A Pharmacological Examination of the Cardiovascular Effects of Malayan Krait (Bungarus candidus) Venoms
Abstract
:1. Introduction
2. Results
2.1. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.2. Anaesthetised Rats
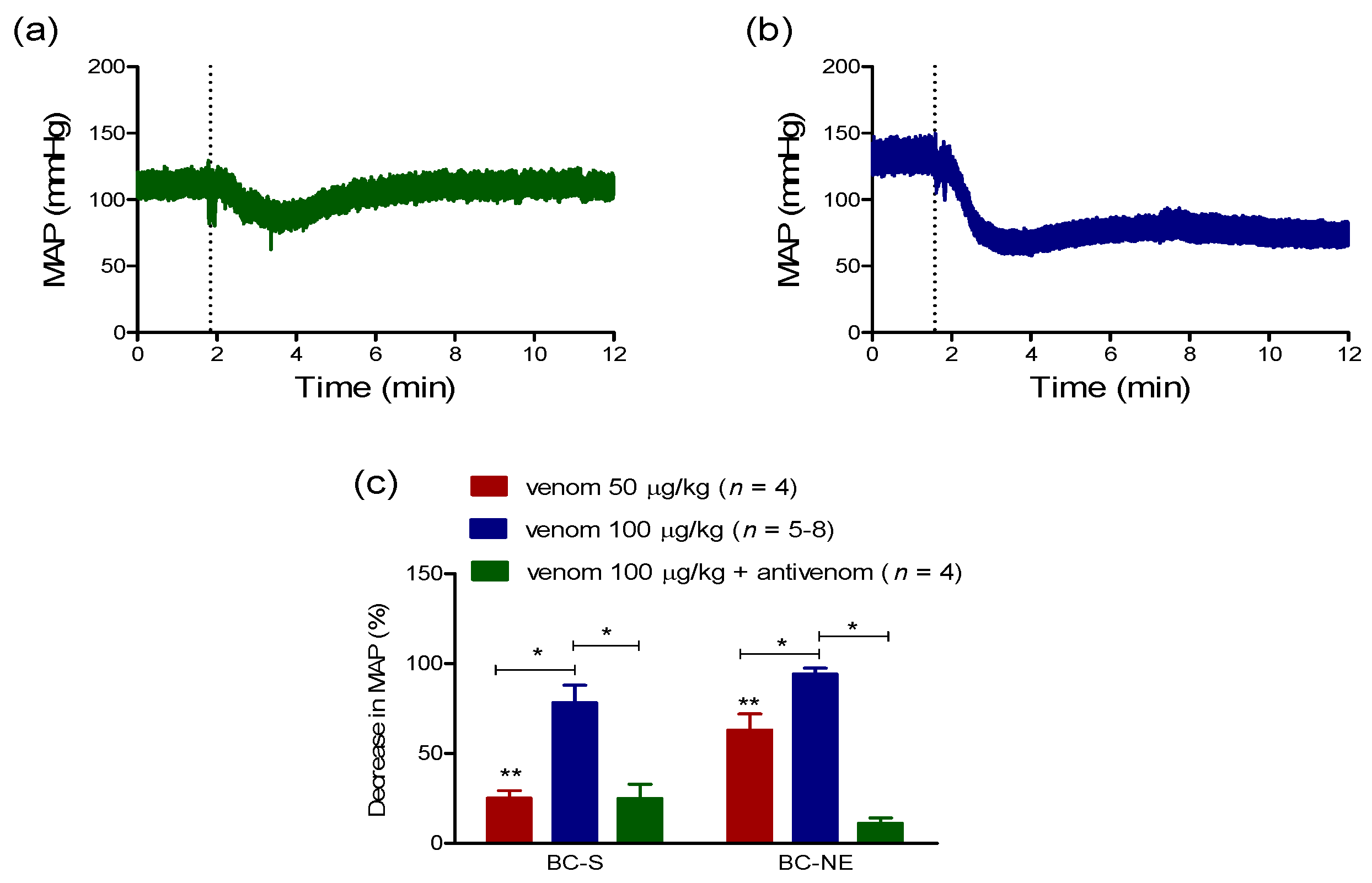
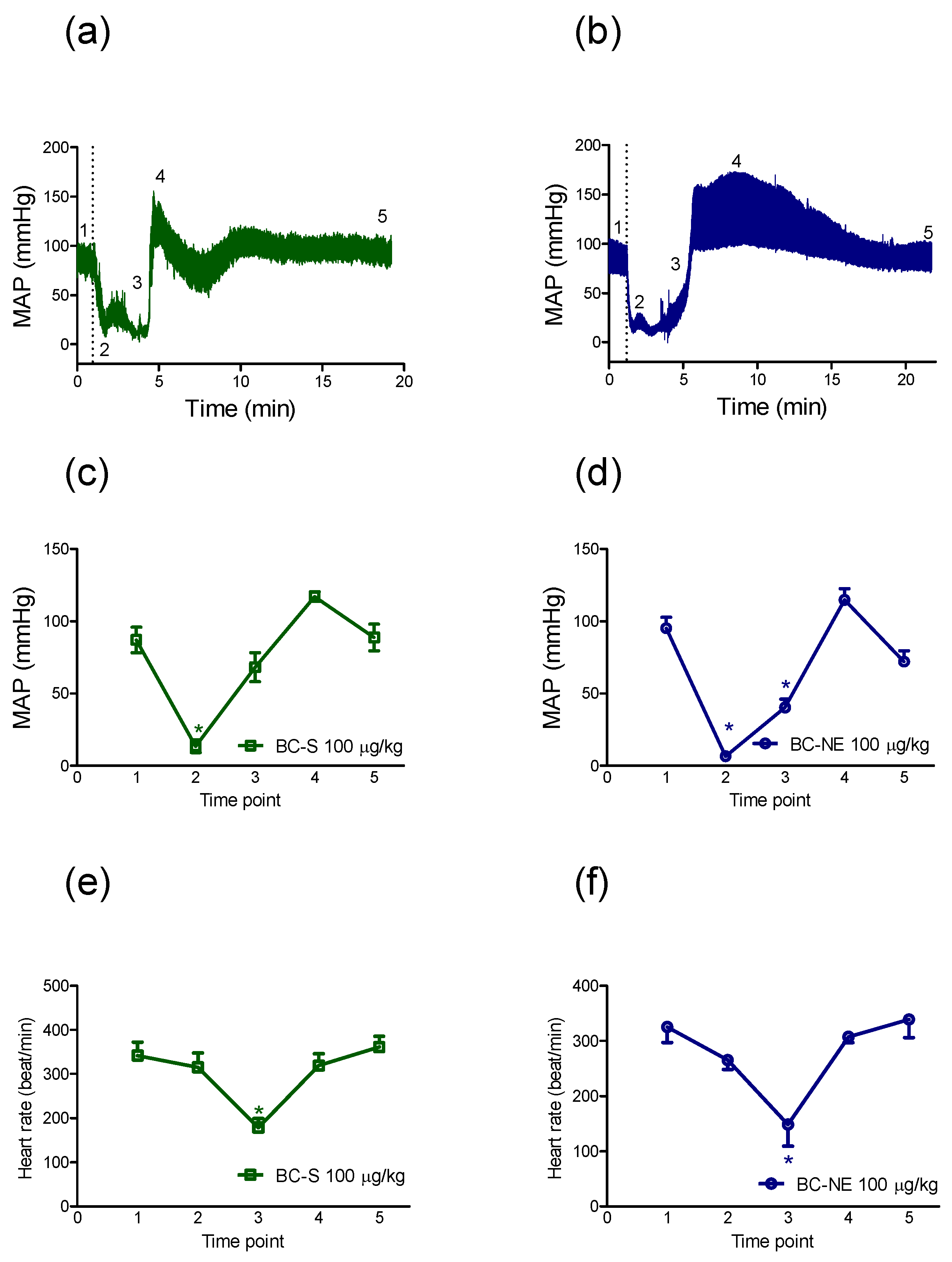
2.2.1. Hypotensive Effect of B. candidus Venoms
2.2.2. The Recovery in MAP Following Hypotensive Effect of B. candidus Venoms
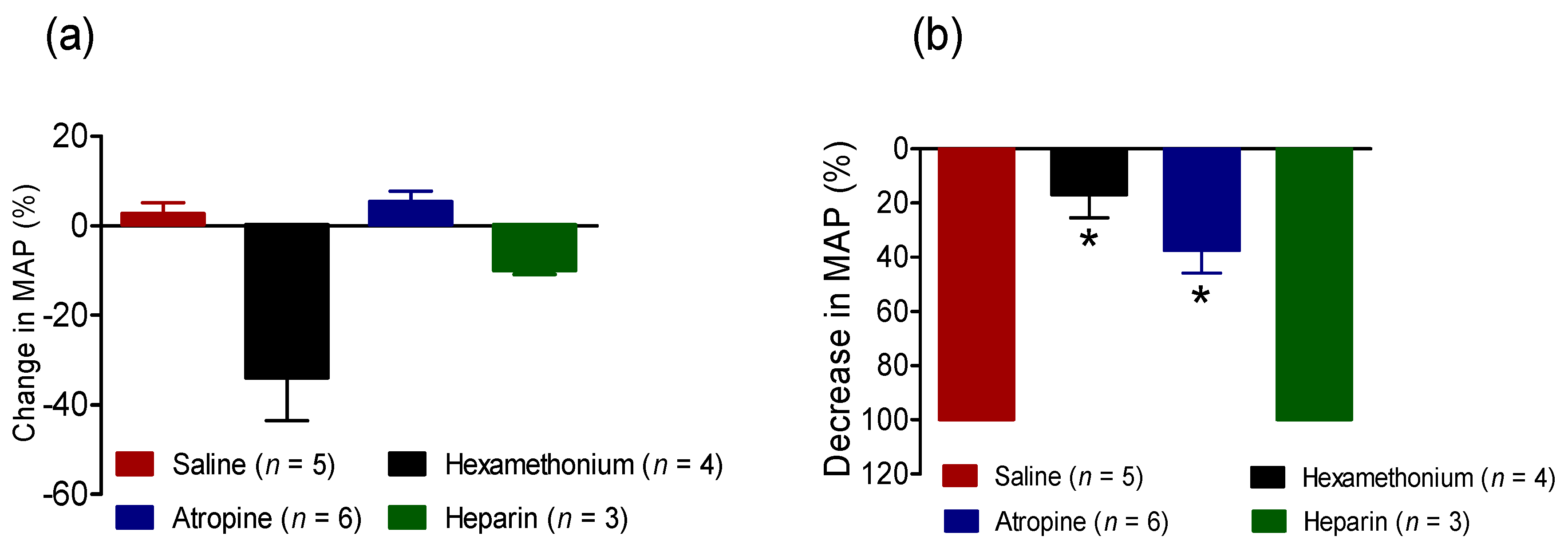
2.2.3. Effect of BC-NE Venom on MAP in the Presence of Receptor Antagonists
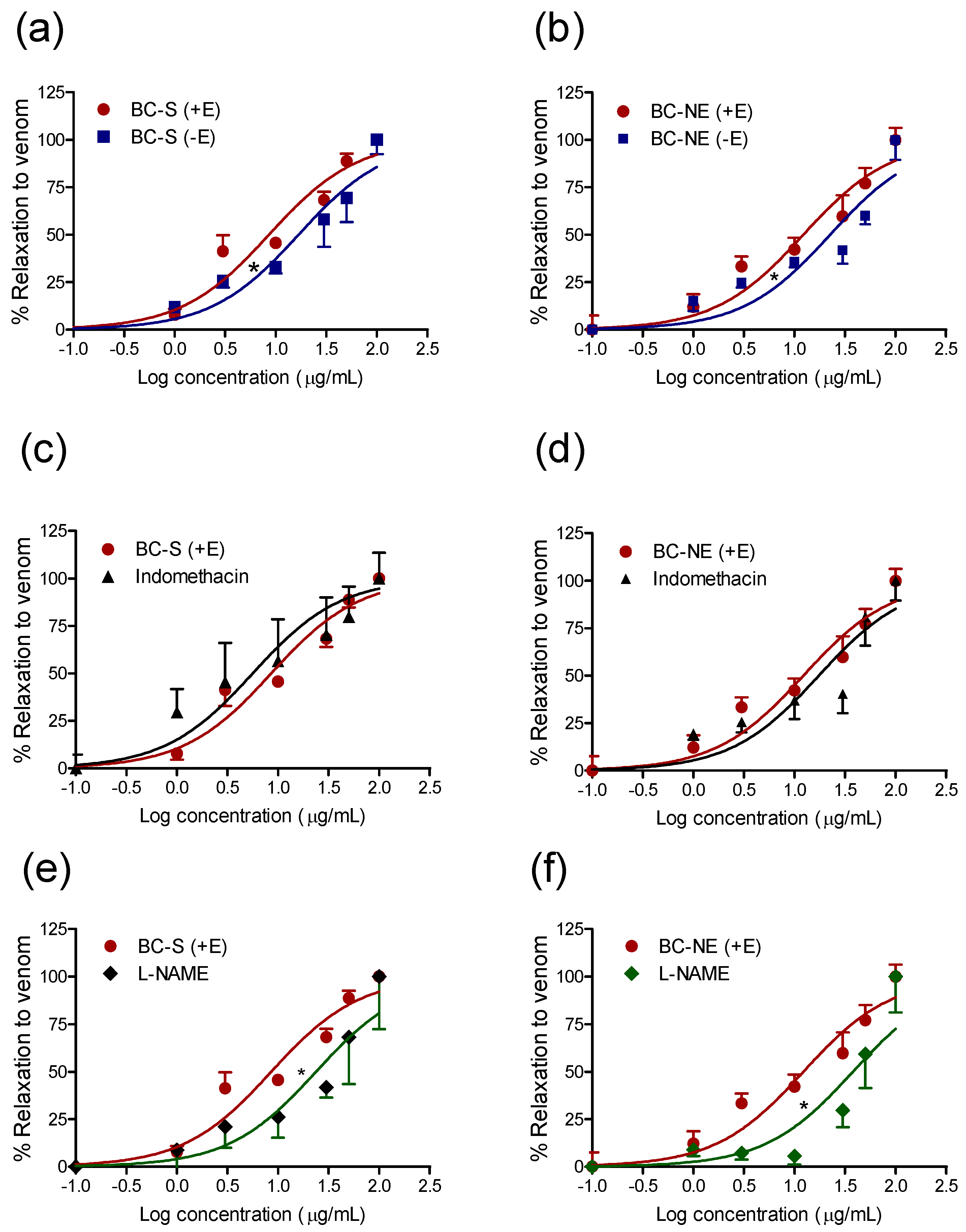
2.3. Effect of B. candidus Venoms on Rat Aortic Rings
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venom Preparation and Storage
5.2. Protein Concentration Determination
5.3. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
5.4. Anaesthetised Rat Preparation
5.5. Isolation and Study of Rat Aortic Ring
5.6. Chemical and Drugs
5.7. Data Analysis and Statistics
5.8. Animal Ethics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.J.; Buckley, N.A.; Johnston, C.; Siribaddana, S.; Isbister, G.K. Neuromuscular effects of common krait (Bungarus caeruleus) envenoming in Sri Lanka. PLoS Negl. Trop. Dis. 2016. [Google Scholar] [CrossRef]
- Trinh, K.X.; Khac, Q.L.; Trinh, L.X.; Warrell, D.A. Hyponatraemia, rhabdomyolysis, alterations in blood pressure and persistent mydriasis in patients envenomed by Malayan kraits (Bungarus candidus) in southern Vietnam. Toxicon 2010, 56, 1070–1075. [Google Scholar] [PubMed]
- Viravan, C.; Looareesuwan, S.; Kosakarn, W.; Wuthiekanun, V.; McCarthy, C.J.; Stimson, A.F.; Bunnag, D.; Harinasuta, T.; Warrell, D.A. A national hospital-based survey of snakes responsible for bites in Thailand. Trans. R. Society. Trop. Med. Hyg. 1992, 86, 100–106. [Google Scholar] [CrossRef]
- Chanhome, L.; Cox, M.J.; Vasaruchapong, T.; Chaiyabutr, N.; Sitprija, V. Characterization of venomous snakes of Thailand. Asian Biomed. 2011, 5, 311–328. [Google Scholar]
- WHO. Venomous snakes of the south-east asia region, their venoms and pathophysiology of human envenoming. In Guidelines for the Management of Snake-Bites, 2nd ed.; WHO: Geneva, Switzerland, 2016; Volume 2. [Google Scholar]
- Khow, O.; Chanhome, L.; Omori-Satoh, T.; Ogawa, Y.; Yanoshita, R.; Samejima, Y.; Kuch, U.; Mebs, D.; Sitprija, V. Isolation, toxicity and amino terminal sequences of three major neurotoxins in the venom of Malayan krait (Bungarus candidus) from Thailand. J. Biochem. 2003, 134, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Rusmili, M.R.; Yee, T.T.; Mustafa, M.R.; Hodgson, W.C.; Othman, I. Proteomic characterization and comparison of Malaysian Bungarus candidus and Bungarus fasciatus venoms. J. Proteom. 2014, 110, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Pilapitiya, S.; Siribaddana, S. Acute myocardial infarction following a possible direct intravenous bite of Russell's viper (Daboia russelli). BMC Res. Notes 2012. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Isbister, G.K.; Kuruppu, S.; Konstantakopoulos, N.; Hodgson, W.C. An examination of cardiovascular collapse induced by eastern brown snake (Pseudonaja textilis) venom. Toxicol. Lett. 2013, 221, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J.; Sutherland, S.K.; Rivera, R.A.; Masci, P.P. The cardiovascular and haematological effects of purified prothrombin activator from the common brown snake (Pseudonaja textilis) and their antagonism with heparin. Anaesth. Intensiv. Care 1992, 20, 28–32. [Google Scholar]
- Chaisakul, J.; Isbister, G.K.; Konstantakopoulos, N.; Tare, M.; Parkington, H.C.; Hodgson, W.C. In vivo and in vitro cardiovascular effects of Papuan taipan (Oxyuranus scutellatus) venom: Exploring "sudden collapse". Toxicol. Lett. 2012, 213, 243–248. [Google Scholar] [PubMed]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Aggarwal, A.N.; Gupta, D. Elapid snakebite as a cause of severe hypertension. J. Emerg. Med. 2006, 30, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Laothong, C.; Sitprija, V. Decreased parasympathetic activities in Malayan krait (Bungarus candidus) envenoming. Toxicon 2001, 39, 1353–1357. [Google Scholar] [CrossRef]
- Joseph, R.; Pahari, S.; Hodgson, W.C.; Kini, R.M. Hypotensive agents from snake venoms. Current. Drug Targets. Cardiovasc. Haematol. Disord. 2004, 4, 437–459. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Winter, K.L.; Isbister, G.K.; McGowan, S.; Konstantakopoulos, N.; Seymour, J.E.; Hodgson, W.C. A pharmacological and biochemical examination of the geographical variation of Chironex fleckeri venom. Toxicol. Lett. 2010, 192, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Rusmili, M.R.; Yee, T.T.; Mustafa, M.R.; Othman, I.; Hodgson, W.C. In-vitro neurotoxicity of two Malaysian krait species (Bungarus candidus and Bungarus fasciatus) venoms: Neutralization by monovalent and polyvalent antivenoms from Thailand. Toxins 2014, 6, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Chanhome, L.; Sitprija, V.; Chaiyabutr, N. Effect of Bungarus candidus (Malayan krait) venom on general circulation and renal hemodynamics in experimental animals. Asian Biomed. 2010, 4, 421–428. [Google Scholar]
- Siang, A.S.; Doley, R.; Vonk, F.J.; Kini, R.M. Transcriptomic analysis of the venom gland of the red-headed krait (Bungarus flaviceps) using expressed sequence tags. BMC Mol. Biol. 2010, 11, 24. [Google Scholar]
- Kim, E.; Lee, S.; Kim, J.S.; Yoon, W.D.; Lim, D.; Hart, A.J.; Hodgson, W.C. Cardiovascular effects of Nemopilema nomurai (scyphozoa: Rhizostomeae) jellyfish venom in rats. Toxicol. Lett. 2006, 167, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.J.; Isbister, G.K.; O'Donnell, P.; Williamson, N.A.; Hodgson, W.C. Species differences in the neuromuscular activity of post-synaptic neurotoxins from two Australian black snakes (Pseudechis porphyriacus and Pseudechis colletti). Toxicol. Lett. 2013, 219, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hanamoto, H.; Niwa, H.; Sugimura, M.; Morimoto, Y. Autonomic and cardiovascular effects of pentobarbital anesthesia during trigeminal stimulation in cats. Int. J. Oral Sci. 2012, 4, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Inase, N.; Schreck, R.E.; Lazarus, S.C. Heparin inhibits histamine release from canine mast cells. Am. J. Physiol. 1993, 264, L387–L390. [Google Scholar] [PubMed]
- Johnston, C.I.; Ryan, N.M.; O’Leary, M.A.; Brown, S.G.; Isbister, G.K. Australian taipan (Oxyuranus spp.) envenoming: Clinical effects and potential benefits of early antivenom therapy - Australian snakebite project (asp-25). Clin. Toxicol. (Phila) 2017, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Mohamed, M.; Sulaiman, S.A.; Wan Ahmad, W.A. Autonomic nervous system mediates the hypotensive effects of aqueous and residual methanolic extracts of Syzygium polyanthum (wight) walp. Var. Polyanthum leaves in anaesthetized rats. Evidence.-Based Complement. Altern. Med.: ECAM 2013. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J.; Sutherland, S.K. The efficacy of heparin in the treatment of common brown snake (Pseudonaja textilis) envenomation. Anaesth. Intensiv. Care 1992, 20, 33–37. [Google Scholar]
- Kamkaew, N.; Scholfield, C.N.; Ingkaninan, K.; Maneesai, P.; Parkington, H.C.; Tare, M.; Chootip, K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J. Ethnopharmacol. 2011, 137, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Crachi, M.T.; Hammer, L.W.; Hodgson, W.C. A pharmacological examination of venom from the Papuan taipan (Oxyuranus scutellatus canni). Toxicon 1999, 37, 1721–1734. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaisakul, J.; Rusmili, M.R.A.; Hodgson, W.C.; Hatthachote, P.; Suwan, K.; Inchan, A.; Chanhome, L.; Othman, I.; Chootip, K. A Pharmacological Examination of the Cardiovascular Effects of Malayan Krait (Bungarus candidus) Venoms. Toxins 2017, 9, 122. https://doi.org/10.3390/toxins9040122
Chaisakul J, Rusmili MRA, Hodgson WC, Hatthachote P, Suwan K, Inchan A, Chanhome L, Othman I, Chootip K. A Pharmacological Examination of the Cardiovascular Effects of Malayan Krait (Bungarus candidus) Venoms. Toxins. 2017; 9(4):122. https://doi.org/10.3390/toxins9040122
Chicago/Turabian StyleChaisakul, Janeyuth, Muhamad Rusdi Ahmad Rusmili, Wayne C. Hodgson, Panadda Hatthachote, Kijja Suwan, Anjaree Inchan, Lawan Chanhome, Iekhsan Othman, and Krongkarn Chootip. 2017. "A Pharmacological Examination of the Cardiovascular Effects of Malayan Krait (Bungarus candidus) Venoms" Toxins 9, no. 4: 122. https://doi.org/10.3390/toxins9040122






