A Simple and Fast Procedure to Determine 3-Nitropropanoic Acid and 3-Nitropropanol in Freeze Dried Canadian Milkvetch (Astragalus canadensis)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of 3-NPA and 3-NPOH from Canadian Milkvetch
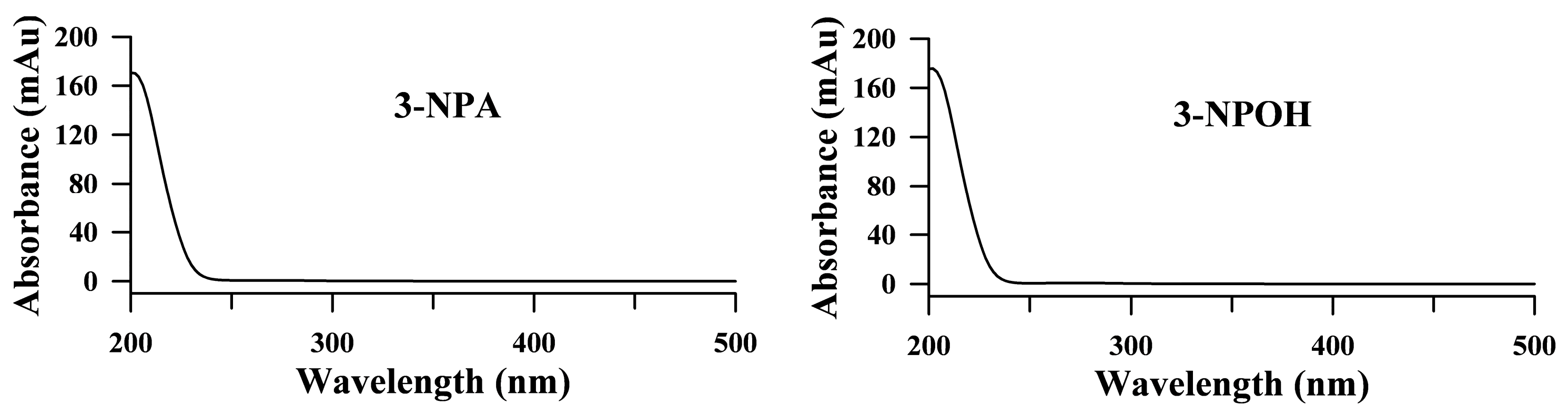
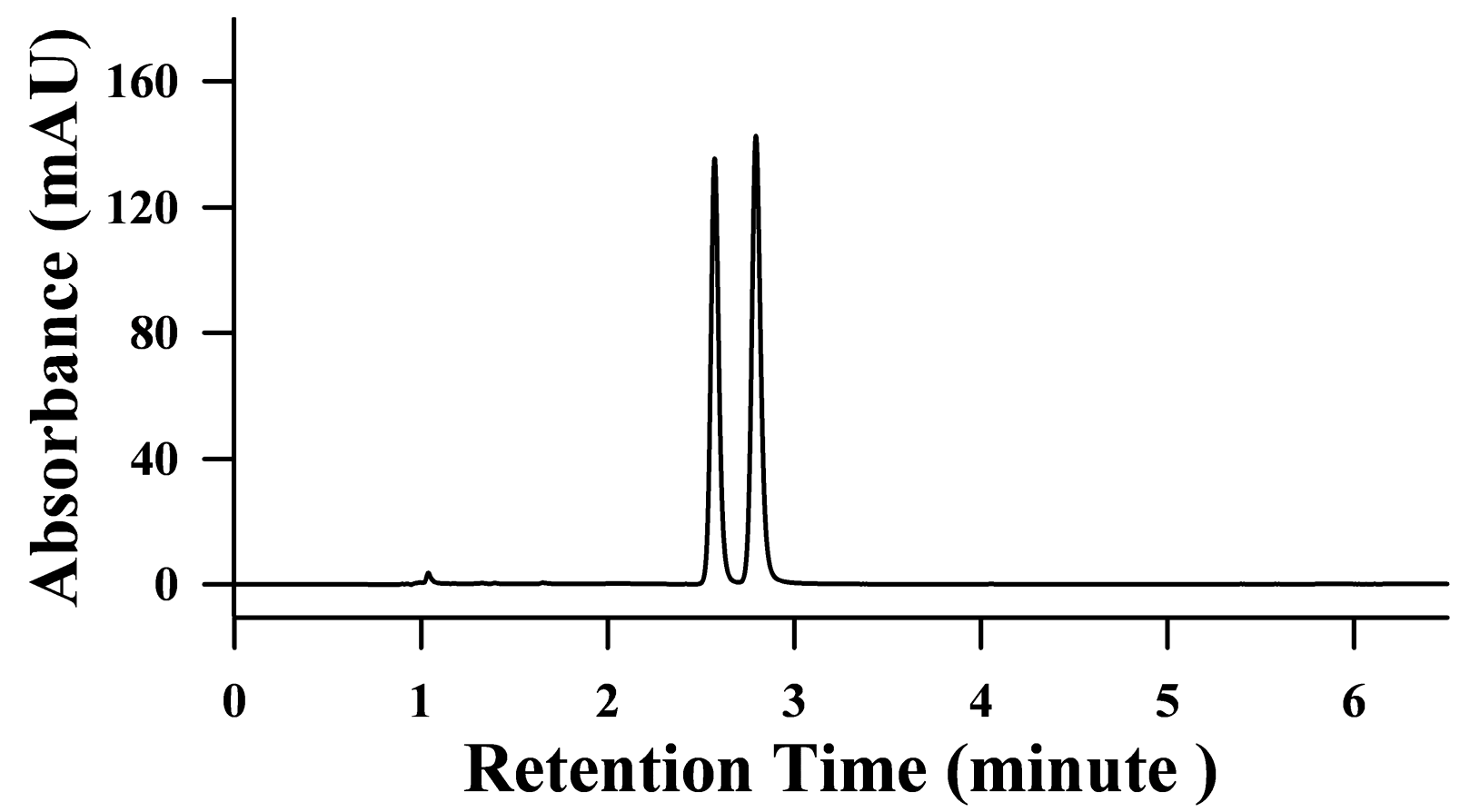
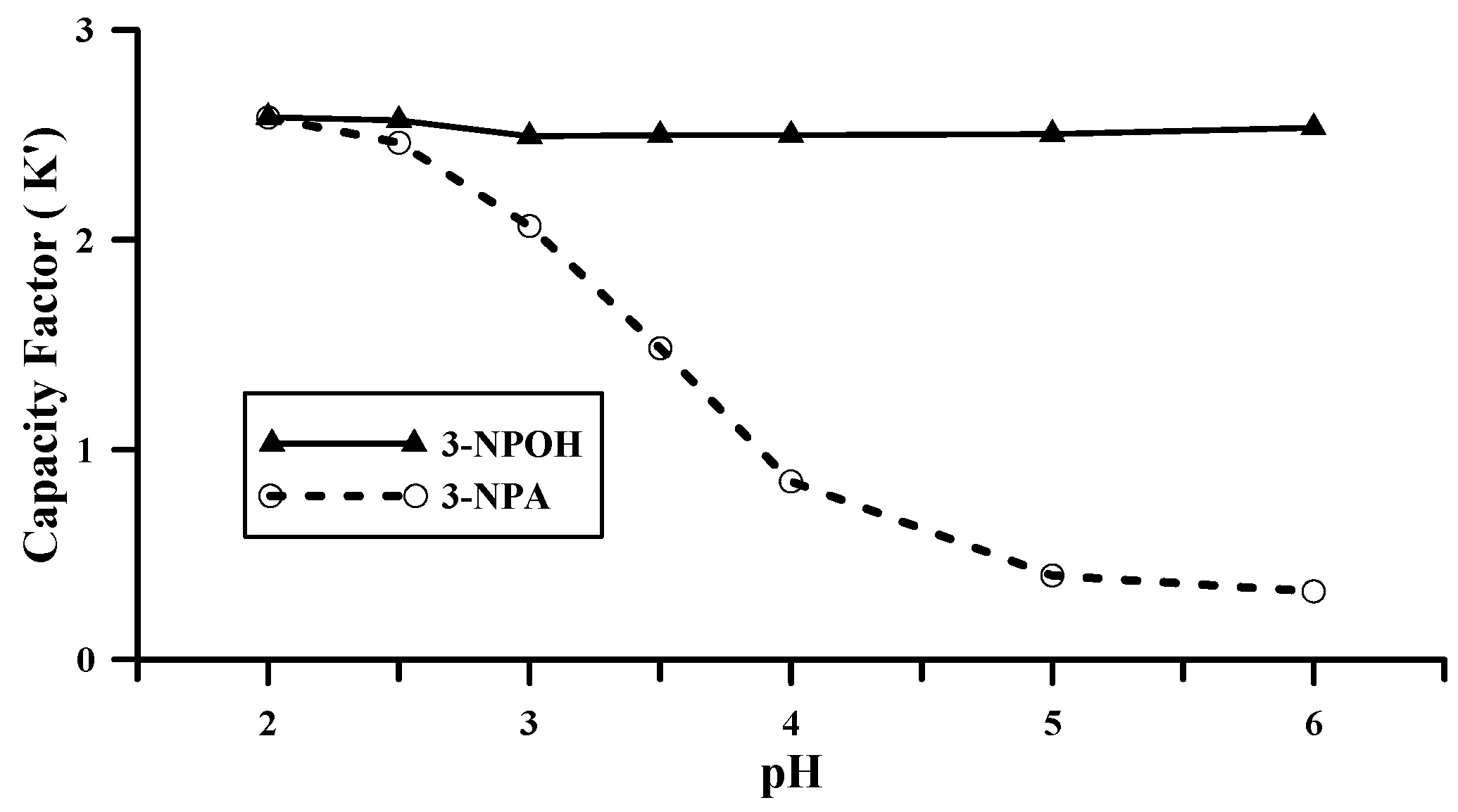
2.2. HPLC Conditions for the Analysis of 3-NPA and 3-NPOH
2.3. Method Validation
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Extraction of 3-NPA and 3-NPOH from A. canadensis
4.3. HPLC Conditions
4.4. Quantification
4.5. Method Validation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, M.C.; James, L.F. Toxicity of nitro-containing astragalus to sheep and chicks. J. Range Manag. 1975, 28, 260–263. [Google Scholar] [CrossRef]
- Williams, M.C.; Stermitz, F.R.; Thomas, R.D. Nitro compounds in astragalus species. Phytochemistry 1975, 14, 2306–2308. [Google Scholar] [CrossRef]
- Benn, M.; Bai, Y.; Majak, W. Aliphatic nitro-compounds in astragalus canadensis. Phytochemistry 1995, 40, 1629–1631. [Google Scholar] [CrossRef]
- Majak, W.; Pass, M.A. Aliphatic nitrocompounds. In Toxicants of Plant Origin; Cheeke, P.R., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 143–159. [Google Scholar]
- Majak, W.; Bose, R.J. Chromatographic methods for the isolation of miserotoxin and detection of aliphatic nitro compounds. Phytochemistry 1974, 13, 1005–1010. [Google Scholar] [CrossRef]
- Matsumoto, H.; Unrau, A.M.; Hylin, J.W.; Temple, B. Spectrophotometric determination of 3-nitropropanoic acid in biological extracts. Anal. Chem. 1961, 33, 1442–1444. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lowry, W.T.; Norris, F.A.; Buckeridge, F.A.; William, M.C. Aliphatic nitro compounds from astragalus species. Phytochemistry 1972, 11, 1117–1124. [Google Scholar] [CrossRef]
- Greenwood, D.R. Determination of aliphatic nitro compounds in roots of lotus pedunculatus-the effect of maceration on leves of components. J. Sci. Food Agric. 1990, 52, 499–508. [Google Scholar] [CrossRef]
- Gilbert, M.; Penel, A.; Kosikowski, F.V.; Henion, J.D.; Maylin, G.A.; Lisk, D.J. Electron affinity gas chromatographic determination of beta nitropropionic acid as its pentafluorobenzyl derivative in cheeses and mold filtrates. J. Food Sci. 1977, 46, 1650–1653. [Google Scholar] [CrossRef]
- Muir, A.D.; Majak, W. Quantitative determination of 3-nitropropionic acid and 3-nitropropanol in plasma by hplc. Toxicol. Lett. 1984, 20, 133–136. [Google Scholar] [CrossRef]
- William, M.C. 3-Nitropropionic acid and 3-nitro-1-propanol in species of astragalus. Can. J. Bot. 1982, 60, 1956–1963. [Google Scholar] [CrossRef]
- Anderson, R.C.; Rasmussen, M.A.; Allison, M.J. Metabolism of the plant toxins nitropropionic acid and nitropropanol by ruminal microorganisms. Appl. Environ. Microbiol. 1993, 59, 3056–3061. [Google Scholar] [PubMed]
- Majak, W.; Clark, L.J. Metabolism of aliphatic nitro compounds in bovine rumen fluid. Can. J. Anim. Sci. 1980, 60, 319–325. [Google Scholar] [CrossRef]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the terms “recovery” and “apparent recovery” in analytical procedures (iupac recommendations 2002). Pure Appl. Chem. 2002, 74, 2201–2205. [Google Scholar] [CrossRef]

| Extract | H2O:Acetone (2:8) | H2O:Acetonitrile (2:8) | H2O:Ethanol (2:8) | 1 mol/L HCL:Acetone (2:8) | 1 mol/L HCL:Acetonitrile (2:8) | 1 mol/L HCL:Ethanol (2:8) | 1 mol/L HCL | H2O |
|---|---|---|---|---|---|---|---|---|
| One-Time Extraction | 1.4 | 2.3 | 2.3 | 2.9 | 3.2 | 2.0 | 4.6 | 9.3 |
| Sequential Extraction | 6.2 | 6.8 | 6.2 | 9.8 | 10.3 | 4.2 |
| Temperature | Time (H) | 3-NPA | 3-NPOH |
|---|---|---|---|
| 95 °C | 1.5 | 2.02 | 0.15 |
| 95 °C | 4 | 2.49 | 0.15 |
| 95 °C | 48 | 2.31 | 0.14 |
| 25 °C | 4 | 8.48 | 0.20 |
| 25 °C | 48 | 9.27 | 0.23 |
| Compounds | Linear Range (μg/mL) | R2 | LOD (μg/mL) | LOQ(μg/mL) |
|---|---|---|---|---|
| 3-NPA | 1–600 | 0.9997 | 0.038 | 0.123 |
| 3-NPOH | 1–600 | 0.9998 | 0.036 | 0.116 |
| Compounds | Percent Recoveries (%) in in Freeze Dried Canadian Milkvetch Samples at Different Spiking Levels (ng/mg DW) | ||
|---|---|---|---|
| 100 | 400 | 1000 | |
| 3-NPA | 100.09 | 102.7 | 106.8 |
| 3-NPOH | 97.4 | 116.8 | 103.5 |
| Compounds | Measured Amount (mg/g) | RSD (%) |
|---|---|---|
| 3-NPA | 16.65 | 1.91 |
| 3-NPOH | 1.20 | 3.28 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Shao, S.; Schellenberg, M. A Simple and Fast Procedure to Determine 3-Nitropropanoic Acid and 3-Nitropropanol in Freeze Dried Canadian Milkvetch (Astragalus canadensis). Toxins 2017, 9, 204. https://doi.org/10.3390/toxins9070204
Liu H, Shao S, Schellenberg M. A Simple and Fast Procedure to Determine 3-Nitropropanoic Acid and 3-Nitropropanol in Freeze Dried Canadian Milkvetch (Astragalus canadensis). Toxins. 2017; 9(7):204. https://doi.org/10.3390/toxins9070204
Chicago/Turabian StyleLiu, Huaizhi, Suqin Shao, and Mike Schellenberg. 2017. "A Simple and Fast Procedure to Determine 3-Nitropropanoic Acid and 3-Nitropropanol in Freeze Dried Canadian Milkvetch (Astragalus canadensis)" Toxins 9, no. 7: 204. https://doi.org/10.3390/toxins9070204




