Effects of Electron Beam Irradiation on Zearalenone and Ochratoxin A in Naturally Contaminated Corn and Corn Quality Parameters
Abstract
:1. Introduction
2. Results and Discussion
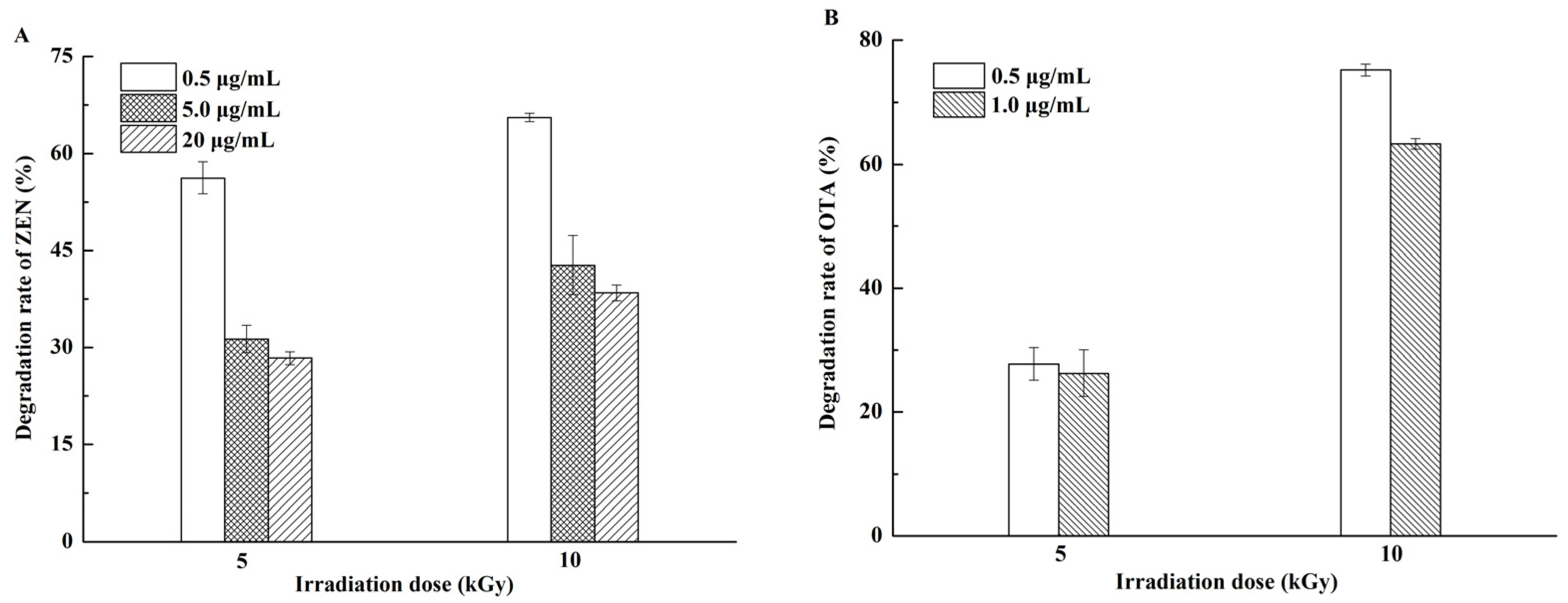
2.1. Effects of EBI on ZEN and OTA Solutions
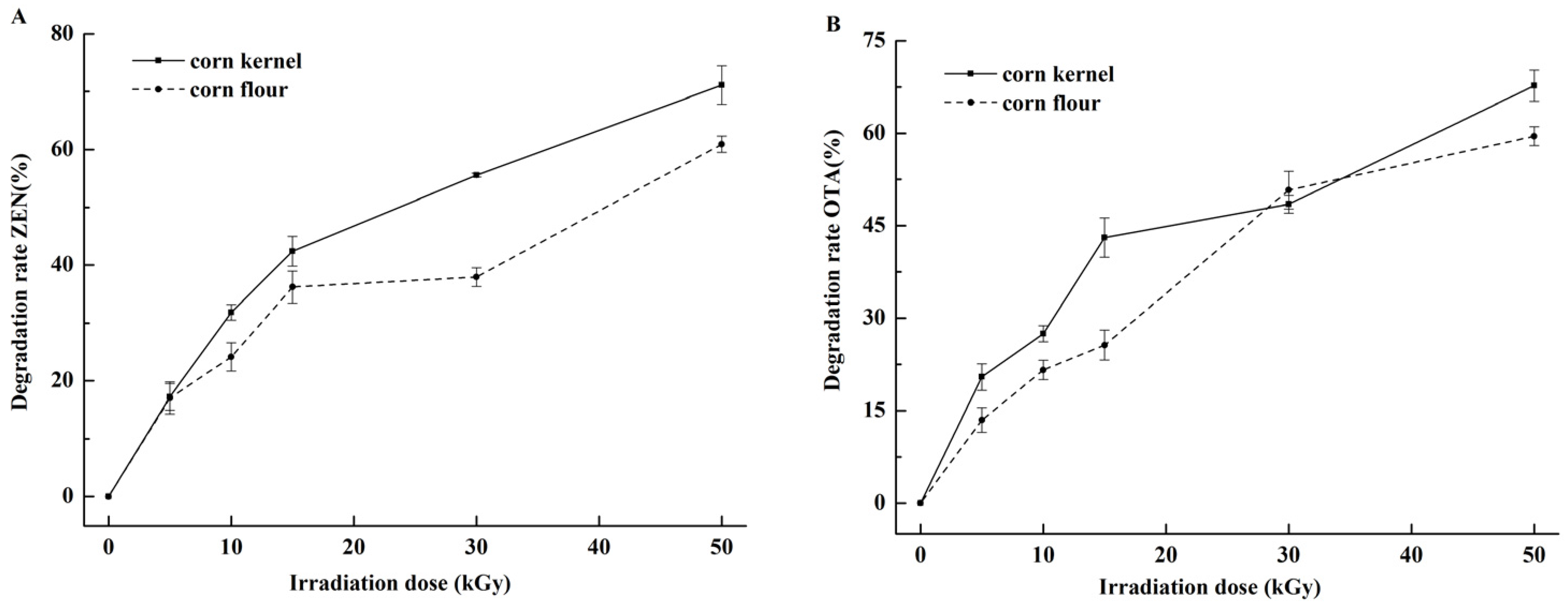
2.2. Effect of EBI on Naturally Contaminated Corn
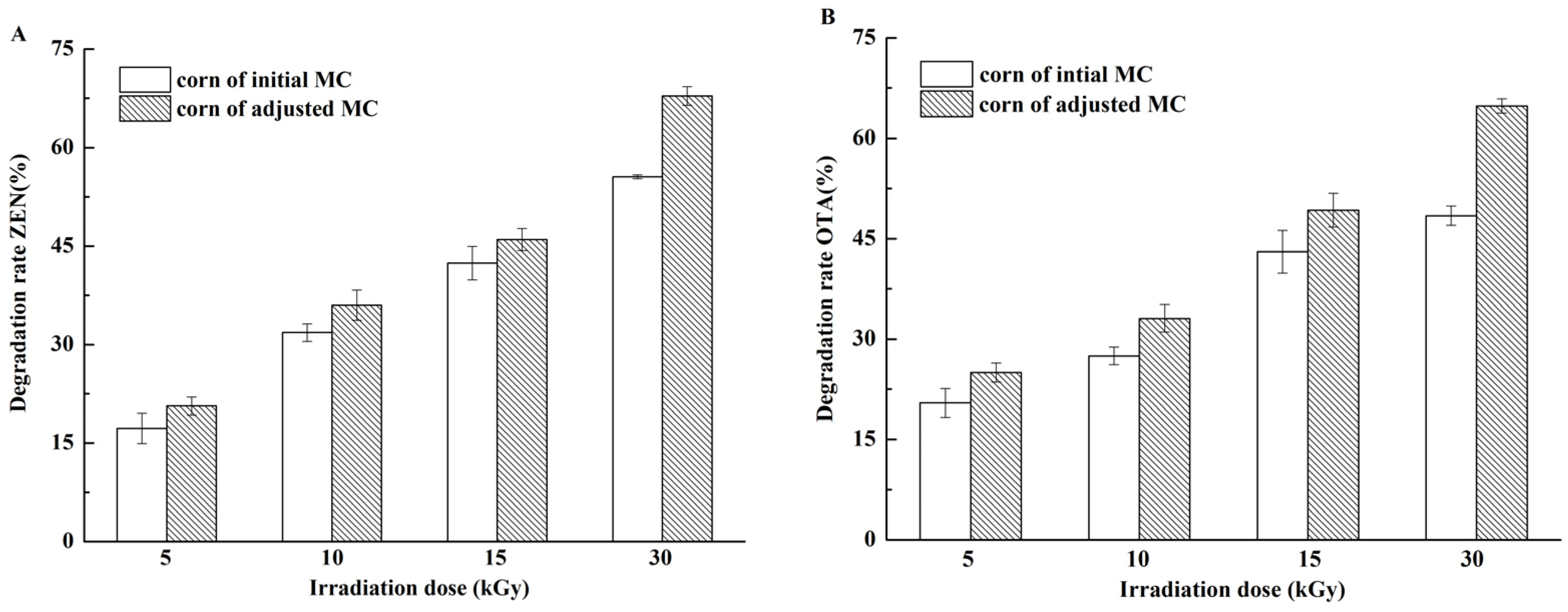
2.3. Effect of MC of Corn Kernel on EBI
2.4. Color of Irradiated Corn Kernel and Flour
2.5. Free Fatty Acid Value of Irradiated Corn Kernel and Flour
2.6. Pasting Properties of Irradiated Corn Kernel and Flour
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Instruments
4.3. EBI of ZEN and OTA Standards
4.4. EBI of Contaminated Corn
4.5. Preparation of ZEN and OTA Extracts
4.6. HPLC Conditions
4.7. MC Determination
4.8. Color Determination of Corn Flour
4.9. Determination of Fatty Acid Values
4.10. Pasting Property Determination of Corn Flour
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biomin. The Global Mycotoxin Threat 2016. Available online: https://info.biomin.net/acton/fs/blocks/showLandingPage/a/14109/p/p-004e/t/page/fm/17 (accessed on 8 March 2016).
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001, 39, 1435–1438. [Google Scholar] [CrossRef]
- Li, Y.J.; He, X.Y.; Yang, X.; Huang, K.L.; Luo, Y.B.; Zhu, L.Y.; Li, Y.Z.; Xu, W.T. Zinc inhibits the reproductive toxicity of zearalenone in immortalized murine ovarian granular KK-1 cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Danicke, S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: A review. Food Addit. Contam. 2007, 24, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Toffa, D.D.; Mahnine, N.; Ouaffak, L.; El Abidi, A.; Faris, F.Z.E.; Zinedine, A. First survey on the presence of ochratoxin A and fungi in raw cereals and peanut available in the Republic of Niger. Food Control 2013, 32, 558–562. [Google Scholar] [CrossRef]
- Janati, S.S.F.; Beheshti, H.R.; Asadi, M.; Mihanparast, S.; Feizy, J. Preliminary survey of aflatoxins and ochratoxin A in dried fruits from Iran. Bull. Environ. Contam. Toxicol. 2012, 88, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Avellone, G.; Pitonzo, R.; Capocchiano, V.G.; Mazza, A.; Cicero, N.; Dugo, G. Natural co-occurrence of ochratoxin A, ochratoxin B and aflatoxins in Sicilian red wines. Food Addit. Contam. Part A 2015, 32, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Pitonzo, R.; Avellone, G.; Di Fiore, A.; Monte, L.; Tabis Ogorka, A.Z. Determination of aflatoxins and ochratoxins in Sicilian sweet wines by high-performance liquid chromatography with fluorometric detection and immunoaffinity cleanup. Food Anal. Methods 2015, 8, 569–577. [Google Scholar] [CrossRef]
- Hadlok, R.M.; Wagner, G. Occurrence of ochratoxin A in men in Germany. Fleischwirtschaft 1993, 73, 1079–1080. [Google Scholar]
- Marquardt, R.R.; Frohlich, A.A. A review of recent advances in understanding ochratoxicosis. J. Anim. Sci. 1992, 70, 3968–3988. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D.; Vitanov, S.; Anguelov, G.; Petkova-Bocharova, T.; Creppy, E.E. Experimental mycotoxic nephropathy in pigs provoked by a diet containing ochratoxin A and penicillic acid. Vet. Res. Commun. 2001, 25, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. The role of ochratoxin A as a possible cause of Balkan endemic nephropathy and its risk evaluation. Vet. Hum. Toxicol. 1998, 40, 352–360. [Google Scholar] [PubMed]
- The International Agency for Research on Cancer. Monograph on the Evaluation of Carcinogenic Risk to Humans; IARC: Lyon, France, 2002; Volume 82, pp. 171–300. [Google Scholar]
- Bordini, J.G.; Borsato, D.; Oliveira, A.S.; Ono, M.A.; Zaninelli, T.H.; Hirooka, E.Y.; Ono, E.Y.S. In vitro zearalenone adsorption by a mixture of organic and inorganic adsorbents: Application of the Box Behnken approach. World Mycotoxin J. 2015, 8, 291–299. [Google Scholar] [CrossRef]
- Diao, E.; Hou, H.; Chen, B.; Shan, C.; Dong, H. Ozonolysis efficiency and safety evaluation of aflatoxin B1 in peanuts. Food Chem. Toxicol. 2013, 55, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Takatori, K.; Sugita-Konishi, Y.; Kim, I.-H.; Lee, M.-H.; Han, D.-W.; Chung, K.-H.; Hyun, S.O.; Park, J.-C. Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Technol. 2007, 201, 5733–5737. [Google Scholar] [CrossRef]
- Trenholm, H.L.; Charmley, L.L.; Prelusky, D.B.; Warner, R.M. Washing procedures using water or sodium-carbonate solutions for the decontamination of 3 cereals contaminated with deoxynivalenol and zearalenone. J. Agric. Food Chem. 1992, 40, 2147–2151. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, H.; Lan, J.; Zhang, R.; Ren, H.; Zhang, X.; Yu, G. Detoxification of zearalenone by three strains of lactobacillus plantarum from fermented food in vitro. Food Control 2015, 54, 158–164. [Google Scholar] [CrossRef]
- FAO/IAEA/WHO. Wholesomeness of Irradiated Food; Joint FAO/IAEA/WHO Expert Committee: Geneva, Switzerland, 1981. [Google Scholar]
- FAO/IAEA/WHO. High-Dose Irradiation: Wholesomeness of Food Irradiated with Doses above 10 kGy; Report of a Joint FAO/IAEA/WHO Study Group: Geneva, Switzerland, 1999; World Health Organization Technical Report Series 890: Geneva, Switzerland, 1999; pp. 1–197. [Google Scholar]
- FDA. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart¼579&showFR¼1 (accessed on 6 November 2006).
- Di Stefano, V.; Pitonzo, R.; Cicero, N.; D’Oca, M.C. Mycotoxin contamination of animal feeding stuff: Detoxification by gamma-irradiation and reduction of aflatoxins and ochratoxin A concentrations. Food Addit. Contam. Part A 2014, 7, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Pitonzo, R.; Bartolotta, A.; D’Oca, M.C.; Fuochi, P. Effects of gamma-irradiation on the alfa-tocopherol and fatty acids content of raw unpeeled almond kernels (Prunus dulcis). LWT Food Sci. Technol. 2014, 59, 572–576. [Google Scholar] [CrossRef]
- Warchalewski, J.R.; Pradzynska, A.; Gralik, J.; Nawrot, J. The effect of gamma and microwave irradiation of wheat grain on development parameters of some stored grain pests. Nahrung Food 2000, 44, 411–414. [Google Scholar] [CrossRef]
- Calado, T.; Venancio, A.; Abrunhosa, L. Irradiation for Mold and Mycotoxin Control: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Scudamore, K.A.; Patel, S. Survey for aflatoxins, ochratoxin A, zearalenone and fumonisins in maize imported into the United Kingdom. Food Addit. Contam. A 2000, 17, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Freita-Silva, O.; de Oliveira, P.S.; Freire, M. Potential of electron beams to control mycotoxigenic fungi in food. Food Eng. Rev. 2015, 7, 160–170. [Google Scholar] [CrossRef]
- Peng, C.; Ding, Y.; An, F.; Wang, L.; Li, S.; Nie, Y.; Zhou, L.; Li, Y.; Wang, C.; Li, S. Degradation of ochratoxin A in aqueous solutions by electron beam irradiation. J. Radioanal. Nucl. Chem. 2015, 306, 39–46. [Google Scholar] [CrossRef]
- Le Caer, S. Water Radiolysis: Influence of Oxide Surfaces on H-2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Van Dyck, P.J.; Tobback, P.; Feyes, M.; Van de Voorde, H. Sensitivity of aflatoxin B1 to ionizing radiation. Appl. Environ. Microb. 1982, 43, 1317–1319. [Google Scholar]
- Liu, R.; Wang, R.; Lu, J.; Chang, M.; Jin, Q.; Du, Z.; Wang, S.; Li, Q.; Wang, X. Degradation of AFB1 in aqueous medium by electron beam irradiation: Kinetics, pathway and toxicology. Food Control 2016, 66, 151–157. [Google Scholar] [CrossRef]
- Stepanik, T.; Kost, D.; Nowicki, T.; Gabay, D. Effects of electron beam irradiation on deoxynivalenol levels in distillers dried grain and solubles and in production intermediates. Food Addit. Contam. A 2007, 24, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.H.; Youssef, B.M. Inactivation of naturally occurring of mycotoxins in some Egyptian foods and agricultural commodities by gamma-irradiation. Egypt. J. Food Sci. 2002, 30, 167–177. [Google Scholar]
- Ghanem, I.; Orfi, M.; Shamma, M. Effect of gamma radiation on the inactivation of aflatoxin B1 in food and feed crops. Braz. J. Microbiol. 2008, 39, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Jinap, S.; Noranizan, M.A. Aflatoxins and ochratoxin A reduction in black and white pepper by gamma radiation. Radiat. Phys. Chem. 2012, 81, 1786–1788. [Google Scholar] [CrossRef]
- Satin, M. Food Irradiation—A Guidebook; Technomic Publishing Company: Lancaster, PA, USA, 1996. [Google Scholar]
- Alldrick, A.J. The effect of processing on the occurrence of ochratoxin A in cereals. Food Addit. Contam. 1996, 13, 27–28. [Google Scholar] [PubMed]
- Qinggang, Y. Research Irradiating Degradation Technology of Zearalenone in Corn. Master’s Thesis, Southwest University Chongqing, Chongqing, China, 2009. [Google Scholar]
- Kumar, S.; Kunwar, A.; Gautam, S.; Sharma, A. Inactivation of A. ochraceusspores and detoxification of ochratoxin A in coffee beans by gamma irradiation. J. Food Sci. 2012, 77, T44–T51. [Google Scholar] [PubMed]
- Frank, H.K. Radiation resistance of aflatoxins. Irradiat. Aliments 1997, 11, 15–20. [Google Scholar]
- Bhat, N.A.; Wani, I.A.; Hamdani, A.M.; Gani, A.; Masoodi, F.A. Physicochemical properties of whole wheat flour as affected by gamma irradiation. LWT Food Sci. Technol. 2016, 71, 175–183. [Google Scholar] [CrossRef]
- Abu, J.O.; Muller, K.; Duodu, K.G.; Minnaar, A. Functional properties of cowpea (Vigna unguiculata L. Walp) flours and pastes as affected by gamma-irradiation. Food Chem. 2005, 93, 103–111. [Google Scholar]
- Wani, A.A.; Wani, I.A.; Hussain, P.R.; Gani, A.; Wani, T.A.; Masoodi, F.A. Physicochemical properties of native and gamma-irradiated wild arrowhead (Sagittaria sagittifolia L.) tuber starch. Int. J. Biol. Macromol. 2015, 77, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Werth, L.; Nelson, S.; Scheerens, J.C.; Pratt, R.C. Variation of Kernel Anthocyanin and Carotenoid Pigment Content in USA/Mexico Borderland Land Races of Maize. Econ. Bot. 2013, 67, 98–109. [Google Scholar] [CrossRef]
- Generally Administration of Quality Supervision (GAQS). Guigelines for Evaluation of Maize Storage Charater; GB/T 20570–2015[S]; GAQS: Beijing, China, 2015.
- Marathe, S.A.; Deshpande, R.; Khamesra, A.; Ibrahim, G.; Jamdar, S.N. Effect of radiation processing on nutritional, functional, sensory and antioxidant properties of red kidney beans. Radiat. Phys. Chem. 2016, 125, 1–8. [Google Scholar] [CrossRef]
- Wu, D.X.; Shu, Q.Y.; Wang, Z.H.; Xia, Y.W. Effect of gamma irradiation on starch viscosity and physicochemical properties of different rice. Radiat. Phys. Chem. 2002, 65, 79–86. [Google Scholar] [CrossRef]
- Rombo, G.O.; Taylor, J.R.N.; Minnaar, A. Effect of irradiation, with and without cooking of maize and kidney bean flours, on porridge viscosity and in vitro starch digestibility. J. Sci. Food. Agric. 2001, 81, 497–502. [Google Scholar] [CrossRef]
- De Kerf, M.; Mondelaers, W.; Lahorte, P.; Vervaet, C.; Remon, J.P. Characterisation and disintegration properties of irradiated starch. Int. J. Pharm. 2001, 221, 69–76. [Google Scholar] [CrossRef]
- Abu, J.O.; Duodu, K.G.; Minnaar, A. Effect of gamma-irradiation on some physicochemical and thermal properties of cowpea (Vigna unguiculata L. Walp) starch. Food Chem. 2006, 95, 386–393. [Google Scholar] [CrossRef]
- Sokhey, A.S.; Chinnaswamy, R. Physicochemical properties of irradiation modified starch extrudates. Food Struct. 1992, 11, 361–371. [Google Scholar]
- Sabularse, V.C.; Liuzzo, J.A.; Rao, R.M.; Grodner, R.M. Physicochemical characteristics of brown rice as influenced by gamma-irradiation. J. Food Sci. 1992, 57, 143–145. [Google Scholar] [CrossRef]
- Qi, L.; Li, Y.; Luo, X.; Wang, R.; Zheng, R.; Wang, L.; Li, Y.; Yang, D.; Fang, W.; Chen, Z. Detoxification of zearalenone and ochratoxin A by ozone and quality evaluation of ozonised corn. Food Addit. Contam. Part A 2016, 33, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Culture of the People’s Republic of China (MHPRC). Determination of Moisture in Food; GB 5009.3-2010[S]; MHPRC: Beijing, China, 2011.
- Generally Administration of Quality Supervision (GAQS). Determination of Fat Acidity of Cereal and Cereal Products; GB/T 29405-2012[S]; GAQS: Beijing, China, 2012.
- Generally Administration of Quality Supervision (GAQS). General Pasting Method for Wheat or Rye Flour or Starch-Using the Rapid Visco Analyzer; GB/T 24853-2010[S]; GAQS: Beijing, China, 2012.

| Sample | Irradiation Dose/kGy | L* | a* | b* |
|---|---|---|---|---|
| Corn kernel | 0 | 89.23 ± 0.72 aa’ | 2.82 ± 0.01 aa’ | 21.75 ± 0.22 aa’ |
| 5 | 89.25 ± 0.09 a | 2.46 ± 0.11 b | 20.44 ± 0.03 b | |
| 10 | 89.90 ± 0.13 a | 2.12 ± 0.08 c | 20.40 ± 0.08 b | |
| 30 | 90.12 ± 0.50 a | 2.08 ± 0.01 d | 19.42 ± 0.16 b | |
| 50 | 89.94 ± 0.05 a | 2.17 ± 0.02 c | 18.70 ± 0.01 c | |
| Corn flour | 5 | 88.73 ± 0.64 a’ | 2.60 ± 0.01 b’ | 21.24 ± 0.20 b’ |
| 10 | 88.76 ± 0.42 a’ | 2.51 ± 0.02 c’ | 20.57 ± 0.42 c’ | |
| 30 | 89.37 ± 0.51 a’ | 1.81 ± 0.02 d’ | 18.70 ± 0.04 d’ | |
| 50 | 90.00 ± 0.61 a’ | 1.57 ± 0.01 e’ | 17.94 ± 0.05 e’ |
| Sample | Irradiation Dose (kGy) | Peak Viscosity (cp) | Trough Viscosity (cp) | Breakdown Viscosity (cp) | Final Viscosity (cp) | Setback Viscosity (cp) |
|---|---|---|---|---|---|---|
| Corn kernel | 0 | 1290 ± 35 aa’ | 673 ± 22 aa’ | 617 ± 32 aa’ | 1939 ± 34 aa’ | 1266 ± 34 aa’ |
| 5 | 1026 ± 28 b | 303 ± 21 b | 723 ± 46 b | 592 ± 41 b | 289 ± 36 b | |
| 10 | 352 ± 21 c | 72 ± 3 c | 281 ± 3 c | 117 ± 4 c | 46 ± 3 c | |
| 30 | 77 ± 2 d | 35 ± 1 d | 42 ± 3 d | 57 ± 1 d | 22 ± 1 d | |
| 50 | 25 ± 1 e | 12 ± 2 e | 13 ± 1 e | 24 ± 1 e | 12 ± 1 e | |
| Corn flour | 5 | 728 ± 38 b’ | 222 ± 21 b’ | 506 ± 43 b’ | 668 ± 27 b’ | 446 ± 42 b’ |
| 10 | 278 ± 46 c’ | 58 ± 2 c’ | 220 ± 22 c’ | 135 ± 25 c’ | 77 ± 12 c’ | |
| 30 | 101 ± 4 d’ | 34 ± 4 d’ | 67 ± 1 d’ | 52 ± 1 d’ | 18 ± 1 d’ | |
| 50 | 35 ± 2 e’ | 18 ± 3 e’ | 17 ± 2 e’ | 28 ± 1 e’ | 10 ± 1 e’ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Qi, L.; Liu, Y.; Wang, R.; Yang, D.; Li, K.; Wang, L.; Li, Y.; Zhang, Y.; Chen, Z. Effects of Electron Beam Irradiation on Zearalenone and Ochratoxin A in Naturally Contaminated Corn and Corn Quality Parameters. Toxins 2017, 9, 84. https://doi.org/10.3390/toxins9030084
Luo X, Qi L, Liu Y, Wang R, Yang D, Li K, Wang L, Li Y, Zhang Y, Chen Z. Effects of Electron Beam Irradiation on Zearalenone and Ochratoxin A in Naturally Contaminated Corn and Corn Quality Parameters. Toxins. 2017; 9(3):84. https://doi.org/10.3390/toxins9030084
Chicago/Turabian StyleLuo, Xiaohu, Lijun Qi, Yuntao Liu, Ren Wang, Dan Yang, Ke Li, Li Wang, Yanan Li, Yuwei Zhang, and Zhengxing Chen. 2017. "Effects of Electron Beam Irradiation on Zearalenone and Ochratoxin A in Naturally Contaminated Corn and Corn Quality Parameters" Toxins 9, no. 3: 84. https://doi.org/10.3390/toxins9030084






