Mycotoxin Decontamination of Food: Cold Atmospheric Pressure Plasma versus “Classic” Decontamination
Abstract
:1. Introduction
2. The Background of CAP Decontamination in Agriculture and Food Industry
3. The Comparison of “Classic” Approaches and CAP Technology in the Field of Mycotoxin Decontamination
4. Conclusions
- -
- Draw firm correlations between different plasma operating parameters and the specific reactive chemical species formed.
- -
- Draw correlations between the composition of the plasma and the structure of the mycotoxin degradation products. As toxicities of the mycotoxin degradation products can be experimentally determined, in this way, the mycotoxin decontamination efficiency would be defined as well.
- -
- Examine the effects of different plasma treatments on the quality of food products, for example on their nutritional value and organoleptic qualities.
- -
- Design plasma-forming systems for efficient mycotoxin decontamination of various types and sizes of food products.
- -
- Test if hybrid plasma-conventional systems for mycotoxin decontamination of food products can be even more effective.
Acknowledgments
Conflicts of Interest
References
- Betina, V. Mycotoxins: Production, Isolation, Separation and Purification; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Vasanthi, S.; Bhat, R.V. Mycotoxins in foods-occurrence, health & economic significance & food control measures. Indian J. Med. Res. 1998, 108, 212–224. [Google Scholar] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Sforza, S.; Dall’Asta, C.; Marchelli, R. Recent advances in mycotoxin determination in food and feed by hyphenated chromatographic techniques/mass spectrometry. Mass Spectrom. Rev. 2006, 25, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (ec) no 1881/2006 of 19 december 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- European Commission. Recommendation 2006/576/ec of 17 august 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- European Commission. Recommendation 2013/165/EU of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 91, 12–15. [Google Scholar]
- Edgewood Parents and Teachers. Directive 2002/32/EC of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Communities 2002, 140, 10–21. [Google Scholar]
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Environmental and developmental factors influencing aflatoxin production by Aspergillus flavus and Aspergillus parasiticus. Mycoscience 2007, 48, 71–80. [Google Scholar] [CrossRef]
- Mousa, W.; Ghazali, F.M.; Jinap, S.; Ghazali, H.M.; Radu, S. Modeling growth rate and assessing aflatoxins production by Aspergillus flavus as a function of water activity and temperature on polished and brown rice. J. Food Sci. 2013, 78, M56–M63. [Google Scholar] [CrossRef] [PubMed]
- Narasaiah, K.V.; Sashidhar, R.; Subramanyam, C. Biochemical analysis of oxidative stress in the production of aflatoxin and its precursor intermediates. Mycopathologia 2006, 162, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Nellemann, C. The Environmental Food Crisis: The Environment's Role in Averting Future Food Crises: A Unep Rapid Response Assessment; UNEP/Earthprint: Nairobi, Kenya, 2009. [Google Scholar]
- Fridman, A.; Kennedy, L.A. Plasma Physics and Engineering; CRC press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kogelschatz, U. Atmospheric-pressure plasma technology. Plasma Phys. Control. Fusion 2004, 46, B63. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons: Chichester, UK, 2005. [Google Scholar]
- Hasan, M.I.; Walsh, J.L. Numerical investigation of the spatiotemporal distribution of chemical species in an atmospheric surface barrier-discharge. J. Appl. Phys. 2016, 119, 203302. [Google Scholar] [CrossRef]
- Locke, B.R.; Lukes, P.; Brisset, J.-L. Elementary chemical and physical phenomena in electrical discharge plasma in gas–liquid environments and in liquids. In Plasma Chemistry and Catalysis in Gases and Liquids; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 185–241. [Google Scholar]
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Misra, N.N. The contribution of non-thermal and advanced oxidation technologies towards dissipation of pesticide residues. Trends Food Sci. Technol. 2015, 45, 229–244. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Laroussi, M.; Kong, M.; Morfill, G. Plasma medicine: Applications of low-temperature gas plasmas in medicine and biology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Takatori, K.; Sugita-Konishi, Y.; Kim, I.H.; Lee, M.H.; Han, D.W.; Chung, K.H.; Hyun, S.O.; Park, J.C. Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Technol. 2007, 201, 5733–5737. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Huang, G.-Q.; Li, Y.-P.; Xiao, J.-X.; Zhang, Y.; Jiang, W.-L. Degradation of aflatoxin B1 by low-temperature radio frequency plasma and degradation product elucidation. Eur. Food Res. Technol. 2015, 241, 103–113. [Google Scholar] [CrossRef]
- Siciliano, I.; Spadaro, D.; Prelle, A.; Vallauri, D.; Cavallero, M.C.; Garibaldi, A.; Gullino, M.L. Use of cold atmospheric plasma to detoxify hazelnuts from aflatoxins. Toxins 2016, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control 2016, 70, 1–8. [Google Scholar] [CrossRef]
- Liang, J.-L.; Zheng, S.-H.; Ye, S.-Y. Inactivation of Penicillium aerosols by atmospheric positive corona discharge processing. J. Aerosol Sci. 2012, 54, 103–112. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [PubMed]
- Suhem, K.; Matan, N.; Nisoa, M.; Matan, N. Inhibition of Aspergillus flavus on agar media and brown rice cereal bars using cold atmospheric plasma treatment. Int. J. Food Microbiol. 2013, 161, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.-Y.; Song, X.-L.; Liang, J.-L.; Zheng, S.-H.; Lin, Y. Disinfection of airborne spores of Penicillium expansum in cold storage using continuous direct current corona discharge. Biosyst. Eng. 2012, 113, 112–119. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.-D. Application of cold oxygen plasma for the reduction of Cladosporium cladosporioides and Penicillium citrinum on the surface of dried filefish (Stephanolepis cirrhifer) fillets. Int. J. Food Sci. Technol. 2015, 50, 966–973. [Google Scholar] [CrossRef]
- Basaran, P.; Basaran-Akgul, N.; Oksuz, L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Patil, S.; Moiseev, T.; Bourke, P.; Mosnier, J.P.; Keener, K.M.; Cullen, P.J. In-package atmospheric pressure cold plasma treatment of strawberries. J. Food Eng. 2014, 125, 131–138. [Google Scholar] [CrossRef]
- Heo, N.S.; Lee, M.-K.; Kim, G.W.; Lee, S.J.; Park, J.Y.; Park, T.J. Microbial inactivation and pesticide removal by remote exposure of atmospheric air plasma in confined environments. J. Biosci. Bioeng. 2014, 117, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Nian, W.; Wu, H.; Feng, H.; Zhang, K.; Zhang, J.; Zhu, W.; Becker, K.; Fang, J. Atmospheric-pressure cold plasma treatment of contaminated fresh fruit and vegetable slices: Inactivation and physiochemical properties evaluation. Eur. Phys. J. D-Atomic Mol. Opt. Plasma Phys. 2012, 66, 1–7. [Google Scholar] [CrossRef]
- Edelblute, C.M.; Malik, M.A.; Heller, L.C. Antibacterial efficacy of a novel plasma reactor without an applied gas flow against methicillin resistant Staphylococcus aureus on diverse surfaces. Bioelectrochemistry 2016, 112, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Min, S.C.; Roh, S.H.; Niemira, B.A.; Sites, J.E.; Boyd, G.; Lacombe, A. Dielectric barrier discharge atmospheric cold plasma inhibits Escherichia coli o157:H7, Salmonella, Listeria monocytogenes, and tulane virus in romaine lettuce. Int. J. Food Microbiol. 2016, 237, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Lynch, M.; Modic, M.; Whalley, R.; Walsh, J. A solar powered handheld plasma source for microbial decontamination applications. J. Phys. D: Appl. Phys. 2016, 49, 355203. [Google Scholar] [CrossRef]
- Doubla, A.; Laminsi, S.; Nzali, S.; Njoyim, E.; Kamsu-Kom, J.; Brisset, J.L. Organic pollutants abatement and biodecontamination of brewery effluents by a non-thermal quenched plasma at atmospheric pressure. Chemosphere 2007, 69, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Hu, Z.; Cao, P.; Zhao, H.J. Decontamination of 2-chloroethyl ethyl sulfide by pulsed corona plasma. Plasma Sci. Technol. 2014, 16, 1054–1058. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Lukes, P.; Brisset, J.-L.; Locke, B.R. Biological effects of electrical discharge plasma in water and in gas–liquid environments. In Plasma Chemistry and Catalysis in Gases and Liquids; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 309–352. [Google Scholar]
- Machala, Z.; Chládeková, L.; Pelach, M. Plasma agents in bio-decontamination by DC discharges in atmospheric air. J. Phys. D: Appl. Phys. 2010, 43, 222001. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar]
- Karaca, H.; Velioglu, Y.S.; Nas, S. Mycotoxins: Contamination of dried fruits and degradation by ozone. Toxin Rev. 2010, 29, 51–59. [Google Scholar] [CrossRef]
- Laurita, R.; Barbieri, D.; Gherardi, M.; Colombo, V.; Lukes, P. Chemical analysis of reactive species and antimicrobial activity of water treated by nanosecond pulsed DBD air plasma. Clin. Plasma Med. 2015, 3, 53–61. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol.-Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar]
- Naitali, M.; Herry, J.M.; Hnatiuc, E.; Kamgang, G.; Brisset, J.L. Kinetics and bacterial inactivation induced by peroxynitrite in electric discharges in air. Plasma Chem. Plasma Process. 2012, 32, 675–692. [Google Scholar] [CrossRef]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A.; Gutsol, A. Nonthermal plasma as a novel food processing technology. Nonthermal Process. Technol. Food 2011, 272–288. [Google Scholar]
- Chirokov, A.; Gutsol, A.; Fridman, A. Atmospheric pressure plasma of dielectric barrier discharges. Pure Appl. Chem. 2005, 77, 487–495. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X. Room-temperature atmospheric pressure plasma plume for biomedical applications. Appl. Phys. Lett. 2005, 87, 113902. [Google Scholar] [CrossRef]
- Smet, C.; Noriega, E.; Rosier, F.; Walsh, J.; Valdramidis, V.; Van Impe, J. Influence of food intrinsic factors on the inactivation efficacy of cold atmospheric plasma: Impact of osmotic stress, suboptimal pH and food structure. Innovative Food Sci. Emerg. Technol. 2016, 38, 393–406. [Google Scholar] [CrossRef]
- Smet, C.; Noriega, E.; Rosier, F.; Walsh, J.; Valdramidis, V.; Van Impe, J. Impact of food model (micro) structure on the microbial inactivation efficacy of cold atmospheric plasma. Int. J. Food Microbiol. 2017, 240, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Ohta, T.; Hori, M. Plasma agriculture. J. Korean Phys. Soc. 2012, 60, 937–943. [Google Scholar] [CrossRef]
- Zahoranova, A.; Henselova, M.; Hudecova, D.; Kalinakova, B.; Kovacik, D.; Medvecka, V.; Cernak, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Pignata, C.; D’Angelo, D.; Basso, D.; Cavallero, M.C.; Beneventi, S.; Tartaro, D.; Meineri, V.; Gilli, G. Low-temperature, low-pressure gas plasma application on Aspergillus brasiliensis, Escherichia coli and pistachios. J. Appl. Microbiol. 2014, 116, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.; Kholif, A. Mycotoxins in animal feeds and prevention strategies: A review. Asian J. Anim. Sci. 2010, 4, 113–131. [Google Scholar] [CrossRef]
- Beaver, R.W. Decontamination of mycotoxin-containing foods and feedstuffs. Trends Food Sci. Technol. 1991, 2, 170–173. [Google Scholar] [CrossRef]
- Varga, J.; Tóth, B. Novel strategies to control mycotoxins in feeds: A review. Acta Veterinaria Hungarica 2005, 53, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A.D. Biological strategies to counteract the effects of mycotoxins. J. Food Prot. 2009, 72, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Girish, C. Prevention and control of animal feed contamination by mycotoxins and reduction of their adverse effects in livestock. In Animal Feed Contamination; Wooodhead Publishing limited: Cambridge, UK, 2012. [Google Scholar]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other adsorbent materials. Food Chem. Toxicol. 2004, 42, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Meister, U.; Springer, M. Mycotoxins in cereals and cereal products—occurrence and changes during processing. J. Appl. Bot. Food Qual. 2004, 78, 168–173. [Google Scholar]
- Park, J.; Scott*, P.; Lau, B.-Y.; Lewis, D. Analysis of heat-processed corn foods for fumonisins and bound fumonisins. Food Addit. Contam. 2004, 21, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Lawrence, G. Stability and problems in recovery of fumonisins added to corn-based foods. J. AOAC Int. 1993, 77, 541–545. [Google Scholar]
- Calado, T.; Venâncio, A.; Abrunhosa, L. Irradiation for mold and mycotoxin control: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1049–1061. [Google Scholar] [CrossRef]
- Kottapalli, B.; Wolf-Hall, C.E.; Schwarz, P.; Schwarz, J.; Gillespie, J. Evaluation of hot water and electron beam irradiation for reducing Fusarium infection in malting barley. J. Food Prot. 2003, 66, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Jubeen, F.; Bhatti, I.A.; Khan, M.Z.; Zahoor-Ul, H.; Shahid, M. Effect of UVC irradiation on aflatoxins in ground nut (Arachis hypogea) and tree nuts (Juglans regia, Prunus duclus and Pistachio vera). J. Chem. Soc. Pak. 2012, 34, 1366–1374. [Google Scholar]
- Mao, J.; He, B.; Zhang, L.X.; Li, P.W.; Zhang, Q.; Ding, X.X.; Zhang, W. A structure identification and toxicity assessment of the degradation products of aflatoxin B1 in peanut oil under UV irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jin, Q.; Tao, G.; Shan, L.; Huang, J.; Liu, Y.; Wang, X.; Mao, W.; Wang, S. Photodegradation kinetics and byproducts identification of the aflatoxin B1 in aqueous medium by ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. J. Mass Spectrom. 2010, 45, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jin, Q.; Tao, G.; Shan, L.; Liu, Y.; Wang, X. LC–MS and UPLC–quadrupole time-of-flight MS for identification of photodegradation products of aflatoxin B1. Chromatographia 2012, 71, 107–112. [Google Scholar] [CrossRef]
- Zhu, Y.; Koutchma, T.; Warriner, K.; Zhou, T. Reduction of patulin in apple juice products by UV light of different wavelengths in the UVC range. J. Food Prot. 2014, 77, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Lescure, G.; Agoulon, A.; Svinareff, P.; Orange, N.; Feuilloley, M. Application of the pulsed light technology to mycotoxin degradation and inactivation. J. Appl.Toxicol. 2013, 33, 357–363. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.L.; Khir, R.; Wu, B.G.; Ma, H.L.; Zhao, L.M. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Grenier, B.; Loureiro-Bracarense, A.-P.; Leslie, J.F.; Oswald, I.P. Physical and chemical methods for mycotoxin decontamination in maize. Mycotoxin Reduct. Grain Chains 2014, 116–129. [Google Scholar]
- Hoogenboom, L.; Tulliez, J.; Gautier, J.-P.; Coker, R.; Melcion, J.-P.; Nagler, M.; Polman, T.H.; Delort-Laval, J. Absorption, distribution and excretion of aflatoxin-derived ammoniation products in lactating cows. Food Addit. Contam. 2001, 18, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Safamehr, A.; Mirhadi, S.A.; Shivazad, M.; Razzaghi-Abyaneh, M.; Afshar-Naderi, A. Evaluation of biochemical and production parameters of broiler chicks fed ammonia treated aflatoxin contaminated maize grains. Anim. Feed Sci. Technol. 2005, 122, 289–301. [Google Scholar] [CrossRef]
- Millán, T.F.; Martinez, Y.A. Efficacy and stability of ammoniation process as aflatoxin B1 decontamination technology in rice. Archivos Latinoamer. Nutr. 2003, 53, 287–292. [Google Scholar]
- Luo, X.; Wang, R.; Wang, L.; Li, Y.; Wang, Y.; Chen, Z. Detoxification of aflatoxin in corn flour by ozone. J. Sci. Food Agric. 2014, 94, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Diao, E.; Hou, H.; Chen, B.; Shan, C.; Dong, H. Ozonolysis efficiency and safety evaluation of aflatoxin B1 in peanuts. Food Chem. Toxicol. 2013, 55, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Y.P.; Luo, X.H.; Wang, R.; Li, Y.F.; Li, Y.N.; Shao, H.L.; Chen, Z.X. Effect of deoxynivalenol detoxification by ozone treatment in wheat grains. Food Control 2016, 66, 137–144. [Google Scholar] [CrossRef]
- Phillips, T.; Clement, B.; Kubena, L.; Harvey, R. Detection and detoxification of aflatoxins: Prevention of aflatoxicosis and aflatoxin residues with hydrated sodium calcium aluminosilicate. Vet. Hum. Toxicol. 1989, 32, 15–19. [Google Scholar]
- Huwig, A.; Freimund, S.; Käppeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Carson, M.S.; Smith, T.K. Role of bentonite in prevention of T-2 toxicosis in rats. J. Anim. Sci. 1983, 57, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghian, J.; Parsons, C.M.; Leeper, R.W.; Harrison, P.C.; Koelkebeck, K.W. Effect of sodium aluminosilicate on phosphorus utilization by chicks and laying hens. Poult. Sci. 1991, 70, 955–962. [Google Scholar] [CrossRef]
- Smith, T.K. Influence of dietary fiber, protein and zeolite on zearalenone toxicosis in rats and swine. J. Anim. Sci. 1980, 50, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; André, G.; Buléon, A.; Jeminet, G.; Canet, I.; François, J.; Bertin, G.; Jouany, J.-P. Comprehensive conformational study of key interactions involved in zearalenone complexation with β-D-glucans. Biomacromolecules 2004, 5, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Hathout, A.S.; Aly, S.E. Biological detoxification of mycotoxins: A review. Ann. Microbiol. 2014, 64, 905–919. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.; Reveron, I.; Doria, F.; Costantini, A.; De las Rivas, B.; Munoz, R.; Garcia-Moruno, E. Degradation of ochratoxin a by Brevibacterium species. J. Agric. Food Chem. 2011, 59, 10755–10760. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Sivaramakrishna, A.; Mehta, A. Degradation and detoxification of aflatoxin B1 by Pseudomonas putida. Int. Biodeterior. Biodegrad. 2014, 86, 202–209. [Google Scholar] [CrossRef]
- El-Deeb, B.A. Isolation and characterization of soil bacteria able to degrade zearalenone. J. Bot. 2005, 32, 3–30. [Google Scholar]
- Teniola, O.D.; Addo, P.A.; Brost, I.M.; Färber, P.; Jany, K.-D.; Alberts, J.F.; Van Zyl, W.H.; Steyn, P.S.; Holzapfel, W.H. Degradation of aflatoxin B1 by cell-free extracts of Rhodococcus erythropolis and Mycobacterium fluoranthenivorans sp. Nov. Dsm44556 t. Int. J. Food Microbiol. 2005, 105, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Möller, A.; Kunte, H.-J.; Koch, M.; Maul, R. Biotransformation of the mycotoxin zearalenone by fungi of the genera Rhizopus and Aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kusumaningtyas, E.; Widiastuti, R.; Maryam, R. Reduction of aflatoxin B1 in chicken feed by using Saccharomyces cerevisiae, Rhizopus oligosporus and their combination. Mycopathologia 2006, 162, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, H.K.; Suman, S.K.; Jha, G.N. Microbial degradation of aflatoxin in maize: A biocontrol approach for management of preharvest aflatoxin contamination. J. Mycol. Plant Pathol. 2011, 41, 408. [Google Scholar]
- Garda-Buffon, J.; Kupski, L.; Badiale-Furlong, E. Deoxynivalenol (DON) degradation and peroxidase enzyme activity in submerged fermentation. Food Sci. Technol. (Camp.) 2011, 31, 198–203. [Google Scholar] [CrossRef]
- Molnar, O.; Schatzmayr, G.; Fuchs, E.; Prillinger, H. Trichosporon mycotoxinivorans sp. nov., a new yeast species useful in biological detoxification of various mycotoxins. Syst. Appl. Microbiol. 2004, 27, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Sangsila, A.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Itsaranuwat, P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Control 2016, 62, 187–192. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Chapter 1 - plasma in food and agriculture. In Cold Plasma in Food and Agriculture; Academic Press: San Diego, CA, USA, 2016; pp. 1–16. [Google Scholar]
- Fellows, P.J. Food Processing Technology: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Stark, A.-A.; Gal, Y.; Shaulsky, G. Involvement of singlet oxygen in photoactivation of aflatoxins B1 and B2 to DNA-binding forms in vitro. Carcinogenesis 1990, 11, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.; Li, J.; Liu, D.; Walsh, J. Optimizing the electrical excitation of an atmospheric pressure plasma advanced oxidation process. J. Hazard. Mater. 2014, 279, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.; Marin, S.; Sanchis, V.; Ramos, A. Fate of mycotoxins in cereals during extrusion cooking: A review. Food Addit. Contam. 2005, 22, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hale, O.; Wilson, D. Performance of pigs on diets containing heated or unheated corn with or without aflatoxin. J. Anim. Sci. 1979, 48, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Petchkongkaew, A.; Taillandier, P.; Gasaluck, P.; Lebrihi, A. Isolation of Bacillus spp. from thai fermented soybean (Thua-nao): Screening for aflatoxin B1 and ochratoxin A detoxification. J. Appl. Microbiol. 2008, 104, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Morgavi, D.P.; Aboab, B.; Lemaire, M.; Boudra, H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria. J. Appl. Microbiol. 2009, 106, 977–985. [Google Scholar] [CrossRef] [PubMed]
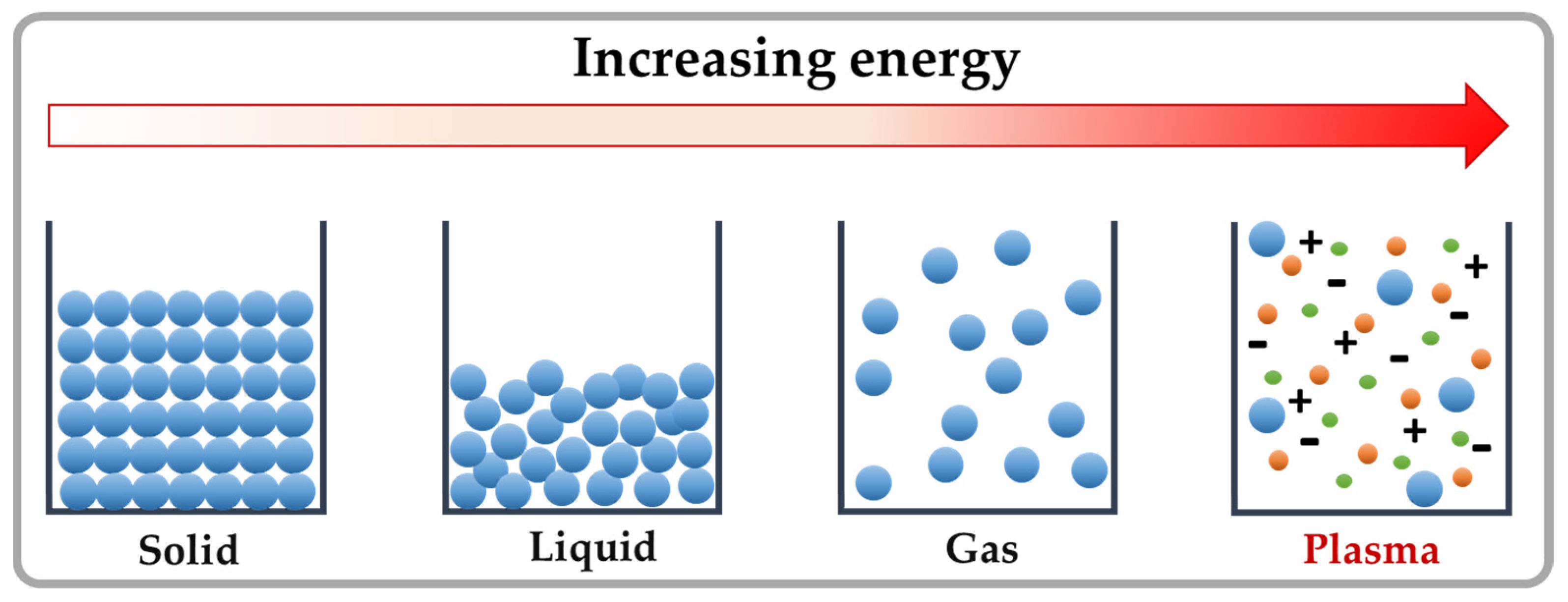


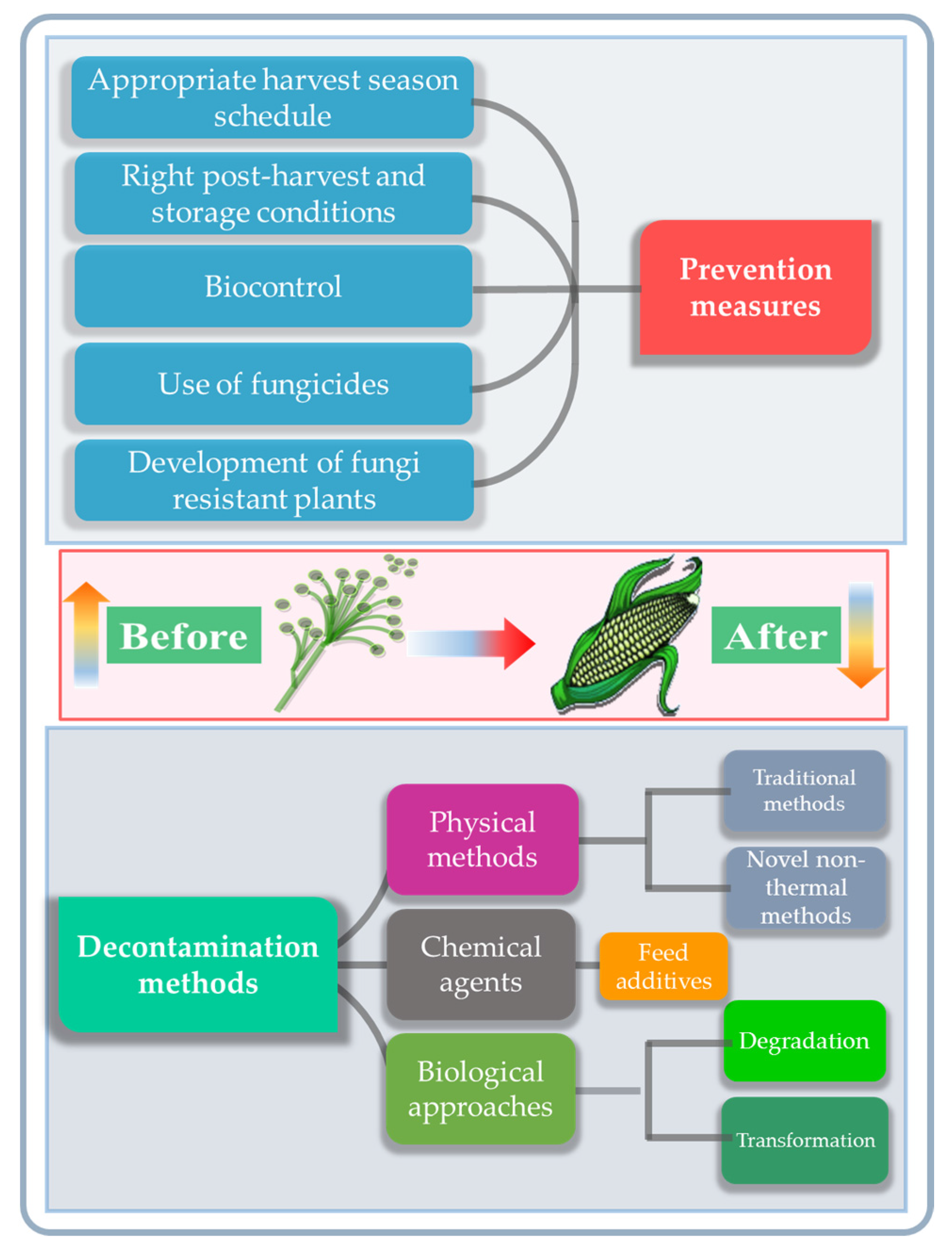

| Type | Representatives | Producing Fungi | Contaminated Foods | Structure Type | Toxicity |
|---|---|---|---|---|---|
| Aflatoxins (AF) | AFB1, AFB2, AFG1, AFG2, AFM1 | Aspergillus spp.: A. flavus A. parasiticus A. nomius A. bombycis A. pseudotamari A. ochraceoreus | Crops, cereals, seeds, nuts, spices | Difuranocoumarins | Carcinogenicity |
| Ochratoxins (OT) | OTA, OTB, OTC | Aspergillus spp. and Penicillium spp.: A. ochraceus A. aliaceus A. auricomus A. carbonarius A. glaucus A. meleus A. niger P. nordicum P. verrucosum | Crops, fruits, beer, wine, juices, coffee | Polyketide-derived dihydroisocoumarins bound to L-β-phenylalanin by amid bond | Nephrotoxicity, mutagenicy, carinogenicity |
| Fumonisins | Series A (FA), B (FB), C (FC) and P (FP) with FB being the most common representatives: FB1, FB2, FB3 | Fusarium spp.: F. verticillioides F. proliferatum F. Napiforme F. dlamini F. nygamai | Maize and its products | 1, 2, 3-propanetricar-boxylic acid | Cytotoxicity, carcinogenicity |
| Zearalenone (ZEN) | ZEN, a-zearalenol, b-zearalenol | Fusarium spp.: F. graminearum F. culmorum F. cerealis F. equiseti F. verticillioides F. incarnatum | Crops, cereals | 6-(10-Hydroxy-6-oxo-trans-1-undecenyl)-β-resorcylic acid lactone | Endocrine disruption |
| Trichothecenes | Deoxynivenol (DON), nivalenol (NIV), T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS) | Fusarium spp. Myrothecium spp. Phomopsis spp. Stachybotrys spp. Trychoderma spp. Trichotecium spp. Verticimonosporium spp. | Crops | Tetracyclic-12,13-epoxy trichothenes | Inhibition of eucaryotic DNA, RNA and protein synthesis; nausea, vomiting, diarrhea, weight loss and loss of appetite, skin inflammation, vomiting, liver damage |
| Ergot alkaloids (EAs) | Ergometrine, ergotamine, ergosine, ergocristine, ergocryptine, ergocornine and the corresponding –inine epimers | Claviceps spp.: C. purpurea | Grains, grass | Tetracyclic ergolines (tryptophan-derived alkaloids) | Neurotoxicity, endocrine disruption |
| Other mycotoxins | Fusaproliferin (FUS), enniatins (ENNs), beauvericin (BEA), moniliformin (MON), patulin (PAT) | Fusarium spp. Penicillium spp. Aspergillus spp. Eupenicillium spp. Paecilomyces spp. Byssochlamys spp. | Crops, fruits, vegetables, cereals | Sesterterpene cyclic hexadepsipeptides, 3-hydroxycyclobut-3-ene-1,2-dione, 4-hydroxy-4H-furo[3,2-c]pyran-2(6H)-one | Cytotoxicity, abnormal gluconeiogenesis, genotoxicity and mutagenicity |
| Decontamination Method | Degradation Products | Reference |
|---|---|---|
| UV | In aqueous solution:  | [75] |
In acetonitrile:  | [76] | |
In peanut oil:  | [74] | |
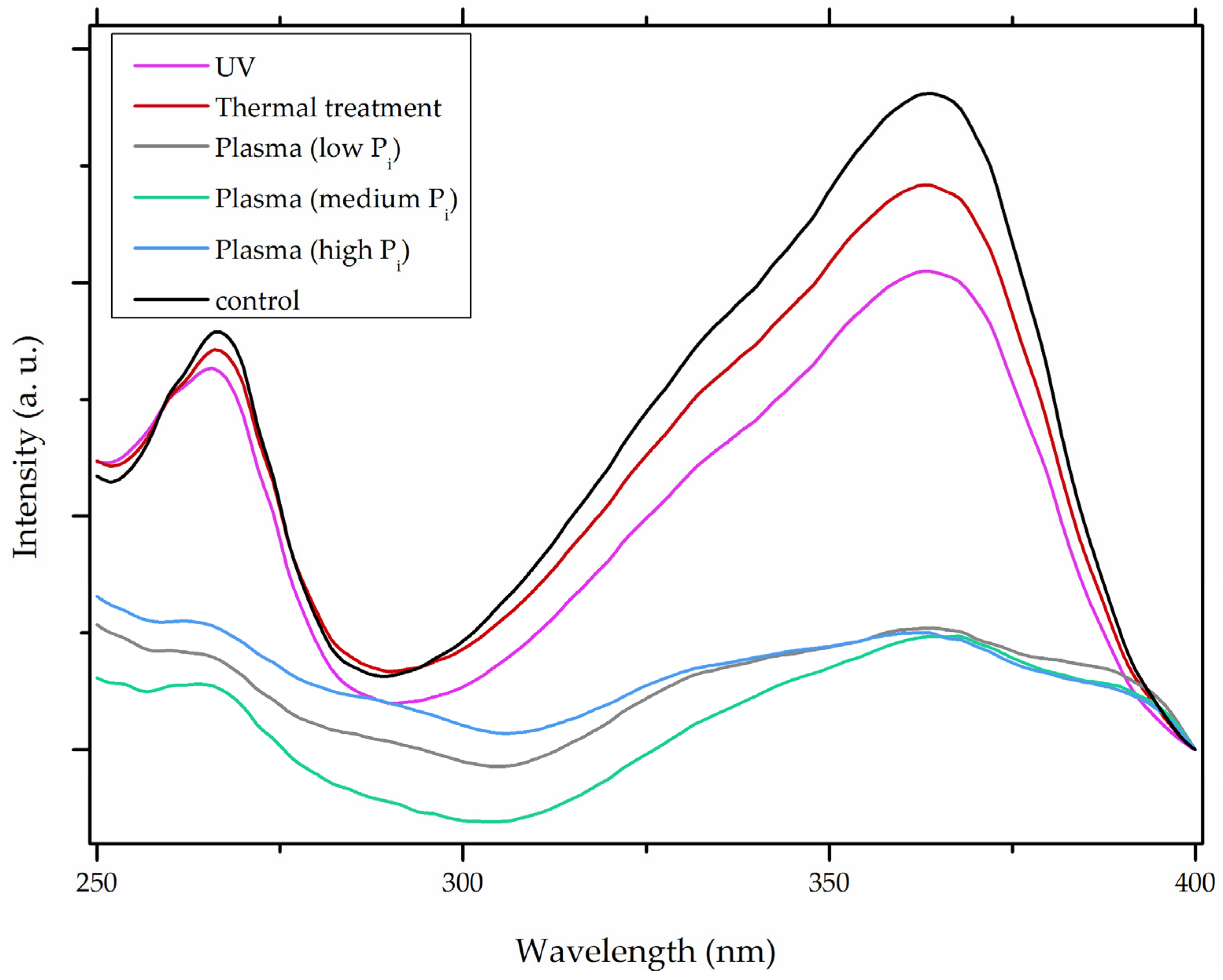
| Plasma |  | [26] |
 | ||
| Ozone | In acetonitrile:  | [85] |
 | ||
| Ammoniation |  | [81] |
| P. putida |  | [96] |
| Decontamination Method | Highest Decontamination Rate Obtained | Food Product | Process Duration | Energy Consumption | Impact on the Food Quality | Reference |
|---|---|---|---|---|---|---|
| Thermal treatment | 85–100% (FBs, ZEN, AFs) | Corn | Long | High | Significant | [70,109,110] |
| Gamma irradiation | 90% (mixture) | Grains, seeds | Short | Low | Significant | [71] |
| UV light irradiation | 90% (AFB1, PAT) | Peanut oil; apple juice; | Short | Low | Negligible | [74,77] |
| Pulsed light technology | 90% (AFB1) | Rice products | Short | Low | Negligible | [79] |
| Ammoniation | 90–100% (AFB1) | Rice | Long | High | Significant | [83] |
| Ozonation | 80% (AFs) | Corn flour, peanuts | Long | Low | Negligible | [84,85] |
| Bacillus spp. | 92.5% (OTA) | / | Long | Low | Negligible-significant | [111] |
| Rhodococcus erythropolis | 90% (AFB1) | / | Long | Low | Negligible | [98] |
| Aspergillus spp. | 100% (ZEN) | / | Long | Low | Negligible-significant | [99] |
| Trichosporon mycotoxinivorans | 100% (OTA) | Animal Feed | Long | Low | Negligible | [103] |
| Lactic acid bacteria | 80–100% (FBs) | / | Long | Low | Negligible | [112] |
| CAP technology | 100% (AFs, DON, NIV) | Seeds, crops, cereals | Short | Low | Negligible | [25,27] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin Decontamination of Food: Cold Atmospheric Pressure Plasma versus “Classic” Decontamination. Toxins 2017, 9, 151. https://doi.org/10.3390/toxins9050151
Hojnik N, Cvelbar U, Tavčar-Kalcher G, Walsh JL, Križaj I. Mycotoxin Decontamination of Food: Cold Atmospheric Pressure Plasma versus “Classic” Decontamination. Toxins. 2017; 9(5):151. https://doi.org/10.3390/toxins9050151
Chicago/Turabian StyleHojnik, Nataša, Uroš Cvelbar, Gabrijela Tavčar-Kalcher, James L. Walsh, and Igor Križaj. 2017. "Mycotoxin Decontamination of Food: Cold Atmospheric Pressure Plasma versus “Classic” Decontamination" Toxins 9, no. 5: 151. https://doi.org/10.3390/toxins9050151







