Food-Borne Outbreak Investigation and Molecular Typing: High Diversity of Staphylococcus aureus Strains and Importance of Toxin Detection
Abstract
:1. Introduction
2. Results
2.1. Epidemiological Investigation
- The first outbreak (outbreak A), took place in an elderly home that housed 111 residents aged between 68 and 99 years. In total 28 residents (24 female and four male), started vomiting within three hours after lunch. Vomiting was followed by diarrhea. Their lunch consisted of meat loaf, cod and mashed potatoes. The mashed potatoes were prepared in the morning and stored for two hours in a water bath at 90 °C until lunch.
- The second outbreak (outbreak B) occurred after a barbecue meal where 18 participants suffered from vomiting and diarrhea upon 6 hours after consumption of various foods. The catering service responsible for the food delivered a similar barbecue meal at a second barbecue event, but no cases were reported there. Interestingly, a current failure of the refrigerating device occurred, but temperature was verified and claimed to be conform by the caterer. None of the food handlers were ill before, during or after the event.
- In the third outbreak (outbreak C) six out of seven children aged between nine months and two years started vomiting within 1 h after eating a mashed carrot-potato mixture with fish at a kinder garten. One child who consumed only one spoon of the meal did not become ill. All children were sent to the hospital and two of them were in shock. All recovered fast and left the hospital the same day. The mashed carrot-potato mixture consisted of leftovers from the lunch of the previous day, which was cooled at room temperature before storage in the fridge. The fish was stored frozen and heated (steamed) just before consumption.
2.2. Laboratory Investigation
2.2.1. Bacterial Investigation of Human Samples
2.2.2. Bacterial Investigation of Food Samples
2.2.3. Toxin Investigation (SE Detection, Identification and Quantification)
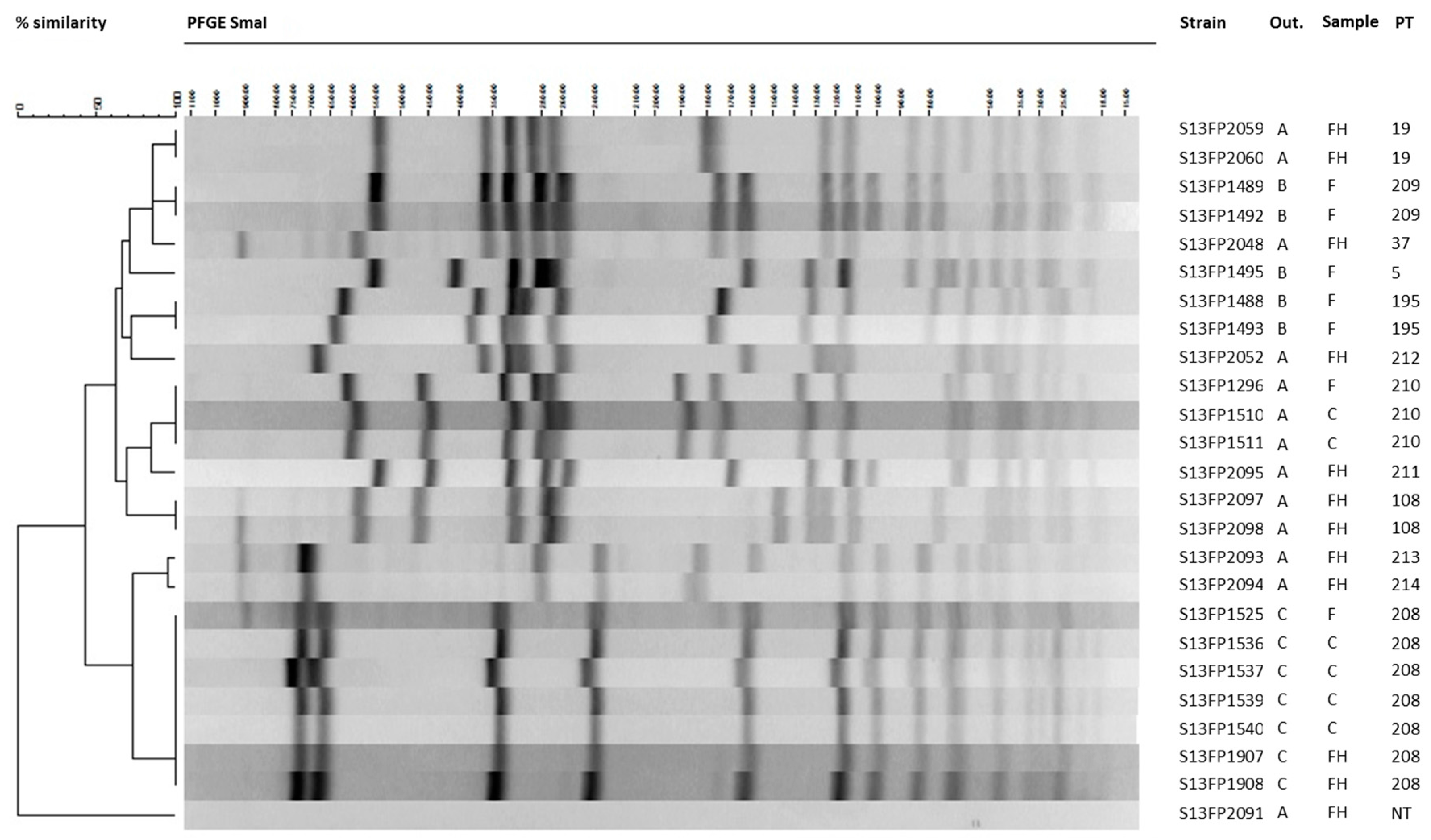
2.2.4. Comparison of CPS Strains from Human and Food Origin
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Outbreak Investigation and Sampling
- Outbreak A: reported to the Health Inspection Service by medical doctors, occurred in an elderly home.
- Outbreak B: reported to the inspector of the FASFC by the head of the catering company, occurred in a temporary mass gathering-barbecue.
- Outbreak C: reported to the Health Inspection Service by medical doctors, occurred in a kindergarten.
- For outbreak A, 18 different composed dishes or food components similar to those consumed the day of the outbreak (mashed potatoes, meat loaf, two samples of cod, cooked liver pasta) or the day before the outbreak (vanilla pudding, cheese, rice, bread, potatoes, mashed potatoes, horse filet, sausages, 3 sauces, mixed chicken with broccolis and leek) were sampled.
- For outbreak B, 15 food items similar to those consumed by the victims were sampled, some of which were stored frozen until sampling. These included meat products (bovine steak, sausages, lamb meat), poultry (chicken chops), vegetables (potatoes, mixture of carrots/cucumber/white cabbage), bakery products (bavarois), fish products (two different salmon preparations, sardines), crustacean (scampi) and mousse based on salmon, ham, duck or crab. For this outbreak, 2 ground water samples were also collected.
- For outbreak C, only one sample containing a small quantity of leftovers consisting in a mashed carrot-potato mixture with frozen fish was collected.
5.2. Laboratory Investigation
5.2.1. Enumeration of Coagulase Positive Staphylococci and B. cereus in Food and Water Samples
5.2.2. Detection and Quantification of Staphylococcal Enterotoxins in Food Samples
5.2.3. Isolation of Coagulase Positive S. aureus and B. cereus from Human Samples
5.2.4. Genotypic and Phenotypic Characterization of Coagulase Positive Staphylococcus aureus
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic shock syndrome and bacterial superantigens: An update. Annu. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; de Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Argudin, M.A.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.K.; Sato’o, Y.; Narita, K.; Naito, I.; Hirose, S.; Hisatsune, J.; Asano, K.; Hu, D.L.; Omoe, K.; Sugai, M.; Nakane, A. Identification and Characterization of a Novel Staphylococcal Emetic Toxin. Appl. Environ. Microbiol. 2015, 81, 7034–7040. [Google Scholar] [CrossRef] [PubMed]
- Omoe, K.; Hu, D.L.; Ono, H.K.; Shimizu, S.; Takahashi-Omoe, H.; Nakane, A.; Uchiyama, T.; Shinagawa, K.; Imanishi, K. Emetic potentials of newly identified staphylococcal enterotoxin-like toxins. Infect. Immun. 2013, 81, 3627–3631. [Google Scholar] [CrossRef] [PubMed]
- Nia, Y.; Mutel, I.; Assere, A.; Lombard, B.; Auvray, F.; Hennekinne, J.A. Review Over a 3-Year Period of European Union Proficiency Tests for Detection of Staphylococcal Enterotoxins in Food Matrices. Toxins 2016, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Nia, Y.; Rodriguez, M.; Zeleny, R.; Herbin, S.; Auvray, F.; Fiebig, U.; Avondet, M.A.; Munoz, A.; Hennekinne, J.A. Organization and ELISA-Based Results of the First Proficiency Testing to Evaluate the Ability of European Union Laboratories to Detect Staphylococcal Enterotoxin Type B (SEB) in Buffer and Milk. Toxins 2016, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Hait, J.M.; Tallent, S.M.; Bennett, R.W. Screening, detection, and serotyping methods for toxin genes and enterotoxins in Staphylococcus strains. J. AOAC Int. 2014, 97, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef]
- Evenson, M.L.; Hinds, M.W.; Bernstein, R.S.; Bergdoll, M.S. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int. J. Food Microbiol. 1988, 7, 311–316. [Google Scholar] [CrossRef]
- Guillier, L.; Bergis, H.; Guillier, F.; Noel, V.; Auvray, F.; Hennekinne, J.-A. Dose-response modelling of staphylococcal enterotoxins using outbreak data. Procedia Food Sci. 2016, 7, 129–132. [Google Scholar] [CrossRef]
- Humphries, R.M.; Linscott, A.J. Laboratory diagnosis of bacterial gastroenteritis. Clin. Microbiol. Rev. 2015, 28, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; Ostyn, A.; Guillier, F.; Herbin, S.; Prufer, A.L.; Dragacci, S. How should staphylococcal food poisoning outbreaks be characterized? Toxins 2010, 2, 2106–2116. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); The European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA J. 2013, 11. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2013.3129/epdf (accessed on 18 December 2017). [CrossRef]
- European Food Safety Authority (EFSA); The European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA J. 2014, 12. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2014.3547/epdf (accessed on 18 December 2017). [CrossRef]
- European Food Safety Authority (EFSA); The European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015, 13. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.3991/epdf (accessed on 18 December 2017). [CrossRef]
- European Food Safety Authority (EFSA); The European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015, 13. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4329/epdf (accessed on 18 December 2017). [CrossRef]
- European Food Safety Authority (EFSA); The European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2016.4634/epdf (accessed on 18 December 2017). [CrossRef]
- Kluytmans, J.A.; Wertheim, H.F. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 2005, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kummel, J.; Stessl, B.; Gonano, M.; Walcher, G.; Bereuter, O.; Fricker, M.; Grunert, T.; Wagner, M.; Ehling-Schulz, M. Staphylococcus aureus Entrance into the Dairy Chain: Tracking S. aureus from Dairy Cow to Cheese. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Roussel, S.; Felix, B.; Vingadassalon, N.; Grout, J.; Hennekinne, J.A.; Guillier, L.; Brisabois, A.; Auvray, F. Staphylococcus aureus strains associated with food poisoning outbreaks in France: Comparison of different molecular typing methods, including MLVA. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.-A.; Kerouanton, A.; Brisabois, A.; De Buyser, M.L. Discrimination of Staphylococcus aureus biotypes by pulsed-field gel electrophoresis of DNA macro-restriction fragments. J. Appl. Microbiol. 2003, 94, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Bartels, M.D.; Petersen, A.; Worning, P.; Nielsen, J.B.; Larner-Svensson, H.; Johansen, H.K.; Andersen, L.P.; Jarløv, J.O.; Boye, K.; Larsen, A.R.; et al. Comparing whole-genome sequencing with Sanger sequencing for spa typing of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2014, 52, 4305–4308. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.R.; Walker, T.M.; Pallen, M.J. Genomics and outbreak investigation: From sequence to consequence. Genome Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Scientific Opinion on the evaluation of molecular typing methods for major food-borne microbiological hazards and their use for attribution modelling, outbreak investigation and scanning surveillance: Part 1 (evaluation of methods and applications). EFSA J. 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Manual for reporting on food-borne outbreaks in accordance with Directive 2003/99/EC for information derived from the year 2013. EFSA J. 2014, 11, 1–46. Available online: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2014.EN-577/full (accessed on 18 December 2017). [CrossRef]
- Jorgensen, H.J.; Mathisen, T.; Løvseth, A.; Omoe, K.; Qvale, K.S.; Loncarevic, S. An outbreak of staphylococcal food poisoning caused by enterotoxin H in mashed potato made with raw milk. FEMS Microbiol. Lett. 2005, 252, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.W. Staphylococcal enterotoxin and its rapid identification in foods by enzyme-linked immunosorbent assay-based methodology. J. Food Prot. 2005, 68, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Dauwalder, O.; Brun, V.; Badiou, C.; Ferry, T.; Etienne, J.; Vandenesch, F.; Lina, G. Staphylococcus aureus superantigens elicit redundant and extensive human Vβ patterns. Infect. Immun. 2009, 77, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, M.; Tsilia, V.; Rajkovic, A.; De Cremer, K.; Van Loco, J. Application of LC-MS/MS MRM to Determine Staphylococcal Enterotoxins (SEB and SEA) in Milk. Toxins 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Gu, H.; Hao, L.; Ye, H.; Gong, W.; Wang, Z. A Review of the Methods for Detection of Staphylococcus aureus Enterotoxins. Toxins 2016, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Anon. EU 2073/2005 Commission Regulation (EC) N_2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 10 November 2017).
- Schubert, J.; Podkowik, M.; Bystron, J.; Bania, J. Production of staphylococcal enterotoxins D and R in milk and meat juice by Staphylococcus aureus strains. Food. Path. Dis. 2017, 14, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, J.; Tang, J.; Tang, C.; Chen, J. Identification and measurement of staphylococcal enterotoxin M from Staphylococcus aureus isolate associated with staphylococcal food poisoning. Lett. Appl. Microbiol. 2017, 65, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.L.; Salsberg, S.P.; Bergdoll, M.S. Production of staphylococcal enterotoxin D in foods by low-enterotoxin-producing staphylococci. Int. J. Food Microbiol. 1991, 14, 19–26. [Google Scholar] [CrossRef]
- Rasooly, R.; Do, P.M.; Herniem, B.J. Rapid cell-based assay for detection an quantification of active staphylococcal enterotoxin D. J. Food. Sci. 2017, 82, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, A. Microbial toxins and low level of foodborne exposure. Trends Food. Sci. Technol. 2014, 38, 149–157. [Google Scholar] [CrossRef]
- Ho, J.; Boost, M.; O’Donoghue, M. Prevalence of enterotoxin genes in Staphylococcus aureus colonising food handlers: Does nasal carriage status matter? Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Ihara, H.; Aoyama, K.; Igarashi, H.; Yanahira, S.; Ohkubo, T.; Asao, T.; Kozaki, S. Characteristics of enterotoxin H-producing Staphylococcus aureus isolated from clinical cases and properties of the enterotoxin productivity. J. Food Prot. 2008, 71, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, E.; Nowrouzian, F.; Adlerberth, I.; Wold, A.E. Long-time persistence of superantigen-producing Staphylococcus aureus strains in the intestinal microflora of healthy infants. Pediatr. Res. 2000, 48, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Muenks, C.E.; Hogan, P.G.; Wang, J.W.; Eisenstein, K.A.; Burnham, C.-A.D.; Fritz, S.A. Diversity of Staphylococcus aureus Strains Colonizing Various Niches of the Human Body. J. Infect. 2016, 72, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Acco, M.; Ferreira, F.S.; Henriques, J.A.P.; Tondo, E.C. Identification of multiple strains of Staphylococcus aureus colonizing nasal mucosa of food handlers. Food Microbiol. 2003, 20, 489–493. [Google Scholar] [CrossRef]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species); ISO 6888-1:1999/Amd.1:2003; International Organization for Standardization: Geneva, Switzerland, 2003; Available online: https://www.iso.org/standard/23036.html (accessed on 10 November 2017).
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony-Count Technique at 30 °C; ISO 7932:2004; International Organization for Standardization: Geneva, Switzerland, 2004; Available online: https://www.iso.org/standard/38219.html (accessed on 10 November 2017).
- EU-RL for CPS. Detection of Staphylococcal Enterotoxins in All Types of Food Matrices. European Screening Method of the EU-RL for Coagulase Positive Staphylococci Including Staphylococcus aureus V5 September 2010. Available online: http://www.piwet.pulawy.pl/piwet7/bieglosc/ZHZ/SET-European.pdf (accessed on 10 November 2017).
| Outbreak | Origin (F, C, FH) * | Matrix | CPS Investigation (cfu/g, D, ND) | SE Detection a | SE Quantification (ng/g) b | se-Genes | Pulsotype | |
|---|---|---|---|---|---|---|---|---|
| Strain Isolate (SE-type, ND) | Food (D, ND) | |||||||
| Outbreak A | F | Mashed potatoes | 270 | SEA, SED | D | SEA 0.019 | sea, sed, seg, sei, sej, ser | 210 |
| F | Diverse (17 samples) | ND | ND | No strain isolated | ||||
| C | Stool 1 | D | SEA | sea, sed, seg, sei, sej, ser | 210 | |||
| C | Stool 2 | D | SEA | sea, sed, seg, sei, sej, ser | 210 | |||
| FH 1 | Swab throat | D | SEA | sea, seh | 37 | |||
| FH 2 | Swab throat | D | SEC | sec, seg, sei | 212 | |||
| FH 3 | Swab Nose/Throat | D | SEA | sea, seh | 19 | |||
| FH 4 | Swab Nose | D | ND | negative for the tested se-genes | N/A | |||
| FH 5 | Swab Nose | D | ND | seg, seh, sei | 213 | |||
| FH 5 | Swab throat | D | ND | seg, seh, sei | 214 | |||
| FH 6 | Swab Nose | D | ND | seg, sei | 211 | |||
| FH 7 | Swab Nose/Throat | D | ND | negative for the tested se-genes | N/A | |||
| 20 FH/7 C | Swab/Stool | ND | No strain isolated | |||||
| Outbreak B | F | Chicken | 1700 | SEA, SEC | ND | N/A | sea, sec | 195 |
| F | Sausage | 1300 | SEA | D | N/A | sea | 209 | |
| F | Bovine meat | 900 | ND | ND | N/A | sea | 209 | |
| F | Potato preparation | 7,200,000 | SEA, SEC | D | SEA 0.015, SEC 0.132 | sea, sec | 195 | |
| F | Dessert (pie) | 100 | ND | ND | N/A | sep | 5 | |
| F | Diverse (11 samples) | ND | N/A | No strain isolated | ||||
| 4 FH/5 C | Stool (9 samples) | ND | No strain isolated | |||||
| Outbreak C | F | Mashed potatoes with carrots | >15,000,000 | SEA | N/A | sea, seg, sei | 208 | |
| F | fish | ND | N/A | No strain isolated | ||||
| C | Stool 1 | D | SEA | sea, seg, sei | 208 | |||
| C | Stool 2 | D | SEA | sea, seg, sei | 208 | |||
| C | Stool 3 | D | SEA | sea, seg, sei | 208 | |||
| C | Stool 4 | D | SEA | sea, seg, sei | 208 | |||
| FH | Swab Nose/Throat | D | SEA | sea, seg, sei | 208 | |||
| C | Vomit | ND | No strain isolated | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denayer, S.; Delbrassinne, L.; Nia, Y.; Botteldoorn, N. Food-Borne Outbreak Investigation and Molecular Typing: High Diversity of Staphylococcus aureus Strains and Importance of Toxin Detection. Toxins 2017, 9, 407. https://doi.org/10.3390/toxins9120407
Denayer S, Delbrassinne L, Nia Y, Botteldoorn N. Food-Borne Outbreak Investigation and Molecular Typing: High Diversity of Staphylococcus aureus Strains and Importance of Toxin Detection. Toxins. 2017; 9(12):407. https://doi.org/10.3390/toxins9120407
Chicago/Turabian StyleDenayer, Sarah, Laurence Delbrassinne, Yacine Nia, and Nadine Botteldoorn. 2017. "Food-Borne Outbreak Investigation and Molecular Typing: High Diversity of Staphylococcus aureus Strains and Importance of Toxin Detection" Toxins 9, no. 12: 407. https://doi.org/10.3390/toxins9120407