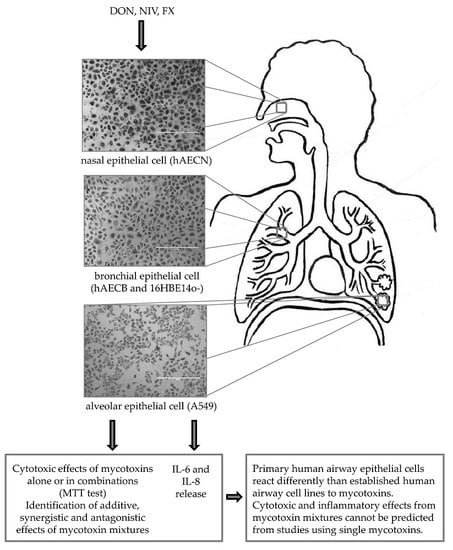
Primary and Immortalized Human Respiratory Cells Display Different Patterns of Cytotoxicity and Cytokine Release upon Exposure to Deoxynivalenol, Nivalenol and Fusarenon-X
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of DON, NIV and FX on Immortalized and Primary Airway Cell Lines
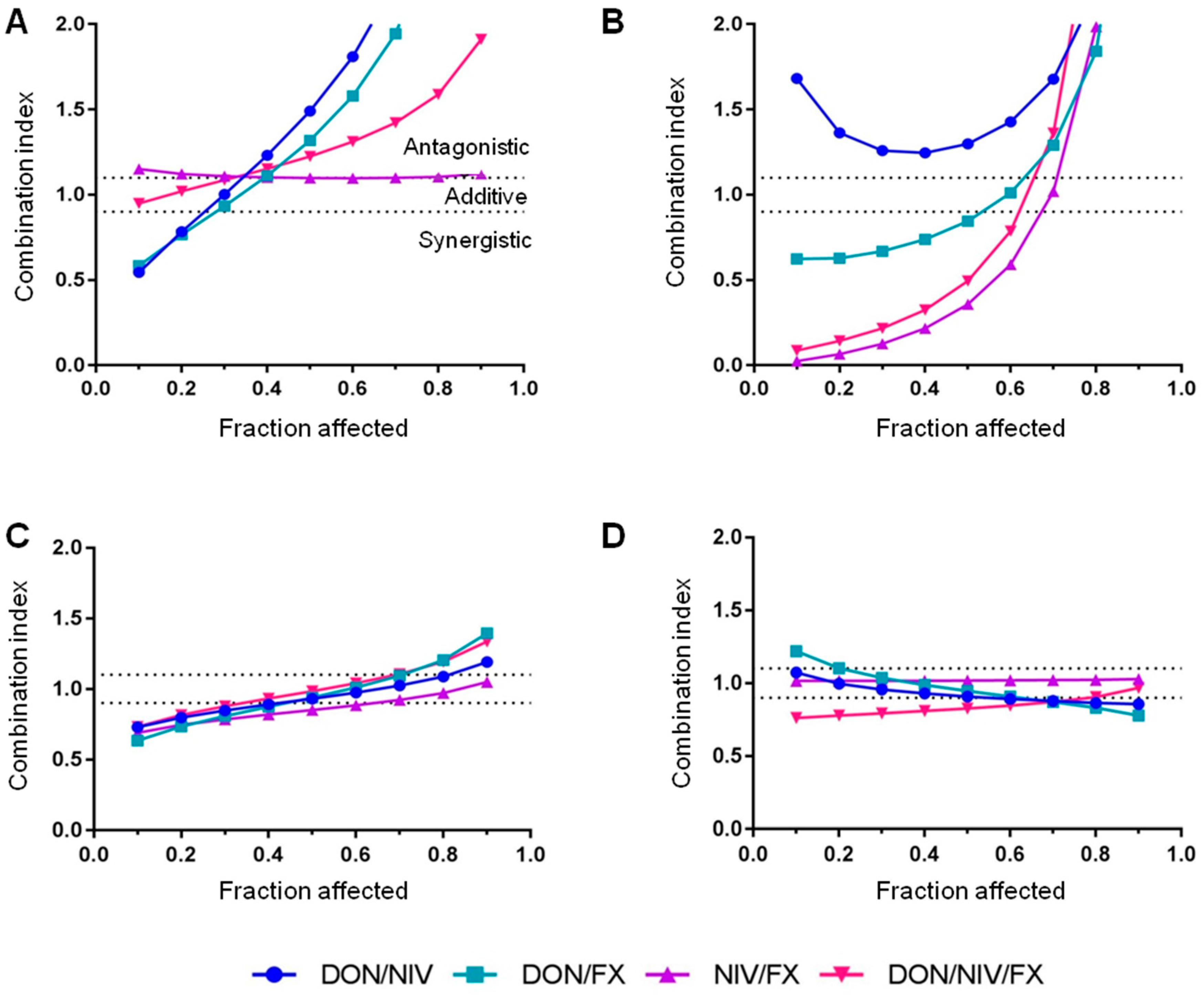
2.2. Cytotoxicity of Binary and Ternary Combinations of DON, NIV and FX on Established and Primary Airway Cell Lines
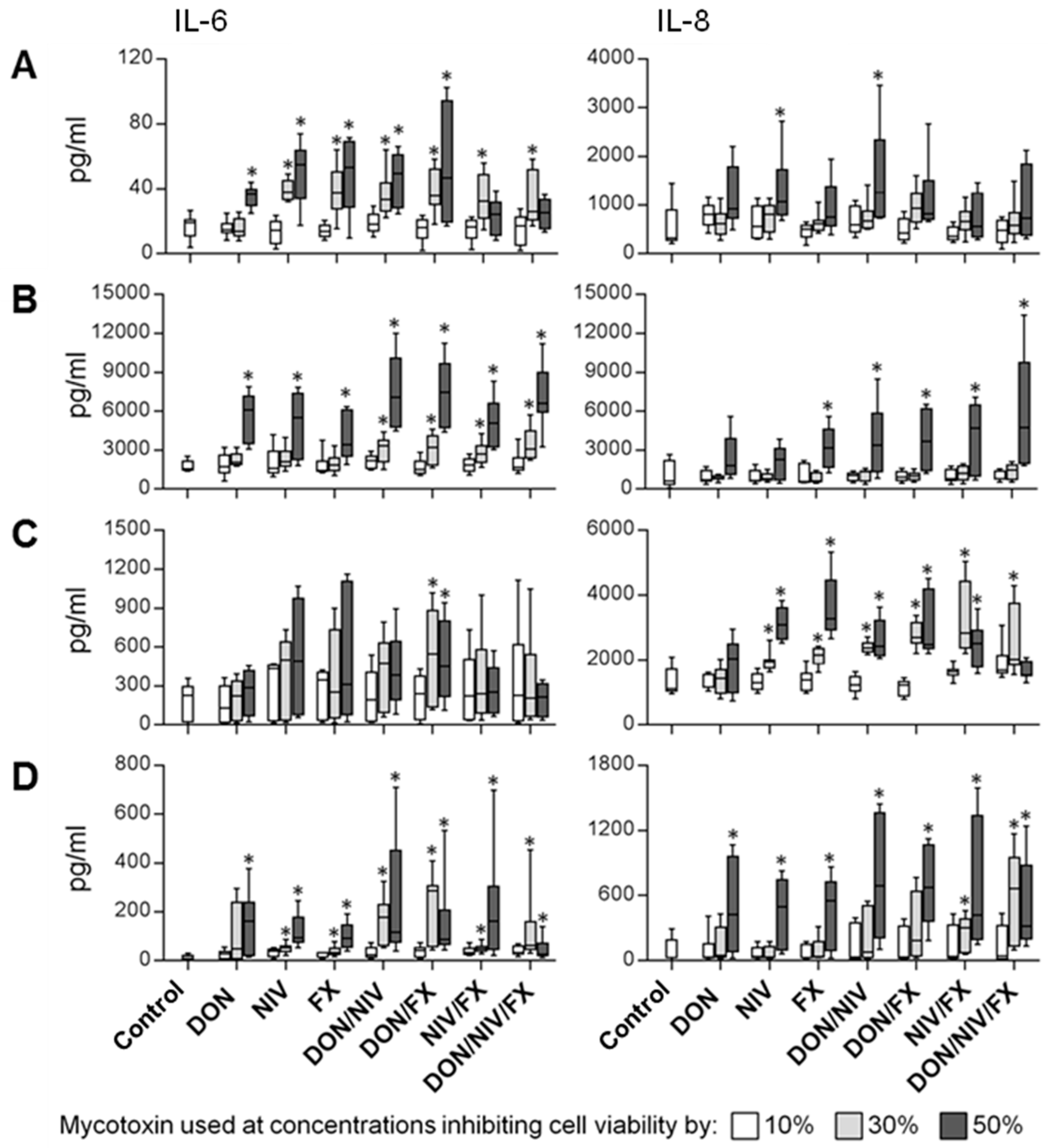
2.3. IL-6 and IL-8 Release by Immortalized and Primary Airway Cell Lines Exposed to DON, NIV and FX
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Regents and Chemicals
4.3. Cell Exposure to Mycotoxins
4.4. Cell Viability Assay
4.5. Mycotoxin Cytotoxicity Assays and Data Analysis
4.6. IL-6 and IL-8 Quantification by ELISA
4.7. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gareis, M.; Wolff, J. Relevance of mycotoxin contaminated feed for farming animals and carry over of mycotoxins in food of animal origin. Mycoses 2000, 43, 79–83. [Google Scholar] [PubMed]
- Krysinska-Traczyk, E.; Perkowski, J.; Dutkiewicz, J. Levels of fungi and mycotoxins in the samples of grain and grain dust collected from five various cereal crops in eastern Poland. Ann. Agric. Environ. Med. AAEM 2007, 14, 159–167. [Google Scholar] [PubMed]
- Niculita-Hirzel, H.; Hantier, G.; Storti, F.; Plateel, G.; Roger, T. Frequent occupational exposure to Fusarium mycotoxins of workers in the Swiss grain industry. Toxins 2016, 8, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. BIOMIN World Mycotoxin Survey 2016; Annual Report No. 13; BIOMIN Holding GmbH: Getzersdorf, Austria, 2016; pp. 1–11. [Google Scholar]
- Anonymous. BIOMIN World Mycotoxin Survey H1 2017; BIOMIN Holding GmbH: Getzersdorf, Austria, 2017; pp. 1–8. [Google Scholar]
- Chandler, E.A.; Simpson, D.R.; Thomsett, M.A.; Nicholson, P. Development of PCR assays to Tri7 and Tri13 trichothecene biosynthetic genes, and characterisation of chemotypes of Fusarium graminearum, Fusarium culmorum and Fusarium cerealis. Physiol. Mol. Plant Pathol. 2003, 62, 355–367. [Google Scholar] [CrossRef]
- De Mers, F. Preliminary assessment of the risk of exposure to trichothecenes produced by Fusarium graminearum in grain dust. In Occupational Health and Safety Branch; HRD: Ottawa, ON, Canada, 1994. [Google Scholar]
- Lappalainen, S.; Nikulin, M.; Berg, S.; Parikka, P.; Hintikka, E.L.; Pasanen, A.L. Fusarium toxins and fungi associated with handling of grain on eight Finnish farms. Atmos. Environ. 1996, 30, 3059–3065. [Google Scholar] [CrossRef]
- Mayer, S.; Curtui, V.; Usleber, E.; Gareis, M. Airborne mycotoxins in dust from grain elevators. Mycotoxin Res. 2007, 23, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Halstensen, A.S.; Nordby, K.C.; Klemsdal, S.S.; Elen, O.; Clasen, P.E.; Eduard, W. Toxigenic Fusarium spp. as determinants of trichothecene mycotoxins in settled grain dust. J. Occup. Environ. Hyg. 2006, 3, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, G.S.; Alexander, J. Fusarium Toxins in Cereals-A Risk Assessment; Nordic Council of Ministers: Copenhagen, Denmark, 1998. [Google Scholar]
- IARC. Toxins Derived from Fusarium graminearum, F. culmorum and F. crookwellense: Zearalenone, Deoxynivalenol, Nivalenol and Fusarenon-X; IARC: Lyon, France, 1993. [Google Scholar]
- Turner, P.C.; Hopton, R.P.; Lecluse, Y.; White, K.L.; Fisher, J.; Lebailly, P. Determinants of urinary deoxynivalenol and de-epoxy deoxynivalenol in male farmers from Normandy, France. J. Agric. Food Chem. 2010, 58, 5206–5212. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.; Irgens, L.M.; Andersen, A.; Bye, A.S.; Sundheim, L. Gestational age, birth weight, and perinatal death among births to Norwegian farmers, 1967–1991. Am. J. Epidemiol. 1997, 146, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.; Andersen, A.; Irgens, L.M. Hormone-dependent cancer and adverse reproductive outcomes in farmers’ families—Effects of climatic conditions favoring fungal growth in grain. Scand. J. Work Environ. Health 2000, 26, 331–337. [Google Scholar] [CrossRef] [PubMed]
- IARC. Improving Public Health though Mycotoxin Control; IARC: Lyon, France, 2012. [Google Scholar]
- Wilkinson, C.F.; Christoph, G.R.; Julien, E.; Kelley, J.M.; Kronenberg, J.; McCarthy, J.; Reiss, R. Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: How to cumulate? Regul. Toxicol. Pharm. 2000, 31, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Male, D.; Wu, W.D.; Mitchell, N.J.; Bursian, S.; Pestka, J.J.; Wu, F. Modeling the emetic potencies of food-borne trichothecenes by benchmark dose methodology. Food Chem. Toxicol. 2016, 94, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.D.; Bates, M.A.; Bursian, S.J.; Link, J.E.; Flannery, B.M.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.B.; Pestka, J.J. Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nvalenol. Toxicol. Sci. 2013, 131, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Laskin, J.D.; Heck, D.E.; Laskin, D.L. The ribotoxic stress response as a potential mechanism for MAP kinase activation in xenobiotic toxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2002, 69, 289–291. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Puel, O.; Pinton, P.; Cossalter, A.M.; Chou, T.C.; Oswald, I.P. Co-exposure to low doses of the food contaminants deoxynivalenol and nivalenol has a synergistic inflammatory effect on intestinal explants. Arch. Toxicol. 2017, 91, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Gerez, J.R.; Cossalter, A.M.; Neves, M.; Laffitte, J.; Naylies, C.; Lippi, Y.; Kolf-Clauw, M.; Bracarense, A.P.L.; Pinton, P.; et al. Intestinal toxicity of the type B trichothecene mycotoxin fusarenon-X: whole transcriptome profiling reveals new signaling pathways. Sci. Rep. UK 2017, 7, 7530. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: the toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. 2017, 57, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Tajima, O.; Schoen, E.D.; Feron, V.J.; Groten, J.P. Statistically designed experiments in a tiered approach to screen mixtures of Fusarium mycotoxins for possible interactions. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2002, 40, 685–695. [Google Scholar] [CrossRef]
- Amuzie, C.J.; Harkema, J.R.; Pestka, J.J. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: comparison of nasal vs. oral exposure. Toxicology 2008, 248, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Instanes, C.; Hetland, G. Deoxynivalenol (DON) is toxic to human colonic, lung and monocytic cell lines, but does not increase the IgE response in a mouse model for allergy. Toxicology 2004, 204, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; Longhin, E.; Caloni, F.; Camatini, M.; Gualtieri, M. Synergistic inflammatory effect of PM10 with mycotoxin deoxynivalenol on human lung epithelial cells. Toxicon 2015, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J. Particle size and pathogenicity in the respiratory tract. Virulence 2013, 4, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A.; Backhaus, T.; Faust, M. State of the Art Report on Mixture Toxicity; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- SCHER; SCENIHR. Opinion on the Toxicity and Assessment of Chemical Mixtures; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Konigs, M.; Schwerdt, G.; Gekle, M.; Humpf, H.U. Effects of the mycotoxin deoxynivalenol on human primary hepatocytes. Mol. Nutr. Food Res. 2008, 52, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.H.; Thrasher, J.D.; Hooper, D. Chronic illness associated with mold and mycotoxins: Is naso-sinus fungal biofilm the culprit? Toxins 2014, 6, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Vacher, G.; Niculita-Hirzel, H.; Roger, T. Immune responses to airborne fungi and non-invasive airway diseases. Semin. Immunopathol. 2015, 37, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sugita-Konishi, Y.; Pestka, J.J. Differential upregulation of TNF-alpha, IL-6, and IL-8 production by deoxynivalenol (vomitoxin) and other 8-ketotrichothecenes in a human macrophage model. J. Toxicol. Environ. Health A 2001, 64, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Aminova, L.; Murayama, Y. Fusarenon-X induced apoptosis in HL-60 cells depends on caspase activation and cytochrome c release. Toxicology 2002, 172, 103–112. [Google Scholar] [CrossRef]
- Bony, S.; Olivier-Loiseau, L.; Carcelen, M.; Devaux, A. Genotoxic potential associated with low levels of the Fusarium mycotoxins nivalenol and fusarenon X in a human intestinal cell line. Toxicol. In Vitro 2007, 21, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Uzarski, R.; Pestka, J.J. Relationship of trichothecene structure to COX-2 induction in the macrophage: Selective action of Type B (8-keto) trichothecenes. J. Toxicol. Environ. Health A 2003, 66, 1967–1983. [Google Scholar] [CrossRef] [PubMed]
- Fornelli, F.; Minervini, F.; Logrieco, A. Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF-9). J. Invertebr. Pathol. 2004, 85, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Puel, O.; Oswald, I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015, 89, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Straumfors, A.; Heldal, K.K.; Eduard, W.; Wouters, I.M.; Ellingsen, D.G.; Skogstad, M. Cross-shift study of exposure-response relationships between bioaerosol exposure and respiratory effects in the Norwegian grain and animal feed production industry. Occup. Environ. Med. 2016, 73, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Galaverna, G.; Dall'Asta, C. In silico analysis sheds light on the structural basis underlying the ribotoxicity of trichothecenes-A tool for supporting the hazard identification process. Toxicol. Lett. 2017, 270, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Shifrin, VI.; Anderson, P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem. 1999, 274, 13985–13992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Islam, Z.; Pestka, J.J. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. Off. J. Soc. Toxicol. 2003, 72, 130–142. [Google Scholar] [CrossRef]
- Sugiyama, K.; Muroi, M.; Kinoshita, M.; Hamada, O.; Minai, Y.; Sugita-Konishi, Y.; Kamata, Y.; Tanamoto, K. NF-kappa B activation via MyD88-dependent Toll-like receptor signaling is inhibited by trichothecene mycotoxin deoxynivalenol. J. Toxicol. Sci. 2016, 41, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kankkunen, P.; Rintahaka, J.; Aalto, A.; Leino, M.; Majuri, M.L.; Alenius, H.; Wolff, H.; Matikainen, S. Trichothecene mycotoxins activate inflammatory response in human macrophages. J. Immunol. 2009, 182, 6418–6425. [Google Scholar] [CrossRef] [PubMed]
- Katika, M.R.; Hendriksen, P.J.M.; Shao, J.; van Loveren, H.; Peijnenburg, A. Transcriptome analysis of the human T lymphocyte cell line Jurkat and human peripheral blood mononuclear cells exposed to deoxynivalenol (DON): New mechanistic insights. Toxicol. Appl. Pharmacol. 2012, 264, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Pestka, J.J. Vomitoxin-induced cyclooxygenase-2 gene expression in macrophages mediated by activation of ERK and p38 but not JNK mitogen-activated protein kinases. Toxicol. Sci. 2002, 69, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Pestka, J.J. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J. Nutr. Biochem. 2003, 14, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Bhoola, K. Ochratoxins-food contaminants: impact on human health. Toxins 2010, 2, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Cremer, B.; Soja, A.; Sauer, J.A.; Damm, M. Pro-inflammatory effects of ochratoxin A on nasal epithelial cells. Eur. Arch. Otorhinolaryngol. 2012, 269, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Vacher, G.; Ciarlo, E.; Savova-Bianchi, D.; Le Roy, D.; Hantier, G.; Niculita-Hirzel, H.; Roger, T. Innate Immune sensing of Fusarium culmorum by mouse dendritic cells. J. Toxicol. Environ. Health Part A 2015, 78, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W.; Workgrp, E.A. Exposure and Health effects of fungi on humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Rand, T.G.; Sun, M.; Gilyan, A.; Downey, J.; Miller, J.D. Dectin-1 and inflammation-associated gene transcription and expression in mouse lungs by a toxic (1,3)-beta-d glucan. Arch. Toxicol. 2010, 84, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
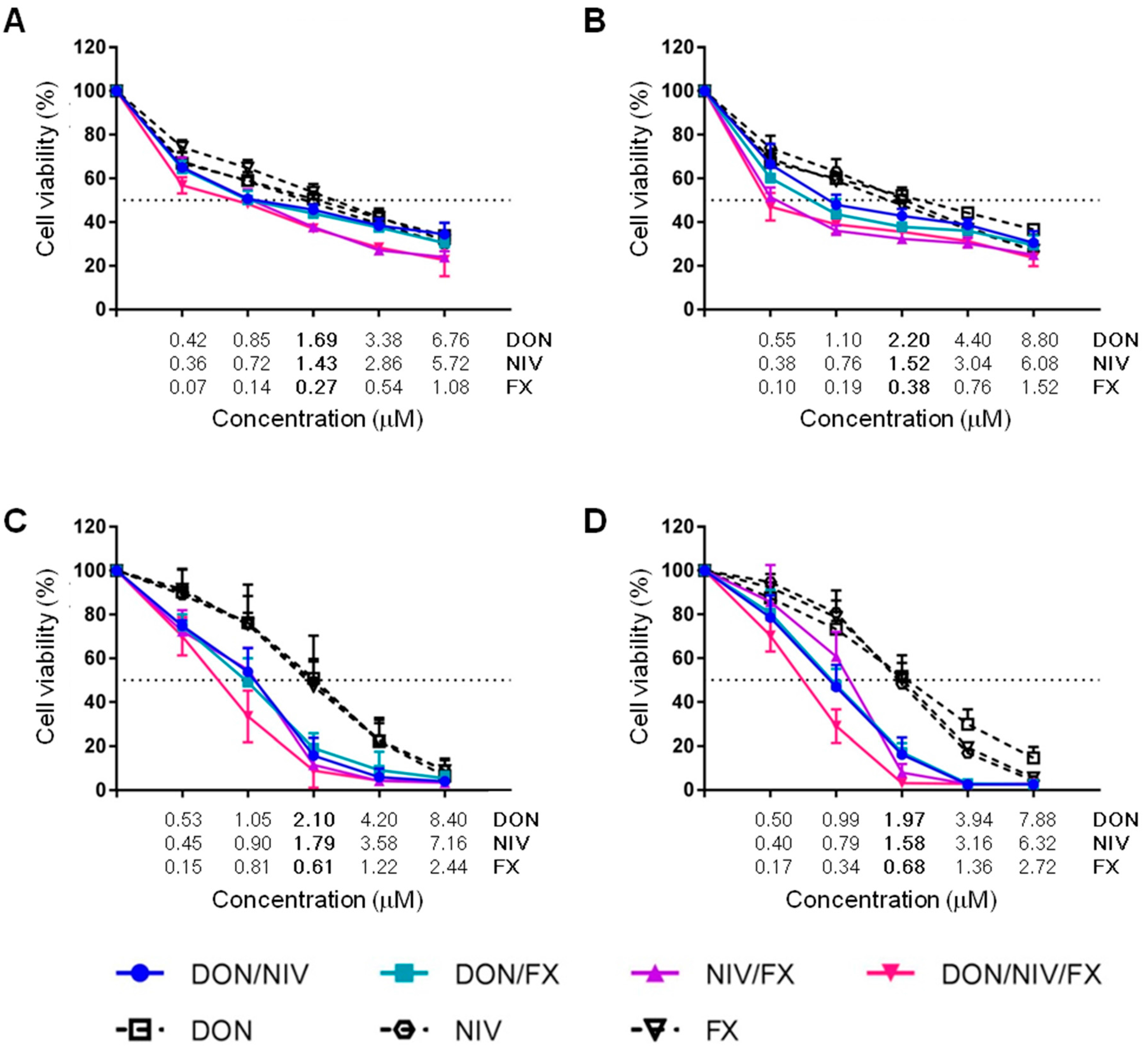

| Mycotoxin | A549 | 16HBE14o- | hAECN | hAECB |
|---|---|---|---|---|
| DON | 1.69 ± 0.49 | 2.2 ± 0.31 | 2.1 ± 0.88 | 1.97 ± 0.40 |
| NIV | 1.43 ± 0.37 | 1.52 ± 0.46 | 1.79 ± 0.45 | 1.58 ± 0.15 |
| FX | 0.27 ± 0.08 | 0.38 ± 0.06 | 0.61 ± 0.12 | 0.68 ± 0.14 |
| Fraction Affected | Mycotoxin | A549 | 16HBE14o- | hAECN | hAECB |
|---|---|---|---|---|---|
| fa = 0.1 | DON | 0.01 | 0.03 | 0.70 | 0.50 |
| NIV | 0.07 | 0.10 | 0.60 | 0.50 | |
| FX | 0.02 | 0.01 | 0.20 | 0.23 | |
| fa = 0.3 | DON | 0.25 | 0.28 | 1.40 | 1.00 |
| NIV | 1.00 | 0.38 | 1.20 | 1.10 | |
| FX | 0.40 | 0.10 | 0.40 | 0.45 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira Lopes, S.; Vacher, G.; Ciarlo, E.; Savova-Bianchi, D.; Roger, T.; Niculita-Hirzel, H. Primary and Immortalized Human Respiratory Cells Display Different Patterns of Cytotoxicity and Cytokine Release upon Exposure to Deoxynivalenol, Nivalenol and Fusarenon-X. Toxins 2017, 9, 337. https://doi.org/10.3390/toxins9110337
Ferreira Lopes S, Vacher G, Ciarlo E, Savova-Bianchi D, Roger T, Niculita-Hirzel H. Primary and Immortalized Human Respiratory Cells Display Different Patterns of Cytotoxicity and Cytokine Release upon Exposure to Deoxynivalenol, Nivalenol and Fusarenon-X. Toxins. 2017; 9(11):337. https://doi.org/10.3390/toxins9110337
Chicago/Turabian StyleFerreira Lopes, Silvia, Gaëlle Vacher, Eleonora Ciarlo, Dessislava Savova-Bianchi, Thierry Roger, and Hélène Niculita-Hirzel. 2017. "Primary and Immortalized Human Respiratory Cells Display Different Patterns of Cytotoxicity and Cytokine Release upon Exposure to Deoxynivalenol, Nivalenol and Fusarenon-X" Toxins 9, no. 11: 337. https://doi.org/10.3390/toxins9110337