Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress
Abstract
:1. Introduction
2. Integration of Nanomaterials in Aptasensors
2.1. Types of Nanomaterials
2.1.1. Gold Nanoparticles
2.1.2. Carbon Based Nanoparticles
2.1.3. Metal Oxide Nanoparticles
2.1.4. Quantum Dots (QDs)
2.2. Functions of Nanomaterials in Aptasensors
2.2.1. Immobilization Support
2.2.2. Mediator
2.2.3. Signal Amplification
2.2.4. Alternative to Enzyme Labels
2.2.5. Optical Signal Generating Probe
2.2.6. Fluorescence Quencher
3. Nano-Aptasensing for Mycotoxins Analysis
3.1. Ochratoxins
3.2. Aflatoxins
3.3. Other Mycotoxins
4. Conclusions and Prospects
Acknowledgments
Conflicts of Interest
References
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of mycotoxins in food: A review of bioanalytical to analytical methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Mejri Omrani, N.; Hayat, A.; Korri-Youssoufi, H.; Marty, J.L. Electrochemical biosensors for food security: Mycotoxins detection. In Biosensors for Security and Bioterrorism Applications; Nikolelis, D.P., Nikoleli, G.-P., Eds.; Springer: Cham, Switzerland, 2016; pp. 469–490. [Google Scholar]
- Arias, R.S.; Dang, P.M.; Sobolev, V.S. Rnai-mediated control of aflatoxins in peanut: Method to analyze mycotoxin production and transgene expression in the peanut/Aspergillus pathosystem. J. Vis. Exp. 2015, 106, 53398. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Lizárraga-Paulín, E.G.; Moreno-Martínez, E.; Miranda-Castro, S.P. Aflatoxins and their impact on human and animal health: An emerging problem. In Aflatoxins-Biochemistry and Molecular Biology; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; MacDonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. 2008, 25, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Welzig, E.; Berthiller, F.; Molinelli, A.; Mizaikoff, B. Advances in the analysis of mycotoxins and its quality assurance. Food Addit. Contam. 2005, 22, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-B. The state-of-the-art in the analysis of type-a trichothecene mycotoxins in cereals. Chin. J. Endemiol. 2003, 6, 035. [Google Scholar]
- Gilbert, J.; Anklam, E. Validation of analytical methods for determining mycotoxins in foodstuffs. TrAC Trends Anal. Chem. 2002, 21, 468–486. [Google Scholar] [CrossRef]
- Magan, N.; Olsen, M. Mycotoxins in Food: Detection and Control; Woodhead Publishing: Sawston, UK, 2004. [Google Scholar]
- Lin, L.; Zhang, J.; Wang, P.; Wang, Y.; Chen, J. Thin-layer chromatography of mycotoxins and comparison with other chromatographic methods. J. Chromatogr. A 1998, 815, 3–20. [Google Scholar] [CrossRef]
- Maragou, N.C.; Rosenberg, E.; Thomaidis, N.S.; Koupparis, M.A. Direct determination of the estrogenic compounds 8-prenylnaringenin, zearalenone, α- and β-zearalenol in beer by liquid chromatography–mass spectrometry. J. Chromatogr. A 2008, 1202, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Songsermsakul, P.; Sontag, G.; Cichna-Markl, M.; Zentek, J.; Razzazi-Fazeli, E. Determination of zearalenone and its metabolites in urine, plasma and faeces of horses by hplc–apci–ms. J. Chromatogr. B 2006, 843, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Foglia, P.; Guarino, C.; Motto, M.; Nazzari, M.; Samperi, R.; Laganà, A.; Berardo, N. Mycotoxins produced by fusarium genus in maize: Determination by screening and confirmatory methods based on liquid chromatography tandem mass spectrometry. Food Chem. 2007, 105, 700–710. [Google Scholar] [CrossRef]
- Paepens, C.; De Saeger, S.; Sibanda, L.; Barna-Vetró, I.; Anselme, M.; Larondelle, Y.; Van Peteghem, C. Evaluation of fumonisin contamination in cornflakes on the belgian market by “flow-through” assay screening and lc−ms/ms analyses. J. Agric. Food Chem. 2005, 53, 7337–7343. [Google Scholar] [CrossRef] [PubMed]
- Cervino, C.; Asam, S.; Knopp, D.; Rychlik, M.; Niessner, R. Use of isotope-labeled aflatoxins for lc-ms/ms stable isotope dilution analysis of foods. J. Agric. Food Chem. 2008, 56, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Reinsch, M.; Töpfer, A.; Lehmann, A.; Nehls, I. Determination of ochratoxin a in wine by liquid chromatography tandem mass spectrometry after combined anion-exchange/reversed-phase clean-up. Anal. Bioanal. Chem. 2005, 381, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Adányi, N.; Levkovets, I.A.; Rodriguez-Gil, S.; Ronald, A.; Váradi, M.; Szendrő, I. Development of immunosensor based on owls technique for determining Aflatoxin B1 and ochratoxin A. Biosens. Bioelectron. 2007, 22, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, H.; Åberg, L. Near infrared spectroscopy for determination of mycotoxins in cereals. Food Control 2003, 14, 229–232. [Google Scholar] [CrossRef]
- Barug, D.; Barug, D.; Bhatnagar, D.; van Egmond, H.P.; van der Kamp, J.W.; van Osenbruggen, W.A.; Visconti, A. The Mycotoxin Factbook; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006. [Google Scholar]
- Van der Gaag, B.; Spath, S.; Dietrich, H.; Stigter, E.; Boonzaaijer, G.; van Osenbruggen, T.; Koopal, K. Biosensors and multiple mycotoxin analysis. Food Control 2003, 14, 251–254. [Google Scholar] [CrossRef]
- Hartl, M.; Humpf, H.-U. Assigning the absolute configuration of fumonisins by the circular dichroism exciton chirality method. Tetrahedron 1998, 9, 1549–1556. [Google Scholar] [CrossRef]
- Anli, E.; Alkis, İ.M. Ochratoxin a and brewing technology: A review. J. Inst. Brew. 2010, 116, 23–32. [Google Scholar] [CrossRef]
- Bazin, I.; Tria, S.A.; Hayat, A.; Marty, J.-L. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Byfield, M.P.; Abuknesha, R.A. Biochemical aspects of biosensors. Biosens. Bioelectron. 1994, 9, 373–399. [Google Scholar] [CrossRef]
- Catanante, G.; Rhouati, A.; Hayat, A.; Marty, J.L. An overview of recent electrochemical immunosensing strategies for mycotoxins detection. Electroanalysis 2016, 28, 1750–1763. [Google Scholar] [CrossRef]
- Ravalli, A.; Voccia, D.; Palchetti, I.; Marrazza, G. Electrochemical, electrochemiluminescence, and photoelectrochemical aptamer-based nanostructured sensors for biomarker analysis. Biosensors 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; Rusconi, C.P.; Sullenger, B.A. Aptamers: An emerging class of therapeutics. Annu. Rev. Med. 2005, 56, 555–583. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [PubMed]
- Poturnayova, A.; Castillo, G.; Subjakova, V.; Tatarko, M.; Snejdarkova, M.; Hianik, T. Optimization of cytochrome c detection by acoustic and electrochemical methods based on aptamer sensors. Sens. Actuators B 2017, 238, 817–827. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Hashim, S.N.; Ise, S.; Furuhata, T.; Kawai, K.; Wakabayashi, R.; Goto, M.; Kamiya, N.; Sando, S. Bodipy-labeled fluorescent aptamer sensors for turn-on sensing of interferon-gamma and adenine compounds on cells. Anal. Sci. 2016, 32, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Ribes, A.; Aznar, E.; Bernardos, A.; Marcos, M.D.; Amorós, P.; Martínez-Máñez, R.; Sancenon, F. Fluorogenic sensing of carcinogenic bisphenol a using aptamer-capped mesoporous silica nanoparticles. Chemistry 2017, 23, 8581–8584. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, S.; Shi, H.; Zhao, G. A highly sensitive and selective aptamer-based colorimetric sensor for the rapid detection of pcb 77. J. Hazard. Mater. 2017, 341, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chen, C.; Che, X.; Wang, W.; Que, L. Detection of plant hormone abscisic acid (aba) using an optical aptamer-based sensor with a microfluidics capillary interface. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 370–373. [Google Scholar]
- Schoukroun-Barnes, L.R.; Macazo, F.C.; Gutierrez, B.; Lottermoser, J.; Liu, J.; White, R.J. Reagentless, structure-switching, electrochemical aptamer-based sensors. Ann. Rev. Anal. Chem. 2016, 9, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y. Functional DNA directed assembly of nanomaterials for biosensing. J. Mater. Chem. 2009, 19, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Yang, C.; Rhouati, A.; Marty, J.L. Recent advances and achievements in nanomaterial-based, and structure switchable aptasensing platforms for ochratoxin a detection. Sensors 2013, 13, 15187–15208. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, S.; Yao, N.; Shi, Y. 1.6 v nanogenerator for mechanical energy harvesting using pzt nanofibers. Nano Lett. 2010, 10, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Maojo, V.; Martin-Sanchez, F.; Kulikowski, C.; Rodriguez-Paton, A.; Fritts, M. Nanoinformatics and DNA-based computing: Catalyzing nanomedicine. Pediatr. Res. 2010, 67, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Shi Kam, N.W.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Aćimović, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. Lspr chip for parallel, rapid, and sensitive detection of cancer markers in serum. Nano Lett. 2014, 14, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Andler, S.M.; Goddard, J.M.; Nugen, S.R.; Rotello, V.M. Integrating recognition elements with nanomaterials for bacteria sensing. Chem. Soc. Rev. 2017, 46, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Plata, M.R.; Contento, A.M.; Ríos, A. State-of-the-art of (bio) chemical sensor developments in analytical spanish groups. Sensors 2010, 10, 2511–2576. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ragavan, K.V.; Thakur, M.S.; Raghavarao, K.S.M.S. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 2015, 74, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Emrani, A.S.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Colorimetric and fluorescence quenching aptasensors for detection of streptomycin in blood serum and milk based on double-stranded DNA and gold nanoparticles. Food Chem. 2016, 190, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rahi, A.; Sattarahmady, N.; Heli, H. Label-free electrochemical aptasensing of the human prostate-specific antigen using gold nanospears. Talanta 2016, 156–157, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ma, X.; Duan, N.; Wu, S.; Xia, Y.; Wang, Z.; Xu, B. Ultrasensitive sers aptasensor for the detection of oxytetracycline based on a gold-enhanced nano-assembly. Talanta 2017, 165, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Pak, Y.; Kim, S.-M.; Jeong, H.; Kang, C.G.; Park, J.S.; Song, H.; Lee, R.; Myoung, N.; Lee, B.H.; Seo, S.; et al. Palladium-decorated hydrogen-gas sensors using periodically aligned graphene nanoribbons. ACS Appl. Mater. Interfaces 2014, 6, 13293–13298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, L. Gold nanoparticle probes. Coord. Chem. Rev. 2009, 253, 1607–1618. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, T.; Gysbers, R.; Tan, Y.; Liu, F.; Lin, S.; Jiang, Y. A simple and sensitive aptasensor for colorimetric detection of adenosine triphosphate based on unmodified gold nanoparticles. Talanta 2017, 168, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Ookubo, K.; Komuro, N.; Shimizu, T.; Uehara, N. Blue-to-red chromatic sensor composed of gold nanoparticles conjugated with thermoresponsive copolymer for thiol sensing. Langmuir 2007, 23, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, C.; Shangguan, L.; Qi, H.; Xue, D.; Gao, Q.; Zhang, C. Click chemistry-assisted self-assembly of DNA aptamer on gold nanoparticles-modified screen-printed carbon electrodes for label-free electrochemical aptasensor. Sens. Actuators B 2014, 192, 558–564. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K. An electrochemical aptasensor based on gold nanoparticles, thionine and hairpin structure of complementary strand of aptamer for ultrasensitive detection of lead. Sens. Actuators B 2016, 234, 462–469. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Wang, Y.; Liu, Z.; Lu, Z. An ultrasensitive aptasensor for detection of ochratoxin a based on shielding effect-induced inhibition of fluorescence resonance energy transfer. Sens. Actuators B 2016, 222, 797–803. [Google Scholar] [CrossRef]
- Sabet, F.S.; Hosseini, M.; Khabbaz, H.; Dadmehr, M.; Ganjali, M.R. Fret-based aptamer biosensor for selective and sensitive detection of Aflatoxin b1 in peanut and rice. Food Chem. 2017, 220, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum hall effect and berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, R.; You, M.; Zhang, X.; Wu, Y.; Tan, W. Single-walled carbon nanotube as an effective quencher. Anal. Bioanal. Chem. 2010, 396, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-E.; Si, S. Aptamer biosensing platform based on carbon nanotube long-range energy transfer for sensitive, selective and multicolor fluorescent heavy metal ion analysis. Anal. Methods 2013, 5, 2947–2953. [Google Scholar] [CrossRef]
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 2004, 34, 151–180. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Liu, B.; Kelly, E.Y.; Servos, M.R.; Liu, J. Adsorption of DNA oligonucleotides by titanium dioxide nanoparticles. Langmuir 2014, 30, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, G.; Hayat, A.; Andreescu, S. A generic amplification strategy for electrochemical aptasensors using a non-enzymatic nanoceria tag. Nanoscale 2015, 7, 13230–13238. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hou, W.; Jiao, Y.; Guo, Y.; Sun, X.; Zhao, J.; Wang, X. Ultra-sensitive aptasensor based on il and Fe3O4 nanoparticles for tetracycline detection. Int. J. Electrochem. Sci. 2017, 12, 7426–7434. [Google Scholar] [CrossRef]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile In Vitro and In Vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum dots in bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed]
- Martín-Palma, R.J.; Manso, M.; Torres-Costa, V. Optical biosensors based on semiconductor nanostructures. Sensors 2009, 9, 5149–5172. [Google Scholar] [CrossRef] [PubMed]
- Oehme, I.; Wolfbeis, O.S. Optical sensors for determination of heavy metal ions. Microchim. Acta 1997, 126, 177–192. [Google Scholar] [CrossRef]
- Hayat, A.; Catanante, G.; Marty, J.L. Current trends in nanomaterial-based amperometric biosensors. Sensors 2014, 14, 23439–23461. [Google Scholar] [CrossRef] [PubMed]
- Azizah, N.; Hashim, U.; Gopinath, S.C.; Nadzirah, S. Gold nanoparticle mediated method for spatially resolved deposition of DNA on nano-gapped interdigitated electrodes, and its application to the detection of the human papillomavirus. Microchim. Acta 2016, 183, 3119–3126. [Google Scholar] [CrossRef]
- Olowu, R.A.; Arotiba, O.; Mailu, S.N.; Waryo, T.T.; Baker, P.; Iwuoha, E. Electrochemical aptasensor for endocrine disrupting 17β-estradiol based on a poly(3,4-ethylenedioxylthiopene)-gold nanocomposite platform. Sensors 2010, 10, 9872–9890. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, B.; Gu, S.; Yang, Y.; Wang, Z.; Xiang, Y. Amperometric low potential aptasensor for the fucosylated golgi protein 73, a marker for hepatocellular carcinoma. Microchim. Acta 2017, 184, 3131–3136. [Google Scholar] [CrossRef]
- Jena, B.K.; Raj, C.R. Enzyme-free amperometric sensing of glucose by using gold nanoparticles. Chemistry 2006, 12, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Solanki, P.R.; Ansari, A.A.; Ahmad, S.; Malhotra, B.D. A nanostructured cerium oxide film-based immunosensor for mycotoxin detection. Nanotechnology 2009, 20, 055105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gan, X.; Liu, T.; Yang, Q.; Li, G. Effect of nano cadmium sulfide on the electron transfer reactivity and peroxidase activity of hemoglobin. J. Biochem. Biophys. Methods 2005, 64, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, F.; Shen, G.; Huang, J.; Wang, X. Aptasensor based on the synergistic contributions of chitosan–gold nanoparticles, graphene–gold nanoparticles and multi-walled carbon nanotubes-cobalt phthalocyanine nanocomposites for kanamycin detection. Analyst 2014, 139, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Abbasi, A.R.; Roushani, M.; Derikvand, Z.; Azadbakht, A. An electrochemical dopamine aptasensor incorporating silver nanoparticle, functionalized carbon nanotubes and graphene oxide for signal amplification. Talanta 2016, 159, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Shahdost-fard, F.; Salimi, A.; Khezrian, S. Highly selective and sensitive adenosine aptasensor based on platinum nanoparticles as catalytical label for amplified detection of biorecognition events through H2O2 reduction. Biosens. Bioelectron. 2014, 53, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Yi, H.; Xue, S.; Chai, Y.; Yuan, R.; Xu, W. A sensitive electrochemical aptasensor based on palladium nanoparticles decorated graphene–molybdenum disulfide flower-like nanocomposites and enzymatic signal amplification. Anal. Chim. Acta 2015, 853, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tan, L.; Hu, L.; Zhang, Y.; Wang, S.; Lv, F. Real colorimetric thrombin aptasensor by masking surfaces of catalytically active gold nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Qian, C.; Hao, N.; Xu, L.; Yao, C. Electrochemical aptasensor for mucin 1 based on dual signal amplification of poly(O-phenylenediamine) carrier and functionalized carbon nanotubes tracing tag. Biosens. Bioelectron. 2015, 64, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, J.; Gui, R.; Jin, H.; Xia, Y. Carbon nanomaterials-based electrochemical aptasensors. Biosens. Bioelectron. 2016, 79, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Hayat Nawaz, M.A.; Badea, M.; Marty, J.L.; Hayat, A. Nano-engineered biomimetic optical sensors for glucose monitoring in diabetes. Sensors 2016, 16, 1931. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, X.; Wang, H.; Zheng, H.; Huang, Y. Analytical and environmental applications of nanoparticles as enzyme mimetics. TrAC Trends Anal.Chem. 2012, 39, 114–129. [Google Scholar] [CrossRef]
- Li, H.; Rothberg, L.J. Label-free colorimetric detection of specific sequences in genomic DNA amplified by the polymerase chain reaction. J. Am. Chem. Soc. 2004, 126, 10958–10961. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Cao, X.; Wang, L.; Li, D.; Song, S.; Ye, B.; Fan, C. Adenosine detection by using gold nanoparticles and designed aptamer sequences. Analyst 2009, 134, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Pan, D.; Song, S.; Boey, F.Y.; Zhang, H.; Fan, C. Visual cocaine detection with gold nanoparticles and rationally engineered aptamer structures. Small 2008, 4, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Jiang, W. Label free DNA detection based on gold nanoparticles quenching fluorescence of rhodamine B. Talanta 2011, 85, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Moulik, S.P.; Bhattacharya, S.C. Understanding the effect of size and shape of gold nanomaterials on nanometal surface energy transfer. J. Colloid Interface Sci. 2017, 491, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Xia, B.; Wang, L.; Ye, J.; Du, G.; Feng, H.; Zhou, X.; Zhang, T.; Wang, W. Fluorescent aptasensor for 17β-estradiol determination based on gold nanoparticles quenching the fluorescence of rhodamine b. Anal. Biochem. 2017, 523, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tianyu, H.; Xu, Y.; Weidan, N.; Xingguang, S. Aptamer-based aggregation assay for mercury(ii) using gold nanoparticles and fluorescent cdte quantum dots. Microchim. Acta 2016, 183, 2131–2137. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Wang, L.; Huang, Y.; Guo, J.; Cao, X.; Shen, F.; Luo, Y.; Sun, C. Aptamer-based fluorescent detection of bisphenol a using nonconjugated gold nanoparticles and cdte quantum dots. Sens. Actuators B 2016, 222, 815–822. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, L.; Huang, Y.; Liu, D.; Sun, R.; Luo, J.; Sun, C. A facile aptamer-based sensing strategy for dopamine through the fluorescence resonance energy transfer between rhodamine b and gold nanoparticles. Dyes Pigments 2015, 123, 55–63. [Google Scholar] [CrossRef]
- Emrani, A.S.; Danesh, N.M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. A novel fluorescent aptasensor based on hairpin structure of complementary strand of aptamer and nanoparticles as a signal amplification approach for ultrasensitive detection of cocaine. Biosens. Bioelectron. 2016, 79, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, H.; Li, H.; Wang, S.; Li, X.; Xu, B.; Tian, W. A label-free aptasensor for turn-on fluorescent detection of atp based on aie-active probe and water-soluble carbon nanotubes. Sens. Actuators B 2016, 230, 556–558. [Google Scholar] [CrossRef]
- Li, C.; Meng, Y.; Wang, S.; Qian, M.; Wang, J.; Lu, W.; Huang, R. Mesoporous carbon nanospheres featured fluorescent aptasensor for multiple diagnosis of cancer In Vitro and In Vivo. ACS Nano 2015, 9, 12096–12103. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hayat, A.; Mishra, R.K.; Catanante, G.; Bhand, S.; Marty, J.L. Titanium dioxide nanoparticles (TiO2) quenching based aptasensing platform: Application to ochratoxin a detection. Toxins 2015, 7, 3771–3784. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.F.; Ning, Y.; Song, Y.; Deng, L. Fluorescent aptasensor for the determination of salmonella typhimurium based on a graphene oxide platform. Microchim. Acta 2014, 181, 647–653. [Google Scholar] [CrossRef]
- Bueno, D.; Valdez, L.F.; Gutiérrez Salgado, J.M.; Marty, J.L.; Muñoz, R. Colorimetric analysis of ochratoxin a in beverage samples. Sensors 2016, 16, 1888. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; García-Campaña, A.M.; Gámiz-Gracia, L. Review of sample treatments and the state-of-the-art of analytical techniques for mycotoxins in food. Anal. Food Toxins Toxic. 2017, 2, 51. [Google Scholar]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Marty, J.-L.; Yang, X. Aptamer-based colorimetric biosensing of ochratoxin a using unmodified gold nanoparticles indicator. Biosens. Bioelectron. 2011, 26, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, D.; Lin, Z.; Qiu, B.; Liu, M.; Guo, L.; Chen, G. Disassembly of gold nanoparticle dimers for colorimetric detection of ochratoxin a. Anal. Methods 2015, 7, 842–845. [Google Scholar] [CrossRef]
- Pons, T.; Medintz, I.L.; Sapsford, K.E.; Higashiya, S.; Grimes, A.F.; English, D.S.; Mattoussi, H. On the quenching of semiconductor quantum dot photoluminescence by proximal gold nanoparticles. Nano Lett. 2007, 7, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Beheshti, H.R.; Ramezani, M.; Abnous, K. A novel fluorescent aptasensor based on gold and silica nanoparticles for the ultrasensitive detection of ochratoxin a. Nanoscale 2016, 8, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qian, J.; Jiang, L.; Yan, Y.; Wang, K.; Liu, Q.; Wang, K. Ultrasensitive electrochemical aptasensor for ochratoxin a based on two-level cascaded signal amplification strategy. Bioelectrochemistry 2014, 96, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qian, J.; Yang, X.; Yan, Y.; Liu, Q.; Wang, K.; Wang, K. Amplified impedimetric aptasensor based on gold nanoparticles covalently bound graphene sheet for the picomolar detection of ochratoxin A. Anal. Chim. Acta 2014, 806, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Shuai, H.-L.; Chen, Y.-X. Layered molybdenum selenide stacking flower-like nanostructure coupled with guanine-rich DNA sequence for ultrasensitive ochratoxin a aptasensor application. Sens. Actuators B 2016, 225, 391–397. [Google Scholar] [CrossRef]
- Wang, L.B.; Ma, W.W.; Chen, W.; Liu, L.Q.; Ma, W.; Zhu, Y.Y.; Xu, L.G.; Kuang, H.; Xu, C.L. An aptamer-based chromatographic strip assay for sensitive toxin semi-quantitative detection. Biosens. Bioelectron. 2011, 26, 3059–3062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.L.; Kong, W.J.; Dou, X.W.; Zhao, M.; Ouyang, Z.; Yang, M.H. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin a in astragalus membranaceus. J. Chromatogr. B 2016, 1022, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Porfireva, A.; Sitdikov, R.; Evtugyn, V.; Stoikov, I.; Antipin, I.; Hianik, T. Electrochemical aptasensor for the determination of ochratoxin a at the au electrode modified with ag nanoparticles decorated with macrocyclic ligand. Electroanalysis 2013, 25, 1847–1854. [Google Scholar] [CrossRef]
- Karimi, A.; Hayat, A.; Andreescu, S. Biomolecular detection at ssdna-conjugated nanoparticles by nano-impact electrochemistry. Biosens. Bioelectron. 2017, 87, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zhou, M.; Huang, K.; Cao, J.; Wang, H.; Chen, Y. Chemiluminescence detection of protein in capillary electrophoresis using aptamer-functionalized gold nanoparticles as biosensing platform. J. Chromatogr. A 2014, 1340, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Tavares, A.J.; Krull, U.J. Beyond labels: A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal. Chim. Acta 2010, 673, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, W.; Ma, W.; Liu, L.; Ma, W.; Zhao, Y.; Zhu, Y.; Xu, L.; Kuang, H.; Xu, C. Fluorescent strip sensor for rapid determination of toxins. Chem. Commun. 2011, 47, 1574–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, J.; Wang, K.; Hua, M.; Liu, Q.; Hao, N.; You, T.; Huang, X. Nitrogen-doped graphene quantum dots@SiO2 nanoparticles as electrochemiluminescence and fluorescence signal indicators for magnetically controlled aptasensor with dual detection channels. ACS Appl. Mater. Interfaces 2015, 7, 26865–26873. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wei, W.; Wang, J.; Ji, S.; Chen, G.; Lu, J. Fluorescence resonance energy transfer aptasensor between nanoceria and graphene quantum dots for the determination of ochratoxin A. Anal. Chim. Acta 2017. [Google Scholar] [CrossRef]
- Chu, X.; Dou, X.; Liang, R.; Li, M.; Kong, W.; Yang, X.; Luo, J.; Yang, M.; Zhao, M. A self-assembly aptasensor based on thick-shell quantum dots for sensing of ochratoxin a. Nanoscale 2016, 8, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Jiang, L.; Qian, J.; Wang, K. Ultrasensitive electrochemical ochratoxin a aptasensor based on cdte quantum dots functionalized graphene/au nanocomposites and magnetic separation. J. Electroanal. Chem. 2016, 781, 332–338. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, X.; Hu, W. A fluorescence aptasensor based on semiconductor quantum dots and MoS2 nanosheets for ochratoxin a detection. Sens. Actuators B 2017, 246, 61–67. [Google Scholar] [CrossRef]
- Bülbül, G.; Hayat, A.; Andreescu, S. Ssdna-functionalized nanoceria: A redox-active aptaswitch for biomolecular recognition. Adv. Healthc. Mater. 2016, 5, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ren, J.; Wang, J.; Wang, E. Single-walled carbon nanotubes based quenching of free fam-aptamer for selective determination of ochratoxin a. Talanta 2011, 85, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Taghdisi, S.M. Amperometric aptasensor for ochratoxin a based on the use of a gold electrode modified with aptamer, complementary DNA, swcnts and the redox marker methylene blue. Microchim. Acta 2017, 184, 1151–1159. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Wang, X.; Duan, Y. Amplified fluorescent aptasensor through catalytic recycling for highly sensitive detection of ochratoxin a. Biosens. Bioelectron. 2015, 65, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Mishra, R.K.; Catanante, G.; Marty, J.L. Development of an aptasensor based on a fluorescent particles-modified aptamer for ochratoxin a detection. Anal. Bioanal. Chem. 2015, 407, 7815–7822. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Chen, Z.; Xie, G.; Chen, J.; Lu, A.; Li, C.; Fu, H.; Ma, Z.; Wang, J. Rapid visual detection of Aflatoxin b1 by label-free aptasensor using unmodified gold nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Chen, J.; Xie, G.; Li, C.; Ping, H.; Ma, Z.; Lu, A. Visual and microplate detection of Aflatoxin b2 based on nacl-induced aggregation of aptamer-modified gold nanoparticles. Microchim. Acta 2015, 182, 995–1001. [Google Scholar] [CrossRef]
- Hosseini, M.; Khabbaz, H.; Dadmehr, M.; Ganjali, M.R.; Mohamadnejad, J. Aptamer-based colorimetric and chemiluminescence detection of Aflatoxin B1 in foods samples. Acta Chim. Slov. 2015, 62, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, Y.; Wu, Y.; Weng, B.; Liu, Y.; Lu, Z.; Li, C.M.; Yu, C. Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of afb 1. Biosens. Bioelectron. 2016, 78, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Teng, J.; Cheng, L.; Ye, Y.; Pan, D.; Wu, J.; Xue, F.; Liu, G.; Chen, W. Hetero-enzyme-based two-round signal amplification strategy for trace detection of Aflatoxin b1 using an electrochemical aptasensor. Biosens. Bioelectron. 2016, 80, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Luo, Y.; Yang, X.; Li, M.; Song, Q. Double detection of mycotoxins based on sers labels embedded ag@ au core–shell nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 21780–21786. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, G.; Mehedi, H.M.; Ouyang, Q.; Chen, Q. A universal sers aptasensor based on DTNB labeled GNTs/Ag core-shell nanotriangle and CS-Fe3O4 magnetic-bead trace detection of Aflatoxin B1. Anal. Chim. Acta 2017, 986, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, Y.-K.; Chen, M.; Wu, D.-Z.; Cai, S.-X.; Liu, M.-M.; He, W.-H.; Chen, J.-H. A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with zn (ii)-ion signal-enhancement for simultaneous detection of ota and afb 1. Sens. Actuators B 2016, 235, 79–85. [Google Scholar] [CrossRef]
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Zhao, S.; Lu, Y. Size-dependent modulation of graphene oxide–aptamer interactions for an amplified fluorescence-based detection of Aflatoxin B 1 with a tunable dynamic range. Analyst 2016, 141, 4029–4034. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.-B.; Kim, M.J.; Mun, H.; Kim, M.-G. An aptamer-based dipstick assay for the rapid and simple detection of Aflatoxin b1. Biosens. Bioelectron. 2014, 62, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, E.; Chughtai, M.F.; Jin, Z.; Irudayaraj, J. Highly sensitive fluorescence sensing of zearalenone using a novel aptasensor based on upconverting nanoparticles. Food Chem. 2017, 230, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Hayat, A.; Satyanarayana, M.; Kumar, V.S.; Catanante, G.; Gobi, K.V.; Marty, J.L. Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim. Acta 2017, 184, 4401–4408. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Li, X.; Tan, G.; Ma, X.; Xia, Y.; Wang, Z.; Wang, H. Homogenous detection of fumonisin B 1 with a molecular beacon based on fluorescence resonance energy transfer between NAYF 4: Yb, Ho upconversion nanoparticles and gold nanoparticles. Talanta 2013, 116, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Y.; Duan, N.; Wu, S.; Xia, Y.; Ma, X.; Zhu, C.; Jiang, Y.; Ding, Z.; Wang, Z. Selection and characterization of single stranded DNA aptamers recognizing fumonisin b1. Microchim. Acta 2014, 181, 1317–1324. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Duan, N.; Wu, S.; Ma, X.; Xia, Y.; Zhu, C.; Jiang, Y.; Wang, Z. Selection and identification of ssdna aptamers recognizing zearalenone. Anal. Bioanal. Chem. 2013, 405, 6573–6581. [Google Scholar] [CrossRef] [PubMed]
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
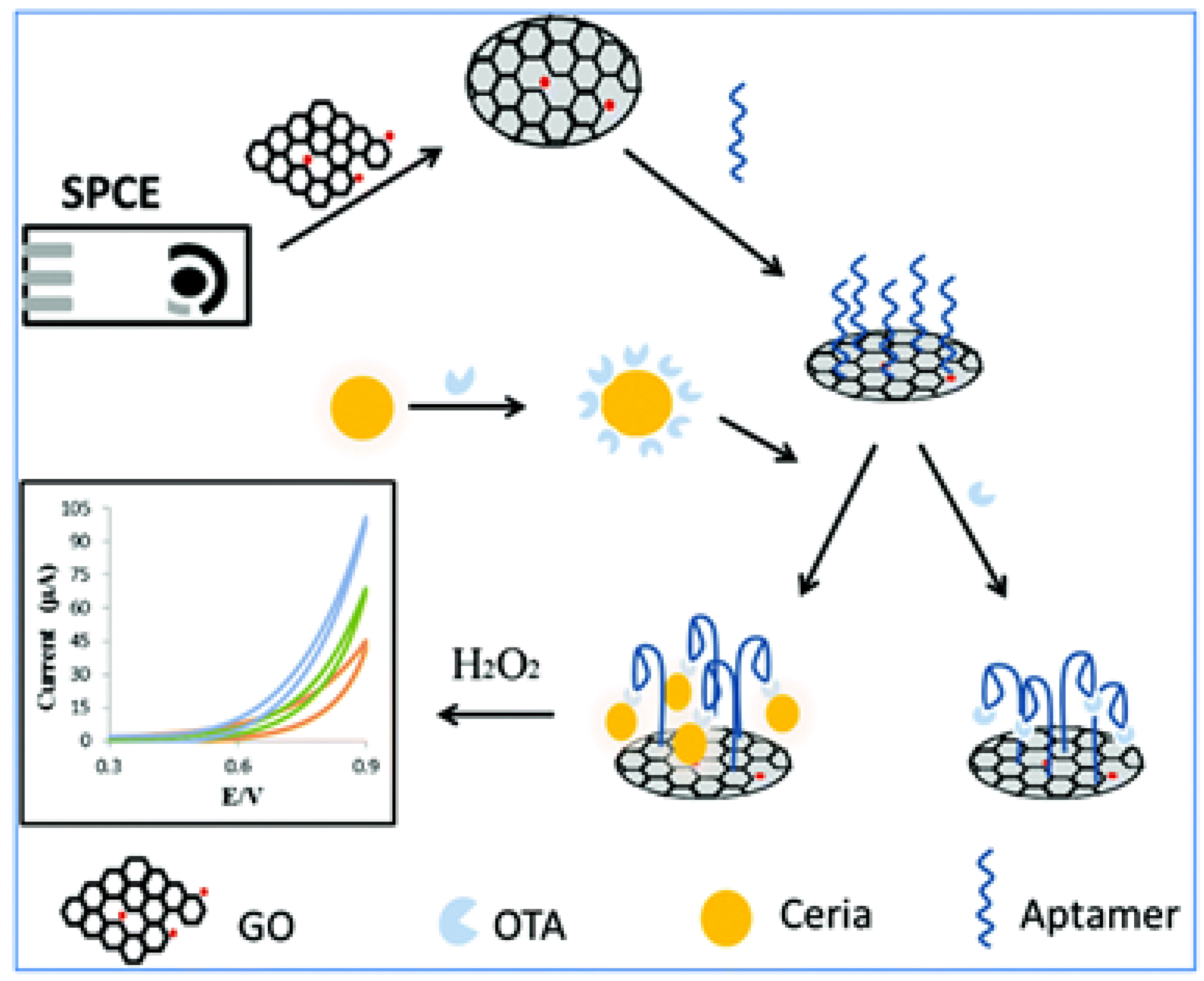

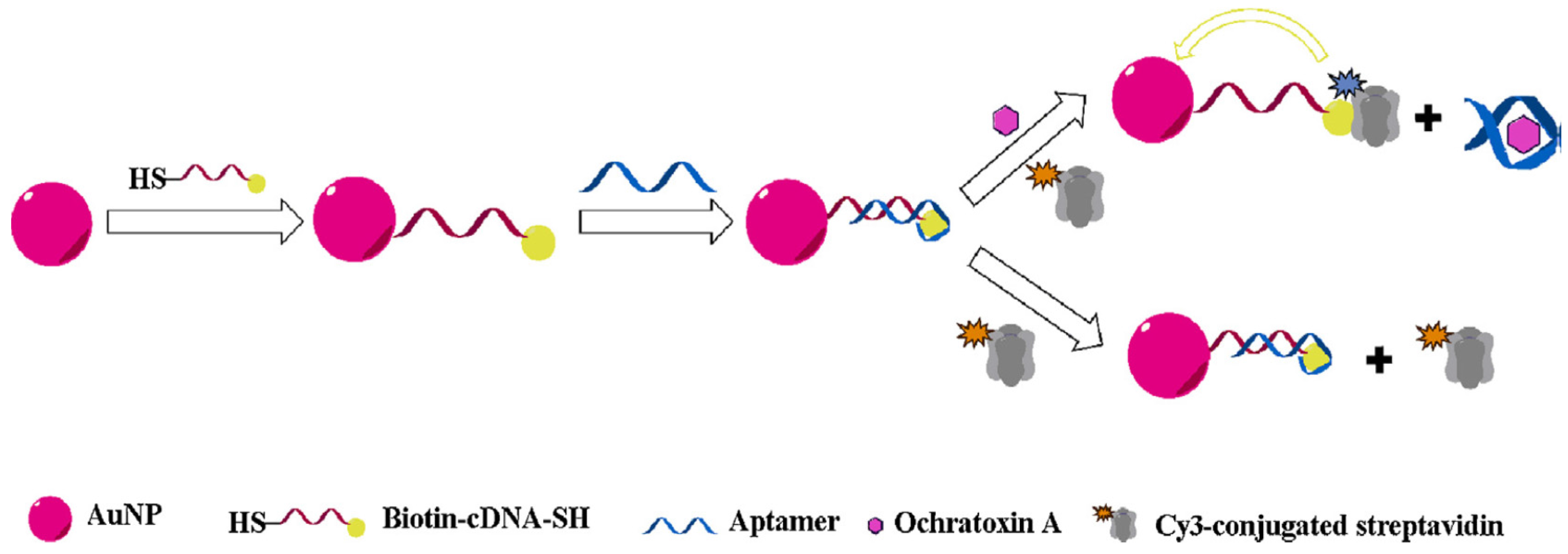
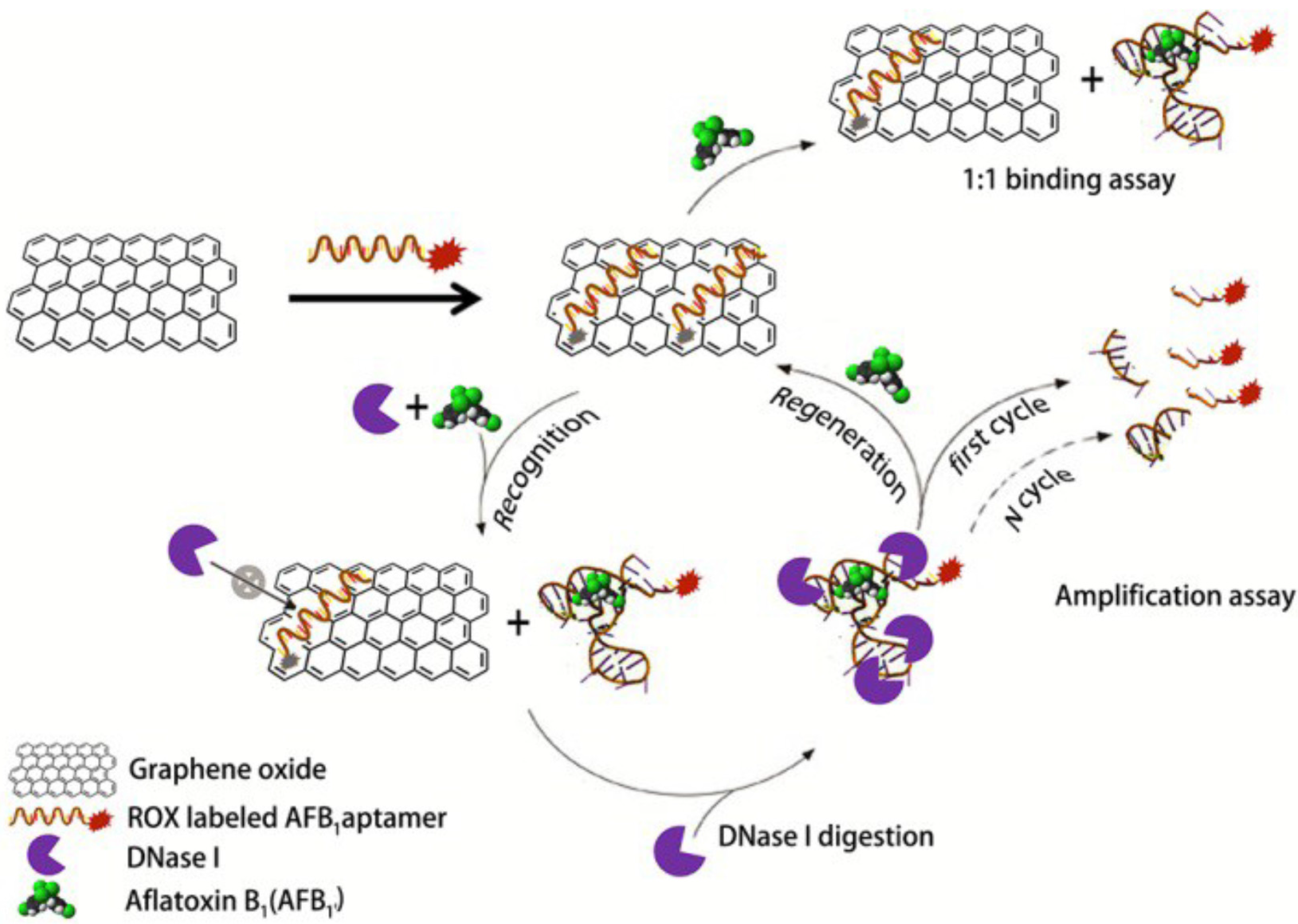
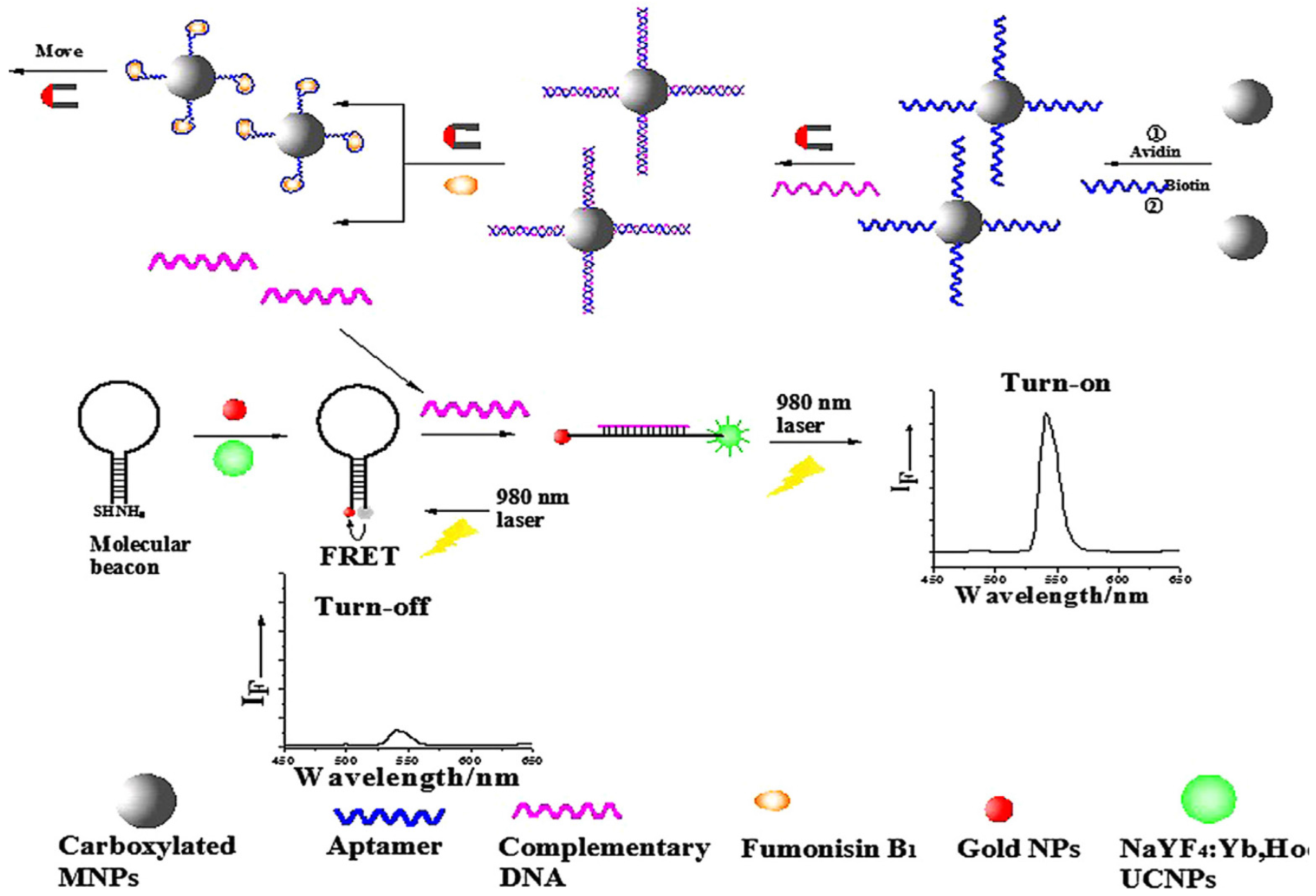
| Nanomaterial | Nanomaterial Role | Limit of Detection (µg/L) | Real Sample | Refs. |
|---|---|---|---|---|
| AuNPs | Colorimetric probe | 8.07 | ------ | [108] |
| Colorimetric probe | 0.02 | Red wine | [109] | |
| LSPR probe | 0.4 | Ground corn samples | [50] | |
| FRET quencher | 1.4 × 10−3 | Wheat and green coffee beans | [56] | |
| AuNPs and silica NPs | Fluorescence quencher | 0.039 | Grape juice and serum | [111] |
| Signal amplifier | 0.0003 | Red wine | [112] | |
| AuNPs-rGo | Signal amplifier | 0.0003 | Red wine | [113] |
| AuNPs-MoS2 | Immobilization | 0.00003 | Wine | [114] |
| AgNPs | Immobilization | 0.02 | Beer | [117] |
| AgNPs | Signal generating probe | 0.02 | ----- | [118] |
| AgNCs | Fluorophore | 0.002 | Wheat | [119] |
| QDs | Fluorescent probe | 1.9 | Wine | [121] |
| NGQDs@SiO2 NPs | ECL and Fluorescent probe | 0.0005 0.012 | Peanut | [122] |
| Graphene quantum dots and nanoceria | FRET probe | 0.0025 | Peanut | [123] |
| Thick shell QDs | FERT probe | 0.5 | Beer | [124] |
| mSiO2@Au QDs-modified graphene/AuNPs | Signal amplifier | 0.00007 | ------ | [125] |
| QDs and MoS2 | Fluorescent probe and quencher | 1 | Red wine | [126] |
| Nanoceria | Redox probe | 0.06 | Milk | [127] |
| SWCNTs | Fluorescence quencher | 9.72 | Beer | [128] |
| SWCNTs | Signal amplifier | 0.02 | Serum and grape juice | [129] |
| Nanographite | Fluorescence quencher | 8.07 | Red wine | [130] |
| Fluorescent NPs | Fluorescence probe | 0.002 | Beer | [131] |
| Aflatoxin | Nanomaterial | Role of the Nanomaterial | LOD (µg/L) | Real Sample | References |
|---|---|---|---|---|---|
| AFB1 | AuNPs | Colorimetric probe | 0.025 | ------ | [132] |
| AFB2 | Colorimetric probe | 0.025 | Beer | [133] | |
| AFB1 | Colorimetric probe | 2.18 | Peanut and rice | [134] | |
| HRP-mimicking activity | 0.15 | Peanut and rice | [134] | ||
| Fluorescence quencher | 0.005 | Corn | [135] | ||
| cDNA carrier | 6 × 10−11 | Corn | [136] | ||
| Ag core and Au shell NPs | SERS signal enhancer | 0.03 | Maize | [137] | |
| GNTs/Ag core–shell | SERS signal enhancer | 0.00054 | Peanut oil | [138] | |
| AgNCs | Fluorescent signal enhancer | 0.0003 | Rice, corn and wheat | [139] | |
| Graphene oxide NPs | Signal amplification | 0.35 | Corn | [141] | |
| QDs/Graphene oxide NPs | Fluorescence probe/Quencher | 0.31 | Peanut oil | [140] | |
| ZEN | UPCNPs | Florescent probe | 0.007 | Beer | [143] |
| Functional graphene oxide | Fluorescence quencher | 0.5 | Wine and beer | [144] | |
| FB1 | UPCNPs and AuNPs | FRET probe | 0.01 | Maize | [145] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.-H.; Marty, J.L. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. https://doi.org/10.3390/toxins9110349
Rhouati A, Bulbul G, Latif U, Hayat A, Li Z-H, Marty JL. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins. 2017; 9(11):349. https://doi.org/10.3390/toxins9110349
Chicago/Turabian StyleRhouati, Amina, Gonca Bulbul, Usman Latif, Akhtar Hayat, Zhan-Hong Li, and Jean Louis Marty. 2017. "Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress" Toxins 9, no. 11: 349. https://doi.org/10.3390/toxins9110349






