Rapid Detection and Identification of Mycotoxigenic Fungi and Mycotoxins in Stored Wheat Grain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Specificity and Sensitivity of Multiplex PCR
2.2. Application to Stored Wheat Grain Samples
2.3. Development of the Analytical Method
2.4. Mycotoxin Detection in Stored Wheat Grain
3. Materials and Methods
3.1. Strains and Media
3.2. Wheat Grain Samples
3.3. Reagents and Solvents
3.4. DNA Extraction
3.5. Multiplex PCR
3.6. Multiplex PCR with Artificially Contaminated Wheat Grain Samples
3.7. Isolation of Fungal Species from Wheat Grain
3.8. Preparation of Mycotoxin Standard Solutions
3.9. Sample Preparation for Mycotoxin Analysis
3.10. Instrumentation for Mycotoxin Analysis
3.11. Validation of Analytical Parameters
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed. Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Miller, J.D. Fungi and mycotoxins in grain-implications for stored-product research. J. Stored Prod. Res. 1995, 31, 1–16. [Google Scholar] [CrossRef]
- Campbell, K.W.; White, D.G. Evaluation of corn genotypes for resistance to Aspergillus ear rot, kernel infection, and aflatoxin production. Plant Dis. 1995, 79, 1039–1045. [Google Scholar] [CrossRef]
- Smith, J.E. Aflatoxins; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Medina, A.; Schmidt-Heydt, M.; Rodríguez, A.; Parra, R.; Geisen, R.; Magan, N. Impacts of environmental stress on growth, secondary metabolite biosynthetic gene clusters and metabolite production of xerotolerant/xerophilic fungi. Curr. Genet. 2015, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Bernaldez, V.; Cordoba, J.J.; Magan, N.; Peromingo, B.; Rodríguez, A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT Food Sci. Technol. 2017, 83, 283–291. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Priyanka, S.R.; Venkataramana, M.; Balakrishna, K.; Murali, H.S.; Batra, H.V. Development and evaluation of a multiplex PCR assay for simultaneous detection of major mycotoxigenic fungi from cereals. J. Food Sci. Technol. 2015, 52, 486–492. [Google Scholar] [CrossRef]
- Suanthie, Y.; Cousin, M.A.; Woloshuk, C.P. Multiplex real-time PCR for detection and quantification of mycotoxigenic Aspergillus, Penicillium and Fusarium. J. Stored Prod. Res. 2009, 45, 139–145. [Google Scholar] [CrossRef]
- Kim, D.M.; Chung, S.H.; Chun, H.S. Multiplex PCR assay for the detection of aflatoxigenic and non-aflatoxigenic fungi in meju, a korean fermented soybean food starter. Food Microbiol. 2011, 28, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Ramana, M.V.; Balakrishna, K.; Murali, H.C.; Batra, H.V. Multiplex PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in rice and fingermillet collected from southern India. J. Sci. Food Agric. 2011, 91, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Ramana, M.V.; Shylaja, R.; Uppalapati, S.R.; Murali, H.S.; Batra, H.V. Evaluation of a multiplex PCR assay for concurrent detection of four major mycotoxigenic fungi from foods. J. Appl. Microbiol. 2013, 114, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Venancio, A.; Kozakiewicz, Z.; Lima, N. A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus Section Flavi isolated from Portuguese almonds. Int. J. Food Microbiol. 2009, 129, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.H.; Zhang, J.B.; Chen, F.F.; Li, H.P.; Ndoye, M.; Liao, Y.C. A multiplex PCR assay for genetic chemotyping of toxigenic Fusarium graminearum and wheat grains for 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol and nivalenol mycotoxin. J. Food Agric. Environ. 2012, 10, 505–511. [Google Scholar]
- Gilbert, J.; Anklam, E. Validation of analytical methods for determining mycotoxins in foodstuffs. Trac-Trend Anal. Chem. 2002, 21, 468–486. [Google Scholar] [CrossRef]
- Krska, R.; Baumgartner, S.; Josephs, R. The state-of-the-art in the analysis of type-A and -B trichothecene mycotoxins in cereals. Fresenius' J. Anal. Chem. 2001, 371, 285–299. [Google Scholar] [CrossRef]
- Soleimany, F.; Jinap, S.; Rahmani, A.; Khatib, A. Simultaneous detection of 12 mycotoxins in cereals using RP-HPLC-PDA-FLD with PHRED and a post-column derivatization system. Food Addit. Contam. A 2011, 28, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Biselli, S.; Hummert, C. Development of a multicomponent method for Fusarium toxins using LC-MS/MS and its application during a survey for the content of T-2 toxin and deoxynivalenol in various feed and food samples. Food Addit. Contam. 2005, 22, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Lagana, A. Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography-tandem mass spectrometry. Comparison of different extraction procedures. J. Chromatogr. A 2014, 1343, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takino, M.; Sugita-Konishi, Y.; Tanaka, T. Development of a liquid chromatography/time-of-flight mass spectrometric method for the simultaneous determination of trichothecenes, zearalenone and aflatoxins in foodstuffs. Rapid Commun. Mass Spectrom. 2006, 20, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.Z.; Shi, L.; Feng, X.Y. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, A.; Tessiot, S.; Bessaire, T.; Racault, L.; Fiorese, E.; Urbani, A.; Chan, W.C.; Cheng, P.; Mottier, P. Combining the quick, easy, cheap, effective, rugged and safe approach and clean-up by immunoaffinity column for the analysis of 15 mycotoxins by isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1337, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Koesukwiwat, U.; Sanguankaew, K.; Leepipatpiboon, N. Evaluation of a modified quechers method for analysis of mycotoxins in rice. Food Chem. 2014, 153, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.; Ciasca, B.; Powers, S.; Visconti, A. Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid chromatography-tandem mass spectrometry after multi-toxin immunoaffinity clean up. J. Chromatogr. A 2014, 1354, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Berthiller, F.; Burdaspal, P.; Crews, C.; Jonker, M.A.; Krska, R.; MacDonald, S.; Malone, B.; Maragos, C.; Sabino, M.; et al. Developments in mycotoxin analysis: An update for 2009–2010. World Mycotoxin J. 2011, 4, 3–28. [Google Scholar] [CrossRef]
- Birck, N.M.; Lorini, I.; Scussel, V.M. Fungus and Mycotoxins in Wheat Grain at Post Harvest. In Proceedings of the 9th International Working Conference on Stored Product Protection, Campinas, SP, Brazil, 15–18 October 2006; pp. 198–205. [Google Scholar]
- Mule, G.; Susca, A.; Logrieco, A.; Stea, G.; Visconti, A. Development of a quantitative real-time PCR assay for the detection of Aspergillus carbonarius in grapes. Int. J. Food Microbiol. 2006, 111, S28–S34. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Rodriguez, M.; Andrade, M.J.; Cordoba, J.J. Development of a multiplex real-time PCR to quantify aflatoxin, ochratoxin a and patulin producing molds in foods. Int. J. Food Microbiol. 2012, 155, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.; Wiseman, G.; Knight, A.; Bramley, P.; Foster, L.; Rollinson, S.; Damant, A.; Primrose, S. Measurement issues associated with quantitative molecular biology analysis of complex food matrices for the detection of food fraud. Analyst 2016, 141, 45–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Salgado, A.; González-Jaén, M.T.; Vázquez, C.; Patiño, B. Highly sensitive PCR-based detection method specific for Aspergillus flavus in wheat flour. Food Addit. Contam. 2008, 25, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Sardinas, N.; Vazquez, C.; Gil-Serna, J.; Gonzalez-Jaen, M.T.; Patino, B. Specific detection of Aspergillus parasiticus in wheat flour using a highly sensitive PCR assay. Food Addit. Contam. A 2010, 27, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Lenart, A.M.; Klimek-Kopyra, A.; Mateusz Boroń, P. Morphological and molecular identification and PCR amplification to determine the toxigenic potential of Fusarium spp. isolated from maize ears in southern Poland. Phytoparasitica 2013, 41, 241–248. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhou, Y.; Du, L.; Wang, Q. Fumonisin detection and analysis of potential fumonisin-producing Fusarium spp. in asparagus (Asparagus officinalis L.) in Zhejiang province of China. J. Sci. Food Agric. 2010, 90, 836–842. [Google Scholar] [PubMed]
- Vaclavikova, M.; MacMahon, S.; Zhang, K.; Begley, T.H. Application of single immunoaffinity clean-up for simultaneous determination of regulated mycotoxins in cereals and nuts. Talanta 2013, 117, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hong, S.Y.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.K.; Lee, C.; Chung, S.H. Simultaneous determination of multi-mycotoxins in cereal grains collected from South Korea by LC/MS/MS. Toxins 2017, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, W.; Zhang, Y.; Hu, X.; Wu, L.; Wang, B. QuEChERS purification combined with ultrahigh-performance liquid chromatography tandem mass spectrometry for simultaneous quantification of 25 mycotoxins in cereals. Toxins 2016, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; Macdonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. 2008, 25, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Galperin, M.; Levy, Y.; Perl-Treves, R. Genetic diversity of Fusarium moniliforme detected by vegetative compatibility groups and random amplified polymorphic DNA markers. Plant Pathol. 1997, 46, 871–881. [Google Scholar] [CrossRef]
- Toth, B.; Mesterhazy, A.; Nicholson, P.; Teren, J.; Varga, J. Mycotoxin production and molecular variability of European and American isolates of Fusarium culmorum. Eur. J. Plant Pathol. 2004, 110, 587–599. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, L.G.; Gargouri, S.; Barreau, C.; Richard-Forget, F.; Hajlaoui, M.R. Trichothecene chemotypes of Fusarium culmorum infecting wheat in Tunisia. Int. J. Food Microbiol. 2010, 140, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Pancaldi, D.; Tonti, S.; Prodi, A.; Salomoni, D.; Dal Pra, M.; Nipoti, P.; Alberti, I.; Pisi, A. Survey of the main causal agents of Fusarium head blight of durum wheat around Bologna, northern Italy. Phytopathol. Mediterr. 2010, 49, 258–266. [Google Scholar]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J.W. Limiting mycotoxins in stored wheat. Food Addit. Contam. A 2010, 27, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; Berni, E.; Dall’Asta, C.; Spotti, E.; Marchelli, R.; Ferrero, I.; Restivo, F.M. A multiplex RT-PCR approach to detect aflatoxigenic strains of Aspergillus flavus. J. Appl. Microbiol. 2007, 103, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Scherm, B.; Palomba, M.; Serra, D.; Marcello, A.; Migheli, Q. Detection of transcripts of the aflatoxin genes aflD, af1O, and aflP by reverse transcription-polymerase chain reaction allows differentiation of aflatoxin-producing and non-producing isolates of Aspergillus flavus and Aspergillus parasiticus. Int. J. Food Microbiol. 2005, 98, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hoorfar, J.; Malorny, B.; Abdulmawjood, A.; Cook, N.; Wagner, M.; Fach, P. Practical considerations in design of internal amplification controls for diagnostic PCR assays. J. Clin. Microbiol. 2004, 42, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Molecular Diagnostic Methods for Infectious Diseases, Proposed Guideline, 2nd ed.; CLSI document MM3-P2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2005; Volume 25. [Google Scholar]
- Nolte, F.S. Novel internal controls for real-time PCR assays. Clin. Chem. 2004, 50, 801–802. [Google Scholar] [CrossRef] [PubMed]
- GeneBank Database. Available online: http://www.ncbi.nlm.nih.gov/BLAST (accessed on 20 August 2017).
- European Commission. Commission regulation (EC) No. 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- Logotheti, M.; Kotsovili-Tseleni, A.; Arsenis, G.; Legakis, N.I. Multiplex PCR for the discrimination of A. fumigatus, A. flavus, A. niger and A. terreus. J. Microbiol. Methods 2009, 76, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Susca, A.; Stea, G.; Mule, G.; Perrone, G. Polymerase chain reaction (PCR) identification of Aspergillus niger and Aspergillus tubingensis based on the calmodulin gene. Food Addit. Contam. A 2007, 24, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Susca, A.; Stea, G.; Mule, G. PCR assay for identification of Aspergillus carbonarius and Aspergillus japonicus. Eur. J. Plant Pathol. 2004, 110, 641–649. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant P. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Parry, D.W.; Nicholson, P. Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 1996, 45, 383–391. [Google Scholar] [CrossRef]
- Demeke, T.; Clear, R.M.; Patrick, S.K.; Gaba, D. Species-specific PCR-based assays for the detection of Fusarium species and a comparison with the whole seed agar plate method and trichothecene analysis. Int. J. Food Microbiol. 2005, 103, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Mule, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Doohan, F.M.; Parry, D.W.; Jenkinson, P.; Nicholson, P. The use of species specific PCR based assays to analyse Fusarium ear blight of wheat. Plant Pathol. 1998, 47, 197–205. [Google Scholar] [CrossRef]
- Casasnovas, F.; Fantini, E.N.; Palazzini, J.M.; Giaj-Merlera, G.; Chulze, S.N.; Reynoso, M.M.; Torres, A.M. Development of amplified fragment length polymorphism (AFLP)-derived specific primer for the detection of Fusarium solani aetiological agent of peanut brown root rot. J. Appl. Microbiol. 2013, 114, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’Keeffe, T.L. Detection and discrimination of four Aspergillus section Nigri species by PCR. Lett. Appl. Microbiol. 2015, 60, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Fox, R.T.V.; Culham, A. Development of a PCR-based assay for rapid and reliable identification of pathogenic Fusaria. FEMS Microbiol. Lett. 2003, 218, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Dombrink-Kurtzman, M.A.; McGovern, A.E. Species-specific identification of Penicillium linked to patulin contamination. J. Food Protect. 2007, 70, 2646–2650. [Google Scholar] [CrossRef]
- Hamamoto, H.; Hasegawa, K.; Nakaune, R.; Lee, Y.J.; Makizumi, Y.; Akutsu, K.; Hibi, T. Tandem repeat of a transcriptional enhancer upstream of the sterol 14 alpha-demethylase gene (CYP51) in Penicillium digitatum. Appl. Environ. Microbiol. 2000, 66, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Bogs, C.; Battilani, P.; Geisen, R. Development of a molecular detection and differentiation system for ochratoxin A producing Penicillium species and its application to analyse the occurrence of Penicillium nordicum in cured meats. Int. J. Food Microbiol. 2006, 107, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.H.; Skouboe, P.; Boysen, M.; Soule, J.; Rossen, L. Detection of Penicillium species in complex food samples using the polymerase chain reaction. Int. J. Food Microbiol. 1997, 35, 169–177. [Google Scholar] [CrossRef]
- Kanbe, T.; Yamaki, K.; Kikuchi, A. Identification of the pathogenic Aspergillus species by nested PCR using a mixture of specific primers to DNA topoisomerase II gene. Microbiol. Immunol. 2002, 46, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Manonmani, H.K.; Anand, S.; Chandrashekar, A.; Rati, E.R. Detection of aflatoxigenic fungi in selected food commodities by PCR. Process Biochem. 2005, 40, 2859–2864. [Google Scholar] [CrossRef]
- Priyanka, S.R.; Venkataramana, M.; Kumar, G.P.; Rao, V.K.; Murali, H.C.S.; Batra, H.V. Occurrence and molecular detection of toxigenic Aspergillus species in food grain samples from India. J. Sci. Food Agric. 2014, 94, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Woloshuk, C.P.; Bhatnagar, D.; Cleveland, T.E. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 2000, 248, 157–167. [Google Scholar] [CrossRef]
- Skory, C.D.; Chang, P.K.; Cary, J.; Linz, J.E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin aflatoxin biosynthesis. Appl. Environ. Microbiol. 1992, 58, 3527–3537. [Google Scholar] [PubMed]
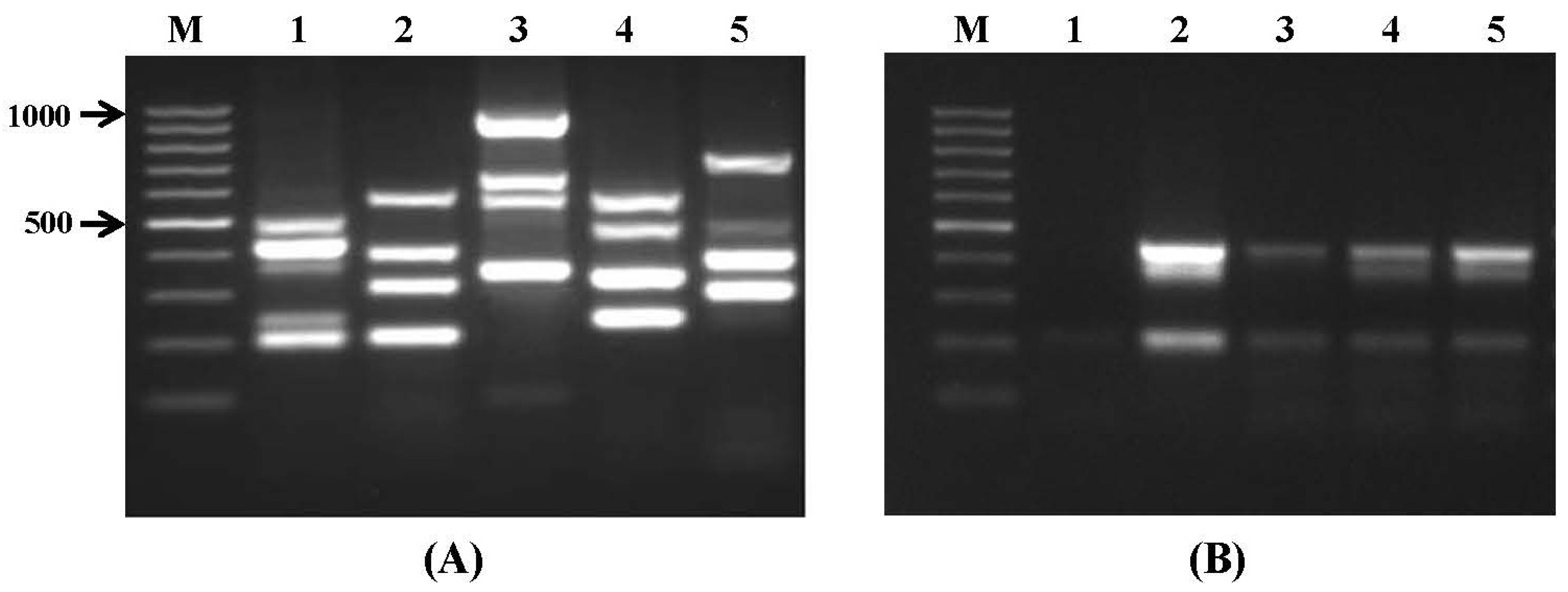
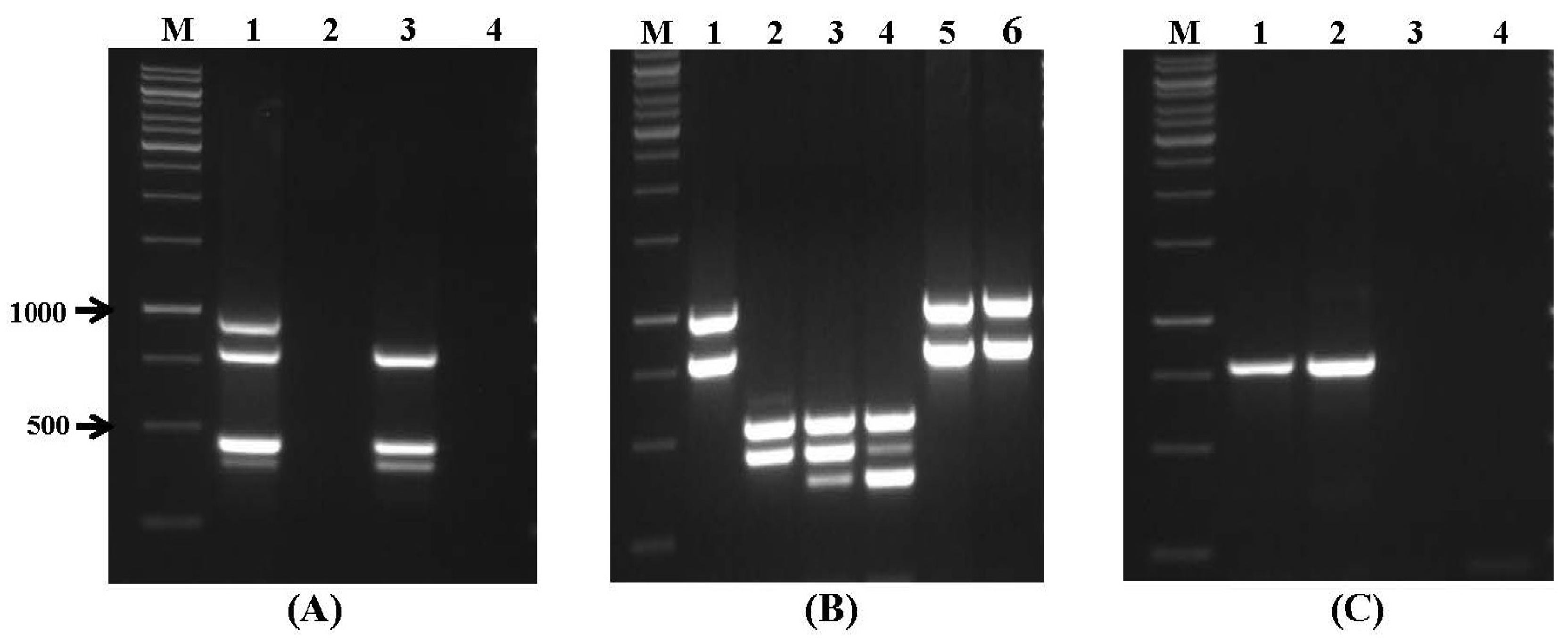
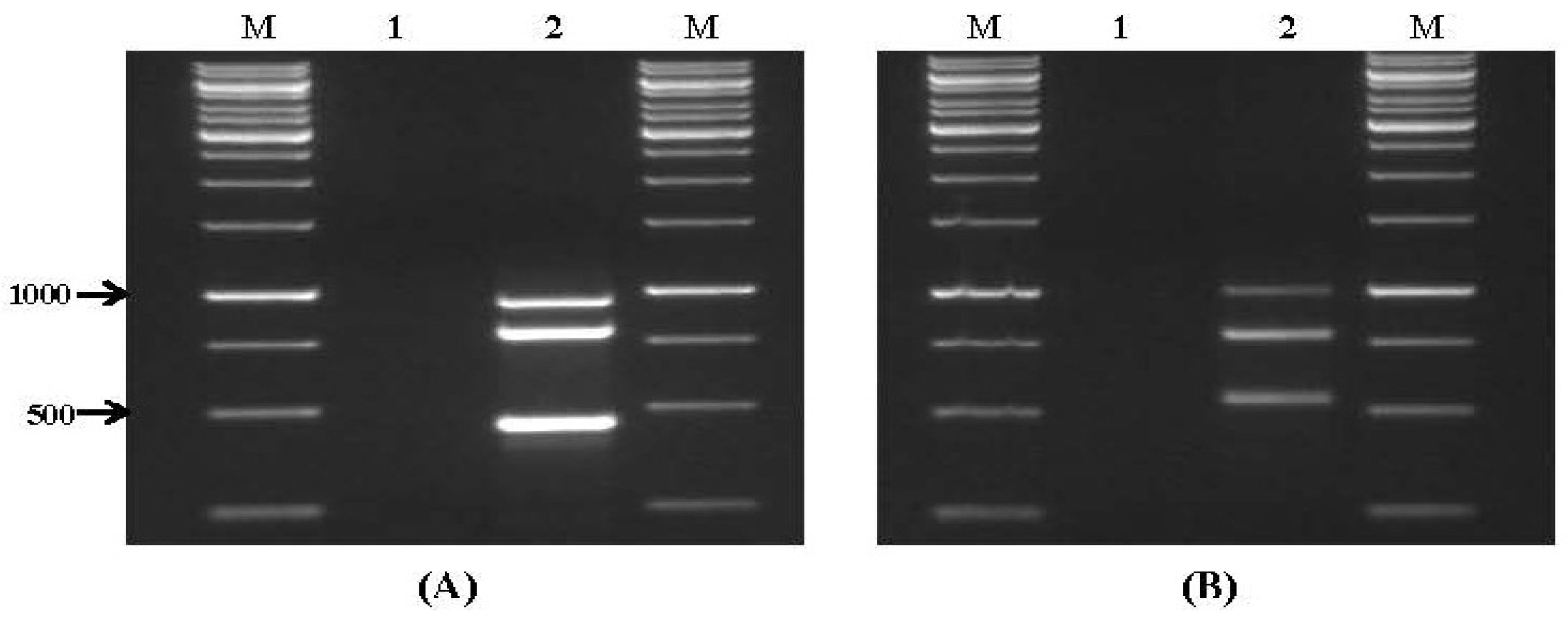
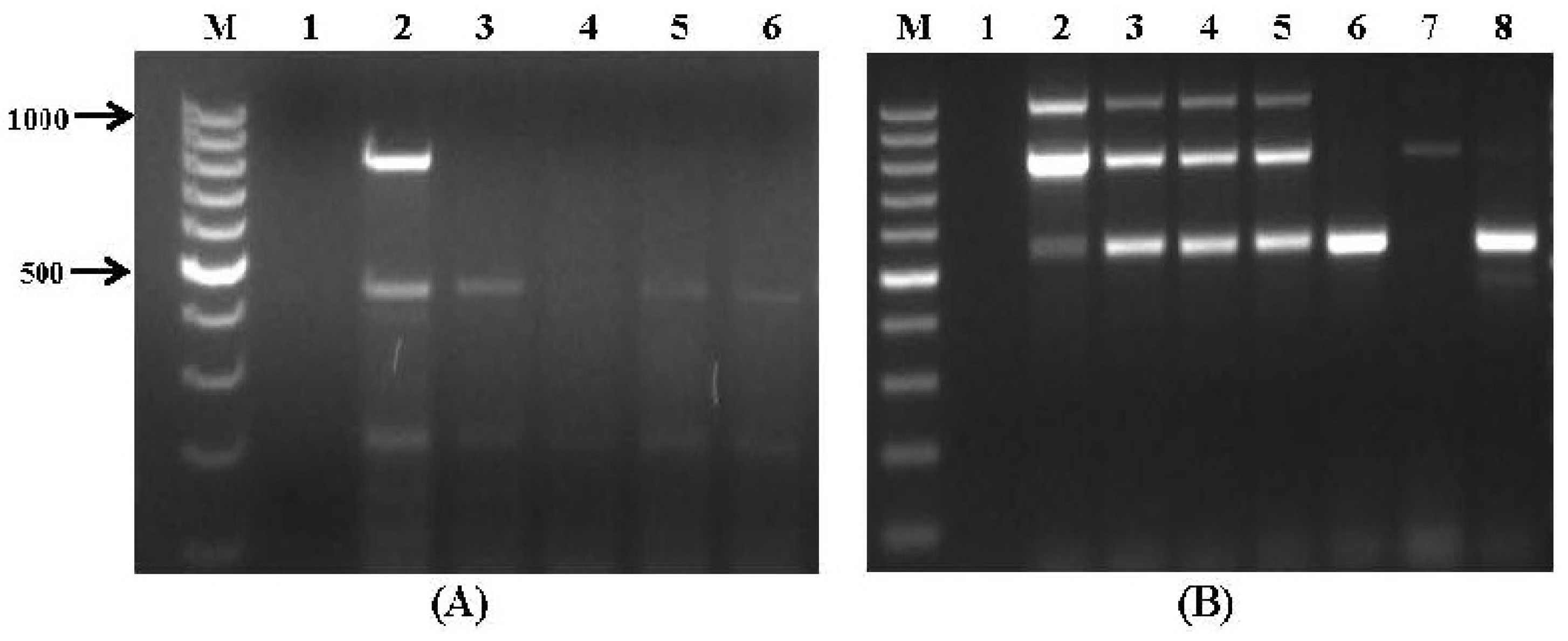
| Set No. | Species | DNA/Gene Target | Amplicon Size (bp) | Annealing Temperature |
|---|---|---|---|---|
| I | A. tubingensis | caM | 505 | |
| A. parasiticus | caM | 430 | ||
| A. carbonarius | caM | 371 | 60 °C | |
| A. fumigatus | pep | 250 | ||
| A. flavus | pepO | 200 | ||
| II | F. culmorum | RAPD 2 marker | 570 | |
| F. graminearum | RAPD marker | 400–500 | ||
| F. sporotrichioides | tri13 | 332 | 55 °C | |
| F. poae | RAPD marker | 220 | ||
| III | F. avenaceum | RAPD marker | 920 | |
| F. verticillioides | caM | 578 | ||
| A. niger | caM | 357 | 55 °C | |
| F. solani | AFLP | 175 | ||
| IV | F. proliferatum | caM | 585 | |
| P. expansum | IDH | 480 | ||
| F. oxysporum | ITS | 340 | 55 °C | |
| P. digitatum | Cyp51 | 250 | ||
| V | P. verrucosum | Otanps | 750 | |
| P. paneum | IDH | 482 | ||
| A. terreus | Topoisomerase II | 386 | 60 °C | |
| P. roqueforti | ITS1-5.8S-ITS2 | 300 |
| Set No. | Mycotoxin | Gene Target | Amplicon Size (bp) | Annealing Temperature |
|---|---|---|---|---|
| VI | Aflatoxins | avfA | 950 | |
| aflR1 | 798 | |||
| ver1 | 452 | 58 °C | ||
| nor1 | 397 | |||
| VII | Fumonisin/Trichothecene/Zearalenone | fum13 | 988 | |
| fum1 | 798 | |||
| tri6 | 546 | 55 °C | ||
| tri5 | 450 | |||
| pks13 | 351 | |||
| VIII | Ochratoxin A | otanps | 788 | |
| 58 °C | ||||
| Wheat Sample # | Mycotoxin Biosynthetic Gene Specific Primer Sets | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aflatoxins (Set No. VI) | Fusarium Toxins (Set No. VII) | OTA (Set No. VIII) | |||||||||
| aflR1 | nor1 | avfA | ver1 | fum1 | fum13 | tri5 | tri6 | pks13 | pks | otanps | |
| 1–3 | - | - | - | - | - | - | - | - | - | - | - |
| 4 | + | - | - | + | + | + | - | - | - | - | - |
| 5 | + | - | - | + | + | + | - | - | - | - | - |
| 6 | + | - | - | + | + | + | - | + | - | - | - |
| 7 | - | - | - | + | - | - | - | - | - | - | - |
| 8 | - | - | - | + | - | - | - | - | - | - | - |
| 9 | - | - | - | + | - | - | - | - | - | - | - |
| 10 | - | - | - | - | - | - | - | - | - | - | - |
| 11 | - | - | - | - | + | + | - | - | - | - | - |
| 12–15 | - | - | - | - | - | - | - | - | - | - | - |
| 16 | - | - | - | + | + | + | - | - | - | - | - |
| 17 | - | - | - | - | - | - | - | - | - | - | - |
| 18 | + | - | - | + | + | + | - | - | - | - | - |
| 19 | + | - | - | + | + | + | - | - | - | + | - |
| 20 | + | - | - | + | + | + | - | - | - | - | - |
| 21 | + | - | - | + | + | + | - | - | - | - | - |
| 22 | - | - | - | - | - | - | - | + | - | + | - |
| 23 | - | - | - | - | - | - | - | - | + | - | |
| 24 | - | - | - | - | - | - | - | + | - | + | - |
| 25 | - | - | - | + | - | - | - | - | - | - | - |
| 26 | + | - | - | + | + | + | - | - | - | - | - |
| 27–34 | - | - | - | - | - | - | - | - | - | - | - |
| Compound | Precursor Ion m/z | Quantifier (Qualifier) Ions m/z | Calibration Level (ppb) | Calculated Concentration ppb (±SD) 1 | RSD (%) 2 | LOQ (LOD) ppb |
|---|---|---|---|---|---|---|
| DON | 355 [M + CH3COO]− | 295 (265) | 40 | 41 (3.6) | 8.9 | 11.4 (3.2) |
| 80 | 82.5 (4.8) | 5.9 | ||||
| 640 | 634.6 (27.4) | 4.4 | ||||
| ZEN | 317 [M − H]− | 131 (175) | 20 | 21.8 (4.9) | 22.5 | 6.3 (1.6) |
| 40 | 36.7 (2.4) | 6.4 | ||||
| 320 | 300 (22.5) | 7.5 | ||||
| T-2 | 484 [M + NH4]+ | 215 (185) | 40 | 37.6 (5.9) | 15.7 | 8.1 (2.4) |
| 80 | 77.5 (8.8) | 11.4 | ||||
| 640 | 650.2 (18.9) | 2.9 | ||||
| OTA | 404 [M + H]+ | 239 (102) | 2 | 2.1 (0.2) | 10.2 | 0.8 (0.2) |
| 4 | 4.3 (0.25) | 5.7 | ||||
| 32 | 32.8 (2.95) | 9.2 | ||||
| FB1 | 722 [M + H]+ | 334 (352) | 20 | 20.2 (0.9) | 4.2 | 8.4 (2.7) |
| 40 | 41.1 (1.5) | 3.6 | ||||
| 320 | 330.4 (27.1) | 8.2 | ||||
| FB2 | 706 [M + H]+ | 336 (318) | 20 | 18.3 (4.0) | 21.7 | 6.8 (2.2) |
| 40 | 38.8 (5.2) | 13.5 | ||||
| 320 | 338.4 (23.7) | 7.0 | ||||
| AFB1 | 313 [M + H]+ | 285 (128) | 1 | 0.98 (0.1) | 10.4 | 0.5 (0.1) |
| 2 | 2.04 (0.16) | 7.8 | ||||
| 16 | 16.3 (0.39) | 2.4 | ||||
| AFB2 | 315 [M + H]+ | 287 (259) | 1 | 1.02 (0.08) | 7.6 | 0.4 (0.1) |
| 2 | 2.07 (0.11) | 5.0 | ||||
| 16 | 15.8 (0.79) | 5.1 | ||||
| AFG1 | 329 [M + H]+ | 243 (200) | 1 | 1.04 (0.12) | 11.2 | 0.5 (0.2) |
| 2 | 2.05 (0.13) | 6.2 | ||||
| 16 | 15.9 (1.01) | 6.4 | ||||
| AFG2 | 331 [M + H]+ | 313 (245) | 1 | 1.1 (0.10) | 9.5 | 1.0 (0.4) |
| 2 | 2.12 (0.11) | 5.3 | ||||
| 16 | 15.7 (0.4) | 2.5 |
| Sample # | AFB1 | AFB2 | AFG1 | AFG2 | FB1 | FB2 | DON | OTA | T-2 | ZEN |
|---|---|---|---|---|---|---|---|---|---|---|
| 1–3 | - | - | - | - | - | - | - | - | - | - |
| 4 | 2.1 | - | - | - | 179.4 | 13.3 | 16.7 | - | - | - |
| 5 | - | - | - | - | 6.2 | 2.2 | - | - | - | - |
| 6 | - | - | - | - | 428.4 | 24.5 | 1.2 | - | - | 0.7 |
| 7 | - | - | - | - | 4.3 | - | - | - | - | - |
| 8 | - | - | - | - | - | - | - | - | - | - |
| 9 | - | - | - | 0.2 | - | - | 2.8 | - | - | - |
| 10 | - | - | - | - | - | - | - | - | - | - |
| 11 | - | - | - | - | - | - | 4.6 | - | - | - |
| 12–17 | - | - | - | - | - | - | - | - | - | - |
| 18 | - | - | - | 1.1 | 2340.7 | 87.9 | - | - | - | - |
| 19 | - | - | - | 0.3 | 53.9 | 4 | - | - | - | - |
| 20 | 6.2 | - | - | 0.4 | 62.6 | 4.8 | - | - | - | 8.9 |
| 21 | 5.2 | - | - | 0.6 | 135.1 | 11.8 | - | 1.9 | - | 3.8 |
| 22 | - | - | - | - | - | - | 263.5 | - | - | 7.6 |
| 23 | - | - | - | - | - | - | - | - | - | |
| 24 | - | - | - | - | - | - | 1746.5 | - | - | 64.8 |
| 25 | - | - | - | - | - | - | - | - | - | - |
| 26 | 32.3 | 1.8 | - | - | 106.7 | 7.5 | - | - | - | - |
| 27 | - | - | - | - | - | - | - | - | - | - |
| 28 | - | - | - | - | - | - | 8.1 | - | - | |
| 29 | - | - | - | - | - | - | - | 22.8 | - | - |
| 30 | - | - | - | - | - | - | - | 41.5 | - | - |
| 31 | - | - | - | - | - | - | 5.7 | - | - | - |
| 32–34 | - | - | - | - | - | - | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadhasivam, S.; Britzi, M.; Zakin, V.; Kostyukovsky, M.; Trostanetsky, A.; Quinn, E.; Sionov, E. Rapid Detection and Identification of Mycotoxigenic Fungi and Mycotoxins in Stored Wheat Grain. Toxins 2017, 9, 302. https://doi.org/10.3390/toxins9100302
Sadhasivam S, Britzi M, Zakin V, Kostyukovsky M, Trostanetsky A, Quinn E, Sionov E. Rapid Detection and Identification of Mycotoxigenic Fungi and Mycotoxins in Stored Wheat Grain. Toxins. 2017; 9(10):302. https://doi.org/10.3390/toxins9100302
Chicago/Turabian StyleSadhasivam, Sudharsan, Malka Britzi, Varda Zakin, Moshe Kostyukovsky, Anatoly Trostanetsky, Elazar Quinn, and Edward Sionov. 2017. "Rapid Detection and Identification of Mycotoxigenic Fungi and Mycotoxins in Stored Wheat Grain" Toxins 9, no. 10: 302. https://doi.org/10.3390/toxins9100302






