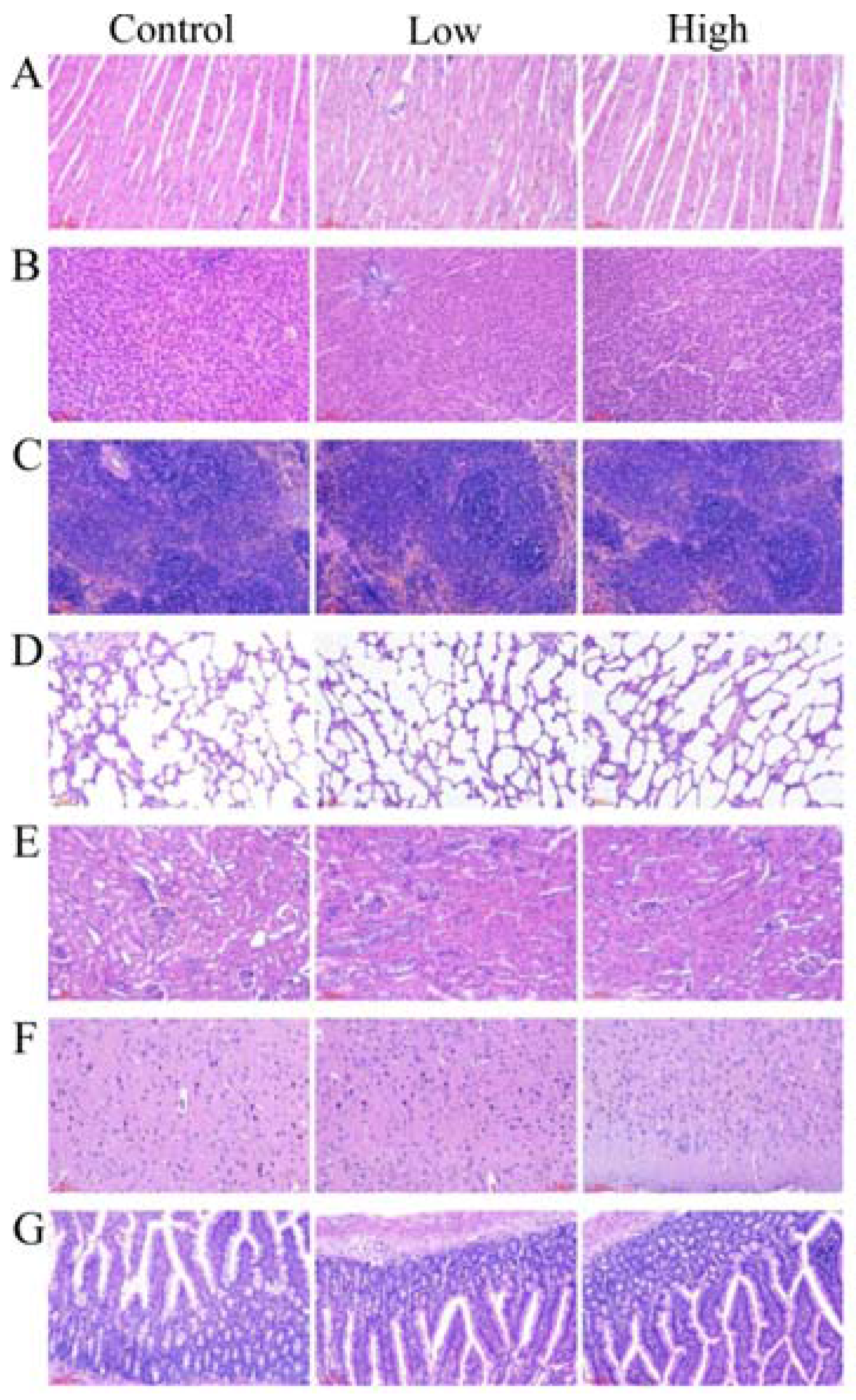
2.6.2. Gross Necropsy and Histopathological Examination
2.6.3. Hematology and Serum Biochemistry Analysis
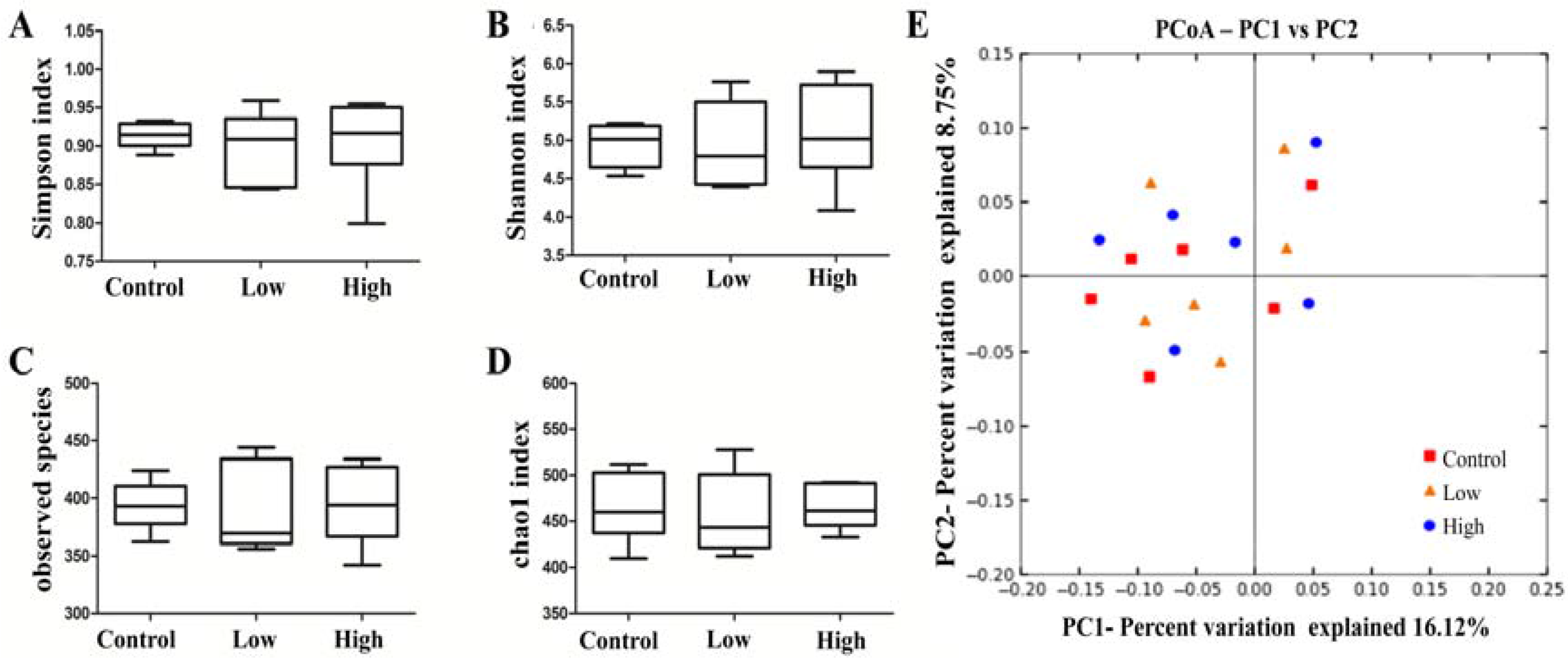
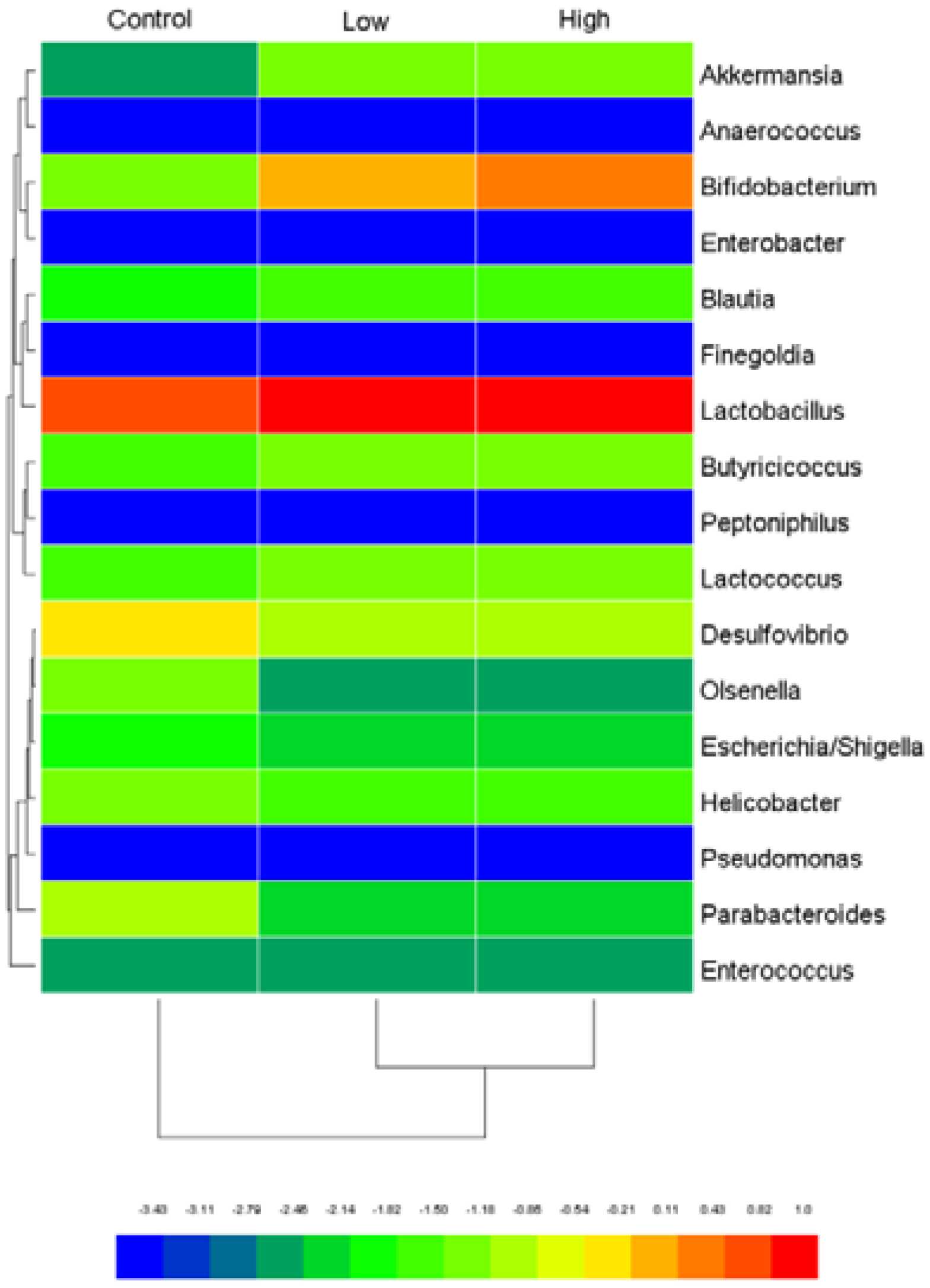
2.6.4. Cecal Microbiota and Harmful Bacterial Enzymes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacteria Strain and Growth Condition
5.2. Genome Sequencing and Taxonomy
5.3. Identification of Safety-Associated Genes in Silico
5.4. Measurement of Antibiotic Resistance Phenotypes
5.5. Measurement of Adverse Metabolites
5.6. Animals
5.7. Experimental Design
5.7.1. Acute Oral Toxicity Study
5.7.2. Subacute Oral Toxicity Study
5.8. Hematological and Serum Biochemistry Analyses
5.9. Gross Necropsy and Histopathological Studies
5.10. Cecal Microbiota and Harmful Bacterial Enzymes
5.11. Statistical Analysisd
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food Agriculture Organization/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food; FAO/WHO: London, UK, 2002. [Google Scholar]
- Taverniti, V.; Guglielmetti, S. Health-promoting properties of Lactobacillus helveticus. Front. Microbiol. 2012, 3, 392. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the scientific committee on a request from EFSA on the introduction of a qualified presumption of safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA J. 2007, 587, 1e16. [Google Scholar]
- Adams, M.; Mitchell, R. Fermentation and pathogen control: A risk assessment approach. Int. J. Food Microbiol. 2002, 79, 75–83. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Routh, J.; Qutub, M.O.; Lischer, G.; Liang, K.V.; Baddour, L.M. Lactobacillus gasseri causing fournier's gangrene. Scand. J. Infect. Dis. 2004, 36, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Gupta, H.; Kapila, S.; Kaur, G.; Vij, S.; Malik, R.K. Safety assessment and evaluation of probiotic potential of bacteriocinogenic Enterococcus faecium KH 24 strain under in vitro and in vivo conditions. Int. J. Food Microbiol. 2010, 141, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Alkema, W.; Boekhorst, J.; Wels, M.; van Hijum, S.A. Microbial bioinformatics for food safety and production. Brief. Bioinform. 2016, 17, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Liu, C.; Zhu, Y.-Z.; Wei, Y.-X.; Tian, F.; Zhao, G.-P.; Guo, X.-K. Safety assessment of Lactobacillus plantarum JDM1 based on the complete genome. Int. J. Food Microbiol. 2012, 153, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-X.; Zhang, Z.-Y.; Liu, C.; Malakar, P.K.; Guo, X.-K. Safety assessment of Bifidobacterium longum JDM301 based on complete genome sequences. World J. Gastroenterol. 2012, 18, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Senan, S.; Prajapati, J.; Joshi, C. Feasibility of genome-wide screening for biosafety assessment of probiotics: A case study of Lactobacillus helveticus MTCC 5463. Probiotics Antimicrob. Proteins 2015, 7, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wang, T.-T.; Xu, M.; Evivie, S.E.; Luo, G.-W.; Liang, H.-Z.; Yu, S.-F.; Huo, G.-C. Effect of Lactobacillus strains on intestinal microflora and mucosa immunity in Escherichia coli O157: H7-induced diarrhea in mice. Curr. Microbiol. 2016, 73, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Evivie, S.E.; Muhammad, Z.; Luo, G.-W.; Liang, H.-Z.; Wang, N.-N.; Huo, G.-C. In vitro assessment of the antimicrobial potentials of Lactobacillus helveticus strains isolated from traditional cheese in sinkiang china against food-borne pathogens. Food Funct. 2016, 7, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Muhammad, Z.; Evivie, S.E.; Luo, G.-W.; Xu, M.; Huo, G.-C. Screening of antifungal potentials of Lactobacillus helveticus KLDS 1.8701 against spoilage microorganism and their effects on physicochemical properties and shelf life of fermented soybean milk during preservation. Food Control 2016, 66, 183–189. [Google Scholar] [CrossRef]
- Li, B.; Liu, F.; Tang, Y.; Luo, G.; Evivie, S.; Zhang, D.; Wang, N.; Li, W.; Huo, G. Complete genome sequence of Lactobacillus helveticus KLDS1. 8701, a probiotic strain producing bacteriocin. J. Biotechnol. 2015, 212, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Poretsky, R.; Rodriguez-R, L.M.; Luo, C.; Tsementzi, D.; Konstantinidis, K.T. Strengths and limitations of 16s rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS ONE 2014, 9, e93827. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rossello-Mora, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Callanan, M.; Kaleta, P.; O’Callaghan, J.; O’Sullivan, O.; Jordan, K.; McAuliffe, O.; Sangrador-Vegas, A.; Slattery, L.; Fitzgerald, G.F.; Beresford, T. Genome sequence of Lactobacillus helveticus, an organism distinguished by selective gene loss and insertion sequence element expansion. J. Bacteriol. 2008, 190, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Muñoz, M.d.C.C.; Lerma, L.L.; Montoro, B.P.; Bockelmann, W.; Pichner, R.; Kabisch, J.; Cho, G.-S.; Franz, C.M.; Galvez, A. New insights in antibiotic resistance of Lactobacillus species from fermented foods. Food Res. Int. 2015, 78, 465–481. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Guo, H.; Pan, L.; Li, L.; Lu, J.; Kwok, L.; Menghe, B.; Zhang, H.; Zhang, W. Characterization of antibiotic resistance genes from Lactobacillus isolated from traditional dairy products. J. Food Sci. 2017, 82, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Botina, S.; Poluektova, E.; Glazova, A.; Zakharevich, N.; Koroban, N.; Zinchenko, V.; Babykin, M.; Zhilenkova, O.; Amerkhanova, A.; Danilenko, V. Antibiotic resistance of potential probiotic bacteria of the genus Lactobacillus from human gastrointestinal microbiome. Microbiology 2011, 80, 164–171. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.; Packman, L.; Burleigh, B.; Dell, A.; Morris, H.; Hartley, B. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature 1979, 282, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin a antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR interference limits horizontal gene transfer in Staphylococci by targeting DNA. Science 2008, 322, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Loret, S.; Deloyer, P.; Dandrifosse, G. Levels of biogenic amines as a measure of the quality of the beer fermentation process: Data from Belgian samples. Food Chem. 2005, 89, 519–525. [Google Scholar] [CrossRef]
- Christiansen, J.K.; Hughes, J.E.; Welker, D.L.; Rodríguez, B.T.; Steele, J.L.; Broadbent, J.R. Phenotypic and genotypic analysis of amino acid auxotrophy in Lactobacillus helveticus CNRZ32. Appl. Environ. Microbiol. 2008, 74, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Jahromi, M.F.; Liang, J.B.; Kalavathy, R.; Sieo, C.C.; Ho, Y.W. Safety assessment of two new Lactobacillus strains as probiotic for human using a rat model. PLoS ONE 2016, 11, e0159851. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Al-Shabanah, O.; El-Hadiyah, T.; Al-Majed, A. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of swiss albino mice. Sci. Pharm. 2002, 70, 135–145. [Google Scholar]
- Petterino, C.; Argentino-Storino, A. Clinical chemistry and haematology historical data in control sprague-dawley rats from pre-clinical toxicity studies. Exp. Toxicol. Pathol. 2006, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696. [Google Scholar] [CrossRef] [PubMed]
- Tham, C.S.-C.; Peh, K.-K.; Bhat, R.; Liong, M.-T. Probiotic properties of Bifidobacteria and Lactobacilli isolated from local dairy products. Ann. Microbiol. 2012, 62, 1079–1087. [Google Scholar] [CrossRef]
- Ouwerkerk, J.; Aalvink, S.; Belzer, C.; De Vos, W. Preparation and preservation of viable Akkermansia muciniphila cells for therapeutic interventions. Benef. Microbes 2017, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Eren, A.M.; Sogin, M.L.; Morrison, H.G.; Vineis, J.H.; Fisher, J.C.; Newton, R.J.; McLellan, S.L. A single genus in the gut microbiome reflects host preference and specificity. ISME J. 2015, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Steppe, M.; Van Nieuwerburgh, F.; Vercauteren, G.; Boyen, F.; Eeckhaut, V.; Deforce, D.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Safety assessment of the butyrate-producing Butyricicoccus pullicaecorum strain 25–3T, a potential probiotic for patients with inflammatory bowel disease, based on oral toxicity tests and whole genome sequencing. Food Chem. Toxicol. 2014, 72, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Coffey, J.C.; O’Connell, P.R. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Boente, R.F.; Ferreira, L.Q.; Falcão, L.S.; Miranda, K.R.; Guimarães, P.L.; Santos-Filho, J.; Vieira, J.M.; Barroso, D.E.; Emond, J.-P.; Ferreira, E.O. Detection of resistance genes and susceptibility patterns in Bacteroides and Parabacteroides strains. Anaerobe 2010, 16, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.; Rôças, I. Uncultivated phylotypes and newly named species associated with primary and persistent endodontic infections. J. Clin. Microbiol. 2005, 43, 3314–3319. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing eztaxon-e: A prokaryotic 16s rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evolut. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evolut. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek 2017, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357, CARD. Available online: http://arpcard.mcmaster.ca/ (accessed on 20 March 2017). [CrossRef] [PubMed]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697, VFDB. Available online: http://www.mgc.ac.cn/VFs/main.htm (accessed on 20 March 2017). [CrossRef] [PubMed]
- Siguier, P.; Pérochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36, ISfinder. Available online: https://www-is.biotoul.fr/ (accessed on 20 March 2017). [CrossRef] [PubMed]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2007, 35, W52–W57, CRISPRFinder. Available online: http://crispr.upsud.fr/ (accessed on 20 March 2017). [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21, PHASTER. Available online: http://phaster.ca/ (accessed on 20 March 2017). [CrossRef] [PubMed]
- International Organization for Standardization. Milk and Milk Products—Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria; ISO 10932/IDF 233 Standard; ISO: Geneva, Switzerland, 2010; p. 31. [Google Scholar]
- Chesson, A.; Franklin, A.; Aumaître, A.; Sköld, O.; Leclercq, R.; von Wright, A.; Guillot, J. Opinion of the Scientific Committee on Animal Nutrition on the Criteria for Assessing the Safety of Microorganisms Resistant to Antibiotics of Human and Veterinary Importance; Directorate C—Scientific Opinions; European Commission Health and Consumer Protection Directorate-General: Brussels, Belgium, 2002. [Google Scholar]
- Dadáková, E.; Křížek, M.; Pelikánová, T. Determination of biogenic amines in foods using ultra-performance liquid chromatography (uplc). Food Chem. 2009, 116, 365–370. [Google Scholar] [CrossRef]
- Lorencová, E.; Buňková, L.; Matoulková, D.; Dráb, V.; Pleva, P.; Kubáň, V.; Buňka, F. Production of biogenic amines by lactic acid bacteria and Bifidobacteria isolated from dairy products and beer. Int. J. Food Sci. Technol. 2012, 47, 2086–2091. [Google Scholar] [CrossRef]
- Smělá, D.; Pechová, P.; Komprda, T.; Klejdus, B.; Kubáň, V. Chromatographic determination of biogenic amines in dry salami during the fermentation and storage. Chem. Pap. 2004, 98, 432–437. [Google Scholar]
- Organisation of Economic Cooperation and Development. Section 4 (Part 423), Acute Oral Toxicity, Acute Toxic Class Method, Guidline for the Testing of Chemicals; OECD: Paris, France, 2001. [Google Scholar]
- Organisation of Economic Cooperation and Development. Section 4 (Part 407), Repeated Dose 28-Day Oral Toxicity Study in Rodents, Guidline for the Testing of Chemicals; OECD: Paris, France, 2008. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Pan, M.; Huang, R.; Tian, X.; Tao, X.; Shah, N.P.; Wei, H.; Wan, C. Modulation of the small intestinal microbial community composition over short-term or long-term administration with Lactobacillus plantarum ZDY2013. J. Dairy Sci. 2016, 99, 6913–6921. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Jang, S.; Baek, E.H.; Kim, M.J.; Lee, K.S.; Shin, H.S.; Chung, M.J.; Kim, J.E.; Lee, K.O.; Ha, N.J. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Dis. 2009, 8, 21. [Google Scholar] [CrossRef] [PubMed]
| Antimicrobials | MIC | Cut-Off Values |
|---|---|---|
| Ampicillin | <0.25 | 1 |
| Vancomycin | 1 | 2 |
| Gentamicin | 16 | 16 |
| Streptomycin | 8 | 16 |
| Kanamycin | >256 | 16 |
| Erythromycin | <0.25 | 1 |
| Clindamycin | >256 | 1 |
| Chloramphenicol | >256 | 4 |
| Tetracycline | 2 | 4 |
| Dalfopristin | 8 | 4 |
| Linezolid; | 2 | 4 |
| Neomycin | 4 | 32 |
| Ciprofloxacin | 16 | 4 |
| Trimethoprim | 64 | 32 |
| Rifampicin | 2 | 32 |
| Treatment | Males | Females | ||||
|---|---|---|---|---|---|---|
| Control | Low | High | Control | Low | High | |
| RBC (×106/µL) | 7.73 ± 0.42 | 7.93 ± 0.55 | 8.26 ± 0.99 | 7.47 ± 0.45 | 7.63 ± 0.32 | 7.64 ± 0.39 |
| WBC (×103/µL) | 13.37 ± 1.2 | 12.10 ± 2.25 | 10.77 ± 1.34 | 11.97 ± 1.56 | 10.63 ± 1.92 | 13.17 ± 1.95 |
| HGB (g/L) | 148.30 ± 6.05 | 150.93 ± 5.47 | 149.47 ± 9.05 | 142.57 ± 2.80 | 142.3 ± 5.51 | 141.87 ± 3.02 |
| PLT (×103/µL) | 909.23 ± 101.57 | 920.80 ± 146.94 | 957.33 ± 193.27 | 1226.67 ± 213.73 | 1350.18 ± 156.90 | 1292.33 ± 237.05 |
| MCV (fL) | 56.03 ± 1.50 | 55.27 ± 2.61 | 57.00 ± 9.54 | 55.12 ± 1.28 | 54.37 ± 1.26 | 55.27 ± 2.78 |
| MCHC (g/L) | 331.43 ± 10.81 | 333.37 ± 11.15 | 336.33 ± 9.61 | 340.83 ± 5.01 | 340.33 ± 7.23 | 337.67 ± 8.02 |
| Neutrophils (%) | 19.47 ± 2.35 | 20.80 ± 0.95 | 21.97 ± 3.24 | 24.84 ± 4.06 | 25.73 ± 0.67 | 21.13 ± 2.90 |
| Lymphocytes (%) | 71.43 ± 5.61 | 72.17 ± 2.20 | 73.97 ± 1.70 | 73.73 ± 3.12 | 75.73 ± 3.17 | 74.21 ± 6.26 |
| Monocytes (%) | 3.83 ± 0.31 | 3.91 ± 0.44 | 4.12 ± 0.43 | 5.17 ± 0.45 | 4.97 ± 1.16 | 4.87 ± 0.67 |
| Eosinophils (%) | 1.40 ± 0.44 | 1.13 ± 0.30 | 1.39 ± 0.47 | 1.30 ± 0.61 | 1.23 ± 0.45 | 1.05 ± 0.13 |
| Treatment | Males | Females | ||||
|---|---|---|---|---|---|---|
| Control | Low | High | Control | Low | High | |
| AST (U/L) | 148.80 ± 5.99 | 151.67 ± 20.03 | 148.67 ± 14.05 | 91.27 ± 8.75 | 94.67 ± 11.85 | 93.47 ± 5.08 |
| ALT (U/L) | 72.77 ± 8.88 | 72.56 ± 6.24 | 75.33 ± 5.69 | 52.53 ± 7.36 | 55.13 ± 4.59 | 53.33 ± 3.79 |
| ALP (U/L) | 159.33 ± 9.29 | 161.67 ± 14.19 | 168.00 ± 10.54 | 88.33 ± 8.50 | 88.67 ± 10.97 | 92.67 ± 5.51 |
| TBIL (μmol/L) | 1.00 ± 0.36 | 0.89 ± 0.11 | 1.07 ± 0.25 | 1.03 ± 0.21 | 1.01 ± 0.29 | 1.02 ± 0.10 |
| TP (g/L) | 53.33 ± 3.68 | 53.16 ± 4.54 | 54.01 ± 6.53 | 67.77 ± 4.50 | 68.53 ± 3.26 | 67.33 ± 7.70 |
| ALB (g/L) | 42.13 ± 3.44 | 38.41 ± 3.61 | 36.73 ± 4.65 | 42.93 ± 2.60 | 43.63 ± 4.40 | 44.87 ± 4.96 |
| GLU (mmol/L) | 5.86 ± 1.60 | 6.37 ± 0.97 | 6.60 ± 0.75 | 8.97 ± 0.87 | 8.37 ± 0.92 | 8.65 ± 0.74 |
| TG (mmol/L) | 0.48 ± 0.05 | 0.53 ± 0.12 | 0.49 ± 0.07 | 0.51 ± 0.02 | 0.51 ± 0.05 | 0.49 ± 0.05 |
| TC (mmol/L) | 1.42 ± 0.11 | 1.47 ± 0.18 | 1.53 ± 0.35 | 2.23 ± 0.25 | 2.17 ± 0.31 | 2.33 ± 0.57 |
| HDL (mmol/L) | 1.68 ± 0.25 | 1.70 ± 0.02 | 1.37 ± 0.31 | 1.77 ± 0.31 | 1.71 ± 0.18 | 1.87 ± 0.35 |
| LDL (mmol/L) | 0.21 ± 0.09 | 0.21 ± 0.10 | 0.15 ± 0.07 | 0.33 ± 0.08 | 0.32 ± 0.03 | 0.31 ± 0.04 |
| UREA (mmol/L) | 6.47 ± 1.32 | 7.03 ± 0.15 | 6.63 ± 0.61 | 6.63 ± 0.38 | 6.47 ± 0.31 | 6.53 ± 0.67 |
| CRE (μmol/L) | 52.70 ± 4.93 | 56.67 ± 5.86 | 52.67 ± 5.69 | 73.17 ± 2.35 | 72.13 ± 3.75 | 71.53 ± 3.18 |
| Na (mmol/L) | 140.93 ± 2.35 | 142.01 ± 3.00 | 143.87 ± 7.96 | 142.17 ± 3.25 | 142.07 ± 7.87 | 144.33 ± 6.03 |
| Cl (mmol/L) | 105.40 ± 2.33 | 100.33 ± 2.52 | 101.33 ± 7.57 | 100.67 ± 2.37 | 98.73 ± 2.28 | 100.73 ± 3.55 |
| Ca (mmol/L) | 2.69 ± 0.13 | 2.45 ± 0.22 | 2.55 ± 0.32 | 2.56 ± 0.07 | 2.55 ± 0.06 | 2.54 ± 0.11 |
| P (mmol/L) | 3.12 ± 0.30 | 2.87 ± 0.21 | 2.83 ± 0.39 | 2.48 ± 0.07 | 2.61 ± 0.12 | 2.52 ± 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Jin, D.; Etareri Evivie, S.; Li, N.; Yan, F.; Zhao, L.; Liu, F.; Huo, G. Safety Assessment of Lactobacillus helveticus KLDS1.8701 Based on Whole Genome Sequencing and Oral Toxicity Studies. Toxins 2017, 9, 301. https://doi.org/10.3390/toxins9100301
Li B, Jin D, Etareri Evivie S, Li N, Yan F, Zhao L, Liu F, Huo G. Safety Assessment of Lactobacillus helveticus KLDS1.8701 Based on Whole Genome Sequencing and Oral Toxicity Studies. Toxins. 2017; 9(10):301. https://doi.org/10.3390/toxins9100301
Chicago/Turabian StyleLi, Bailiang, Da Jin, Smith Etareri Evivie, Na Li, Fenfen Yan, Li Zhao, Fei Liu, and Guicheng Huo. 2017. "Safety Assessment of Lactobacillus helveticus KLDS1.8701 Based on Whole Genome Sequencing and Oral Toxicity Studies" Toxins 9, no. 10: 301. https://doi.org/10.3390/toxins9100301






