Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles
Abstract
:1. Introduction
2. Results
2.1. In Vitro Oxidation of CTXs by rhCYP3A4
2.2. In Vitro Oxidation of CTXs by Fish Liver S9 from Cigauteric and Non-Ciguateric Fishes
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Preparation of Fish Liver S9 and Microsomal Fractions
4.3. In Vitro CTX Oxidation Assay and Sample Preparation for LC-MS/MS Analysis
4.4. LC-MS/MS Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yasumoto, T. The chemistry and biological function of natural marine toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Kiernan, M.C. Neurotoxic marine poisoning. Lancet Neurol. 2005, 4, 219–228. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fleming, L.E.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.Y.; Backer, L.; Ayyar, R.; Weisman, R.; et al. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–479. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.P.; Brewer, T.D.; Johnstone, R.; Fleming, L.E.; Lewis, R.J. Ciguatera fish poisoning in the pacific islands (1998 to 2008). PLoS Negl. Trop. Dis. 2011, 5, e1416. [Google Scholar] [CrossRef] [PubMed]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Murata, M. Marine toxins. Chem. Rev. 1993, 93, 1897–1909. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon Off. J. Int. Soc. Toxinol. 2010, 56, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Yasumoto, T. Structures of ciguatoxin and its congener. J. Am. Chem. Soc. 1989, 111, 8929–8931. [Google Scholar] [CrossRef]
- Yasumoto, T.; Igarashi, T.; Legrand, A.-M.; Cruchet, P.; Chinain, M.; Fujita, T.; Naoki, H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectroscopy. J. Am. Chem. Soc. 2000, 122, 4988–4989. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.M.; Yasumoto, T. Isolation and structure of ciguatoxin-4a, a new ciguatoxin precursor, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem. 1996, 60, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Fukui, M.; Legrand, A.-M.; Cruchet, P.; Yasumoto, T. Isolation and structures of new ciguatoxin analogs, 2,3-dihydroxyCTX3C and 51-hydroxyCTX3C, accumulated in tropical reef fish. Tetrahedron Lett. 1998, 39, 1197–1198. [Google Scholar] [CrossRef]
- Lewis, R.J. Ciguatera: Australian perspectives on a global problem. Toxicon Off. J. Int. Soc. Toxinol. 2006, 48, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Sakugawa, S.; Oshiro, N.; Ikehara, T.; Sugiyama, K.; Yasumoto, T. Determination of toxins involved in ciguatera fish poisoning in the pacific by LC/MS. J. AOAC Int. 2014, 97, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Anzenbacher, P.; Anzenbacherova, E. Cytochromes p450 and metabolism of xenobiotics. Cell. Mol. Life Sci. CMLS 2001, 58, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, T.D.; Callaghan, J.T.; Einolf, H.J.; Fischer, V.; Gan, L.; Grimm, S.; Kao, J.; King, S.P.; Miwa, G.; Ni, L.; et al. The conduct of in vitro and in vivo drug-drug interaction studies: A PhRMA perspective. J. Clin. Pharm. 2003, 43, 443–469. [Google Scholar] [CrossRef]
- Fisher, M.B.; Campanale, K.; Ackermann, B.L.; VandenBranden, M.; Wrighton, S.A. In vitro glucuronidation using human liver microsomes and the pore-forming peptide alamethicin. Drug Metab. Dispos. Boil. Fate Chem. 2000, 28, 560–566. [Google Scholar]
- Spatzenegger, M.; Jaeger, W. Clinical importance of hepatic cytochrome p450 in drug metabolism. Drug Metab. Rev. 1995, 27, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, N.; Yogi, K.; Asato, S.; Sasaki, T.; Tamanaha, K.; Hirama, M.; Yasumoto, T.; Inafuku, Y. Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon Off. J. Int. Soc. Toxinol. 2010, 56, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Hung, P.; Lo, J.Y. Ciguatera fish poisoning in Hong Kong—A 10-year perspective on the class of ciguatoxins. Toxicon Off. J. Int. Soc. Toxinol. 2014, 86, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Rajeish, M.; Malathi, S.; Madhushree, H.N.; Venugopal, M.N. Presumptive case of ciguatera fish poisoning in Mangalore, India. Curr. Sci. 2016, 111, 1543–1547. [Google Scholar] [CrossRef]
- Hirama, M.; Oishi, T.; Uehara, H.; Inoue, M.; Maruyama, M.; Oguri, H.; Satake, M. Total synthesis of ciguatoxin CTX3C. Science 2001, 294, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Miyazaki, K.; Uehara, H.; Maruyama, M.; Hirama, M. First- and second-generation total synthesis of ciguatoxin CTX3C. Proc. Natl. Acad. Sci. USA 2004, 101, 12013–12018. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Miyazaki, K.; Ishihara, Y.; Tatami, A.; Ohnuma, Y.; Kawada, Y.; Komano, K.; Yamashita, S.; Lee, N.; Hirama, M. Total synthesis of ciguatoxin and 51-hydroxyCTX3C. J. Am. Chem. Soc. 2006, 128, 9352–9354. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Oshiro, N.; Matsuda, S.; Sakugawa, S.; Matsuo, T.; Yasumoto, T. Toxin profiles in fish implicated in ciguatera fish poisoning in Amami and Kakeroma islands, Kagoshima prefecture, Japan. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 2013, 54, 385–391. [Google Scholar] [CrossRef]

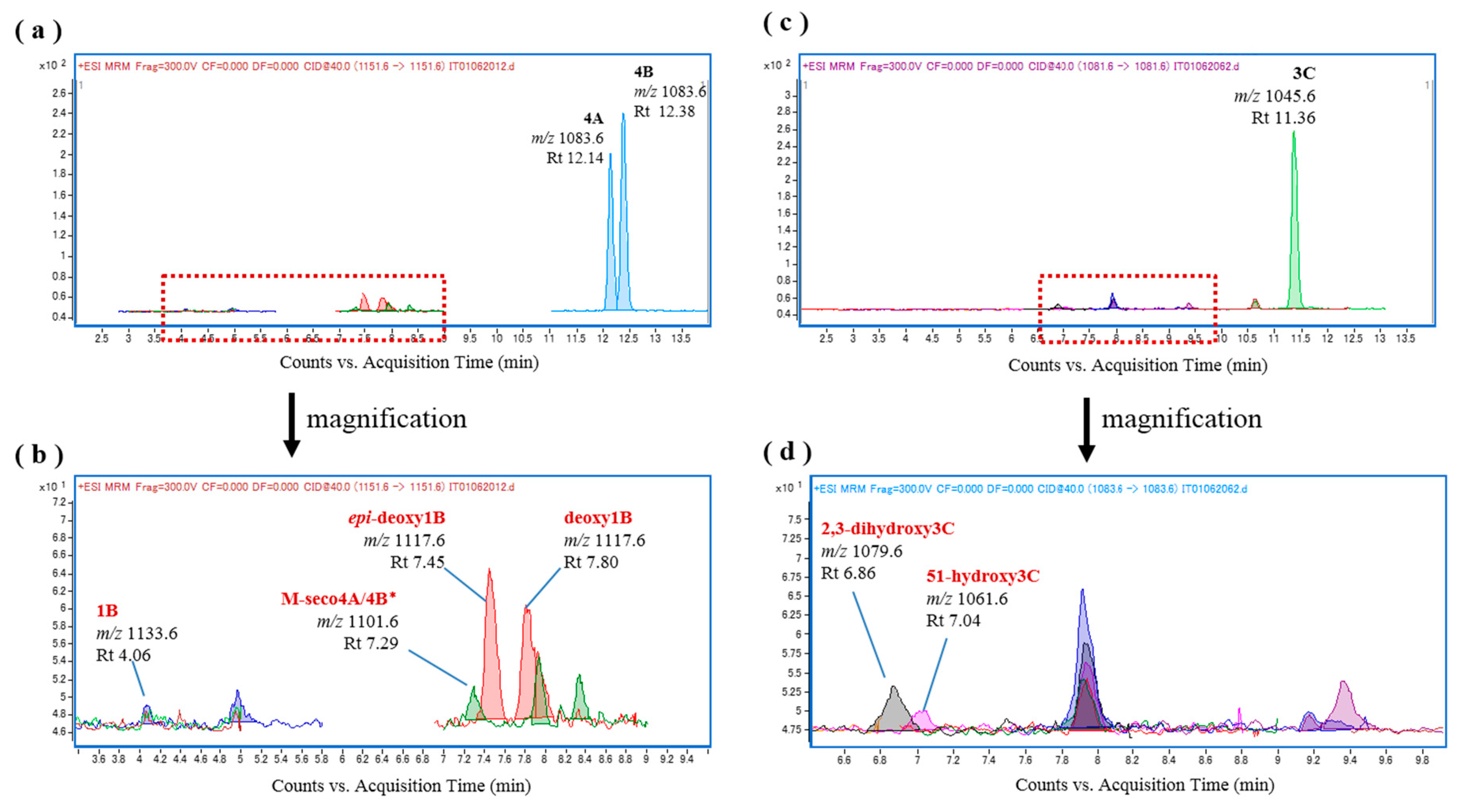
| Abbreviations | CTX Congeners | [M + Na]+ m/z | Abbreviations | CTX Congeners | [M + Na]+ m/z |
|---|---|---|---|---|---|
| 4A | CTX4A | 1083.6 | 3C | CTX3C | 1045.6 |
| 4B | CTX4B | 1083.6 | 2-oxo3C | 2-oxoCTX3C | 1061.6 |
| M-seco4A/4B | M-secoCTX4A/CTX4B | 1101.6 | 2-oxo-epi-3C | 2-oxo-epi-CTX3C | 1061.6 |
| deoxy1B | 54-deoxyCTX1B | 1117.6 | 2,3-dihydroxy3C | 2,3-dihydroxyCTX3C | 1079.6 |
| epi-deoxy1B | 52-epi-54-deoxyCTX1B | 1117.6 | 51-hydroxy3C | 51-hydroxyCTX3C | 1061.6 |
| 1B | CTX1B | 1133.6 | 2-hydroxy3C | 2-hydroxyCTX3C | 1063.6 |
| 4-hydroxy-7-oxo-1B | 4-hydroxy-7-oxo-CTX1B | 1167.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins 2017, 9, 205. https://doi.org/10.3390/toxins9070205
Ikehara T, Kuniyoshi K, Oshiro N, Yasumoto T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins. 2017; 9(7):205. https://doi.org/10.3390/toxins9070205
Chicago/Turabian StyleIkehara, Tsuyoshi, Kyoko Kuniyoshi, Naomasa Oshiro, and Takeshi Yasumoto. 2017. "Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles" Toxins 9, no. 7: 205. https://doi.org/10.3390/toxins9070205






