Impact of a Single Oral Acute Dose of Aflatoxin B1 on Liver Function/Cytokines and the Lymphoproliferative Response in C57Bl/6 Mice
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Effect of Aflatoxin B1 on Serum ALT, γ-GT, and Total Protein Levels
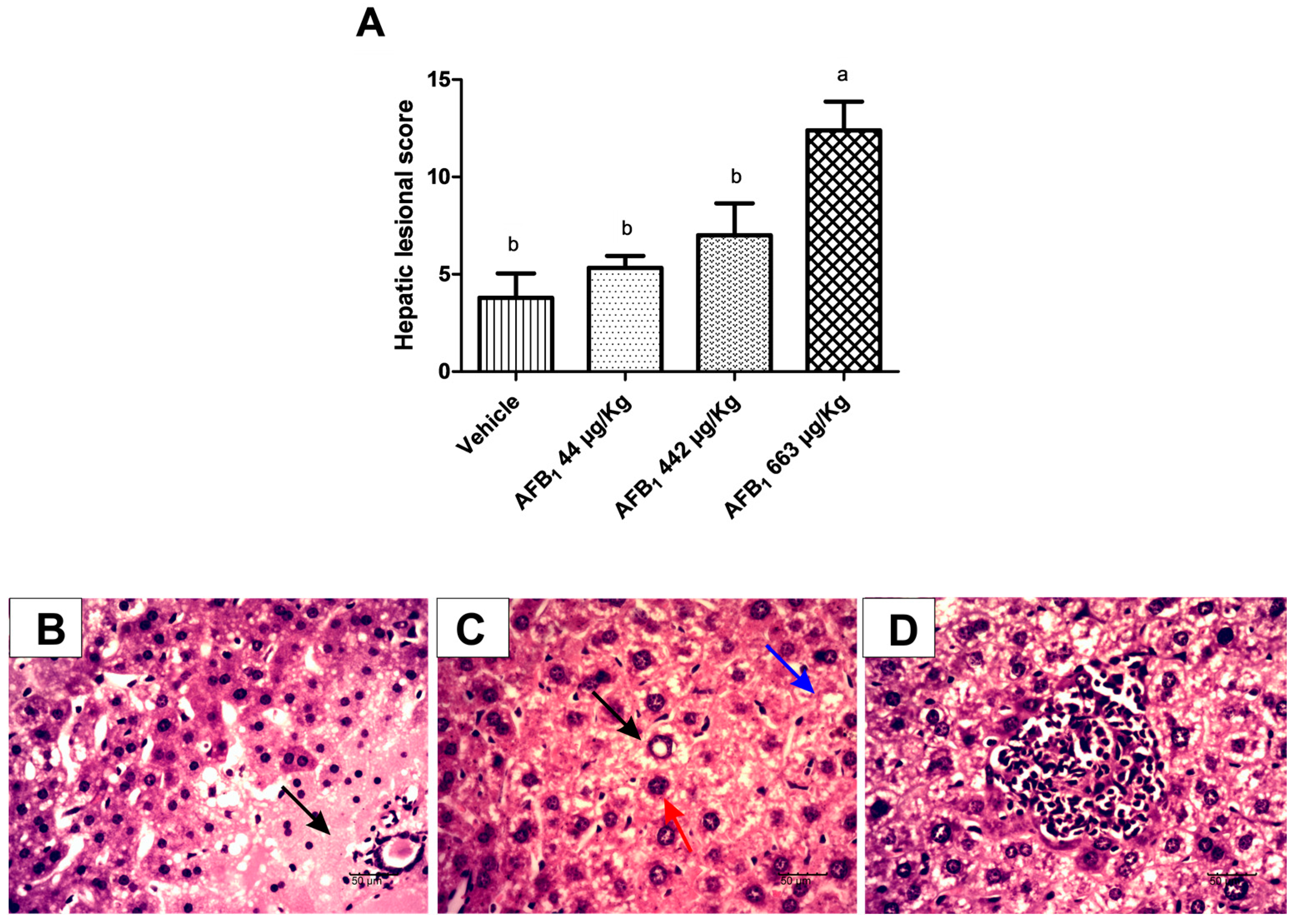
2.2. Evaluation of Aflatoxin B1 on Histological Lesions in Liver Tissue
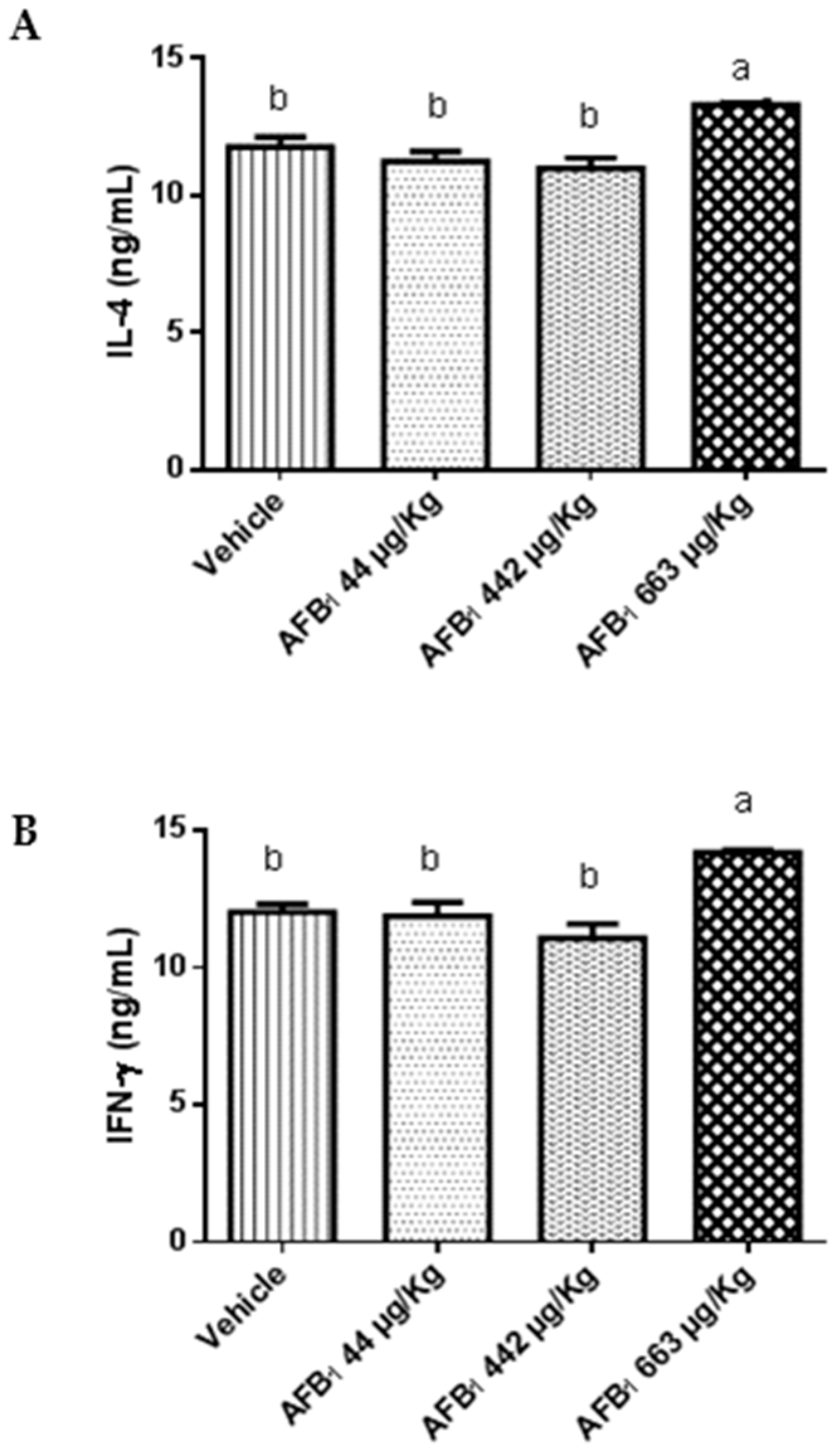
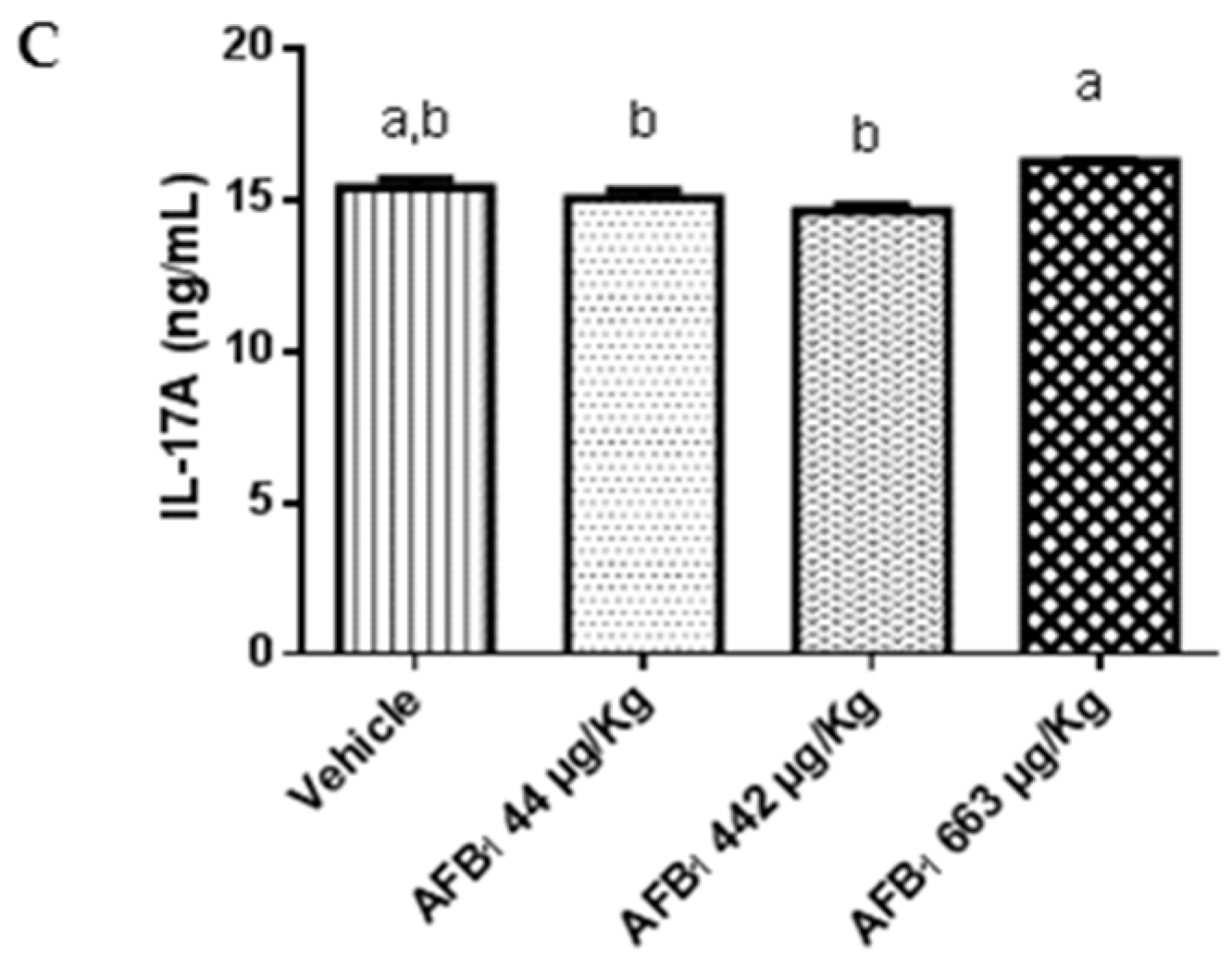
2.3. Effects of Aflatoxin B1 on Cytokine Expression in the Liver
2.4. Effects of Aflatoxin B1 on Lymphoproliferation Assay
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Aflatoxin B1 Standard, Dose Criteria, and Duration of Exposure
5.2. Animals, Housing, and Experimental Design
5.3. Measurements of Liver Function from Serum
5.4. Histopathological Analysis
5.5. Cytokine Assays
5.6. Lymphoproliferation Assay
5.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFB1 | aflatoxin B1 |
| AFs | aflatoxins |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| b.w. | body weight |
| ConA | concanavalin A |
| DMSO | dimethyl sulfoxide |
| ELISA | enzyme-linked immunosorbent assay |
| LD50 | median lethal dose |
| LPS | lipopolysaccharide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| P. I. | proliferation index |
| PBS | phosphate buffered saline |
| RPMI | Roswell Park Memorial Institute |
| γ-GT | γ-glutamyl transpeptidase |
References
- Picinin, L.C.A.; Cerqueira, M.M.O.P.; Vargas, E.A.; Lana, A.M.Q.; Toaldo, I.M.; Bordignon-Luiz, M.T. Influence of climate conditions on aflatoxin M1 contamination in raw milk from Minas Gerais State, Brazil. Food Control 2013, 31, 419–424. [Google Scholar] [CrossRef]
- Santili, A.B.N.; Camargo, A.C.; Nunes, R.S.R.; Glória, E.M.; Machado, P.F.; Cassoli, L.D.; Dias, C.T.S.; Calori-Domingues, M.A. Aflatoxin M1 in raw mik from different regions of São Paulo State—Brazil. Food Addit. Contam. Part B 2015, 25, 1–8. [Google Scholar]
- Liska, D.J. The detoxification enzyme systems. Altern. Med. Rev. 1998, 3, 187–198. [Google Scholar] [PubMed]
- Liang, N.; Wang, F.; Peng, X.; Fang, J.; Cui, H.; Chen, Z.; Lai, W.; Zhou, Y.; Geng, Y. Effect of sodium selenite on pathological changes and renal functions in broilers fed a diet containing aflatoxin B1. Int. J. Res. Public Health 2015, 12, 11196–11208. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Chemical Agents and Related Occupations; World Health Organization: Lyon, France, 2012; Volume 100F. [Google Scholar]
- Diao, E.; Hou, H.; Chen, B.; Shan, C.; Dong, H. Ozonolysis efficiency and safety evaluation of aflatoxin B1 in peanuts. Food Chem. Toxicol. 2013, 55, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Mehrzar, J.; Devriendt, B.; Baert, K.; Cox, E. Aflatoxin B1 interferes with the antigen-presenting capacity of porcine dendritic cells. Toxicol. In Vitro 2014, 28, 521–537. [Google Scholar]
- Mohammadi, A.; Mehrzad, J.; Mahmoudi, M.; Scheneider, M. Environmentally relevant level of aflatoxin B1 dysregulates human dendritic cells through signaling on key Toll-Like Receptors. Int. J. Toxicol. 2014, 33, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Russo, R.; Marzocco, S.; Velotto, S.; Autore, G.; Severino, L. Modulation of macrophage activity by aflatoxins B1 and B2 and their metabolites aflatoxins M1 and M2. Toxicon 2012, 59, 644–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Ilic, Z.; Crawford, D.; Egner, P.A.; Sell, S. Glutathione-S-transferase A3 knockout mice are sensitive to acute cytotoxic and genotoxic effects of aflatoxin B1. Toxicol. Appl. Pharmacol. 2010, 242, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzaq, Y.M.; Padmanabhan, R.; Bastaki, S.; Kochyil, J.; Shafiullah, M. Teratogenic effects of aflatoxin B1 in mice exposed in early and late gestation. Pediatr. Res. 2011, 70, 405. [Google Scholar] [CrossRef]
- Jha, A.; Krithika, R.; Manjeet, D.; Verma, R.J. Protective effect of black tea infusion on aflatoxin-induced hepatotoxicity in mice. J. Clin. Exp. Hepatol. 2013, 3, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.E.; Bondy, G.S.; Mehta, R.; Massey, T.E. The impact of chronic aflatoxin B1 exposure and p53 genotype on base excision. Mutat. Res. 2015, 773, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.M.A.; Correa, B.; Xavier, J.G.; Mallozzi, M.A.B.; Gambale, W.; Paula, C.R. Acute effect of aflatoxin B1 on different inbred mouse strains. Mycopathologia 1996, 133, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Peña, A.; Del Teso, N.; Pérez, V.; Rodrígues-Cuesta, J. Clinical Biochemistry Parameters in C57Bl/6 mice after blood collection from the submandibular vein and retroorbital plexus. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 202–206. [Google Scholar] [PubMed]
- Baptista, A.S.; Abdalla, A.L.; Aguiar, C.L.; Baptista, A.A.S.; Micheluchi, D.; Zampronio, A.C.; Pires, D.S.; Glória, E.M.; Calori-Domingues, M.A.; Walder, J.M.M.; et al. Utilization of diets amended with yeast and amino acids for the control of aflatoxicosis. World J. Microbiol. Biotechnol. 2008, 24, 2547–2554. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.W.; Kyegombe, D.B. Review of the biological and health effects of aflatoxins on body organs and body systems. In Aflatoxins-Recent Advances and Future Prospects; Intech: London, UK, 2013; Chapter 12. [Google Scholar]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolty, C.M.; Aggarwal, D. Humans aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [PubMed]
- Barrett, J.R. Liver cancer and aflatoxin: New information from the Kenyan Outbreak. Environ. Health Perspect. 2005, 113, A837–A838. [Google Scholar] [CrossRef]
- Colakoglu, F.; Donmez, H.H. Effects of aflatoxin on liver and protective effectiveness of esterified glucomannan in merino rams. Sci. World J. 2012, 2012, 462925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.-G.; Zhao, L.-H.; Wei, H.; Duan, G.-X.; Zhang, J.-Y.; Ji, C. Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int. J. Mol. Sci. 2014, 15, 5649–5662. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Tang, L.; Guo, X.; Wang, F.; Massey, M.E.; Su, J.; Guo, T.L.; Williams, J.H.; Phillips, T.D.; Wang, J.-S. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 rats. J. Appl. Toxicol. 2014, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.S.; Coulombe, R.A.; Reed, K.M. Aflatoxicosis: Lessons from toxicity and responses to aflatoxin B1 in poultry. Agriculture 2015, 5, 742–777. [Google Scholar] [CrossRef]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.V.; Sharma, R.P. Effects of aflatoxin B1 on murine lymphocytic functions. Toxicology 1989, 54, 31–44. [Google Scholar] [CrossRef]
- Peraica, M.; Radic, B.; Lucic, A.; Pavlovic, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. Suppl. 1999, 77, 754–766. [Google Scholar]
- Ishikawa, A.T.; Weese, J.S.; Bracarense, A.P.F.R.L.; Alfieri, A.A.; Oliveira, G.G.; Kawamura, O.; Hirooka, E.Y.; Itano, E.N.; Costa, M.C. Single aflatoxin B1 exposure induces changes in gut microbiota community in C57Bl/6 mice. World Mycotoxin J. 2017, 10, 249–254. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz (IAL). Métodos Físico-Químicos Para Análise de Alimentos; Instituto Adolfo Lutz: São Paulo, Brazil, 2008; 1020p.
- European Community (EC). Commission Regulation (EC) n°165/2010 of 26 February of 2010 amending regulation (EC) n°1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, L50, 8–12. [Google Scholar]
- Agência Nacional de Vigilância Sanitária (ANVISA). Resolution of Board of Directors-RDC 07/2011, Technical Regulation on Maximum Tolerated for Mycotoxins in Food; Editora do Ministério da Saúde: Brasília, Brazil, 2011.
- Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa de Orçamentos Familiares 2008–2009, Análise do Consumo Alimentar Pessoal no Brasil; IBGE: Rio de Janeiro, Brazil, 2011; 150p. [Google Scholar]
- Feitah, H.A.; Dessouki, A.A.; Hassanin, A.A.I.; Tahan, A.S. Toxopathological and cytogenic effects of aflatoxin B1 (AFB1) on pregnant rats. Pathol. Res. Pract. 2014, 12, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, R.A., Jr.; Sharma, R.P. Clearance and excretion of intratracheally and orally administered aflatoxin B1 in the rat. Food Chem. Toxicol. 1985, 23, 827–830. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Massuda, T.Y.C.; Nagashima, L.A.; Leonello, P.C.; Kaminami, M.S.; Mantovani, M.S.; Sano, A.; Uno, J.; Venancio, E.J.; Camargo, Z.P.; Itano, E.N. Cyclosporin A treatment and decreaded fungal load/antigenemia in experimental murine paracoccidioidomycosis. Mycoptahologia 2011, 171, 161–169. [Google Scholar] [CrossRef] [PubMed]

| Group | Parameters | ||
|---|---|---|---|
| ALT (U/L) | γ-GT (U/L) | Total Protein (g/dL) | |
| Control | 26.67 ± 9.37 a | 6.83 ± 0.75 a | 5.63 ± 0.49 a |
| Vehicle | 43.25 ± 15.52 a | 6.5 ± 0.71 a | 5.46 ± 0.54 a |
| AFB1 44 μg/kg | 37.33 ± 8.64 a | 6.3 ± 1.03 a | 5.83 ± 0.27 a |
| AFB1 442 μg/kg | 28.33 ± 7.23 a | 5.50 ± 0.71 a | 5.12 ± 0.34 a |
| AFB1 663 μg/kg | 22.80 ± 3.70 a | 7.00 ± 2.00 a | 5.46 ± 0.30 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, A.T.; Hirooka, E.Y.; Alvares e Silva, P.L.; Bracarense, A.P.F.R.L.; Flaiban, K.K.M.d.C.; Akagi, C.Y.; Kawamura, O.; Costa, M.C.d.; Itano, E.N. Impact of a Single Oral Acute Dose of Aflatoxin B1 on Liver Function/Cytokines and the Lymphoproliferative Response in C57Bl/6 Mice. Toxins 2017, 9, 374. https://doi.org/10.3390/toxins9110374
Ishikawa AT, Hirooka EY, Alvares e Silva PL, Bracarense APFRL, Flaiban KKMdC, Akagi CY, Kawamura O, Costa MCd, Itano EN. Impact of a Single Oral Acute Dose of Aflatoxin B1 on Liver Function/Cytokines and the Lymphoproliferative Response in C57Bl/6 Mice. Toxins. 2017; 9(11):374. https://doi.org/10.3390/toxins9110374
Chicago/Turabian StyleIshikawa, Angélica Tieme, Elisa Yoko Hirooka, Paula Leonello Alvares e Silva, Ana Paula Frederico Rodrigues Loureiro Bracarense, Karina Keller Marques da Costa Flaiban, Claudia Yuri Akagi, Osamu Kawamura, Marcio Carvalho da Costa, and Eiko Nakagawa Itano. 2017. "Impact of a Single Oral Acute Dose of Aflatoxin B1 on Liver Function/Cytokines and the Lymphoproliferative Response in C57Bl/6 Mice" Toxins 9, no. 11: 374. https://doi.org/10.3390/toxins9110374






