Prenylflavonoid Isoxanthohumol Sensitizes MCF-7/ADR Cells to Doxorubicin Cytotoxicity via Acting as a Substrate of ABCB1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Cells
2.2. Cell Viability Assessment
2.3. Calculation of Drug Combination and Synergism
2.4. Intracellular DOX and Rhodamine123 (Rho123) Accumulation Detection
2.5. Western Blotting Assay
2.6. ABCB1 ATPase Activity Assay
2.7. Molecule Docking
2.8. Data Analysis
3. Results
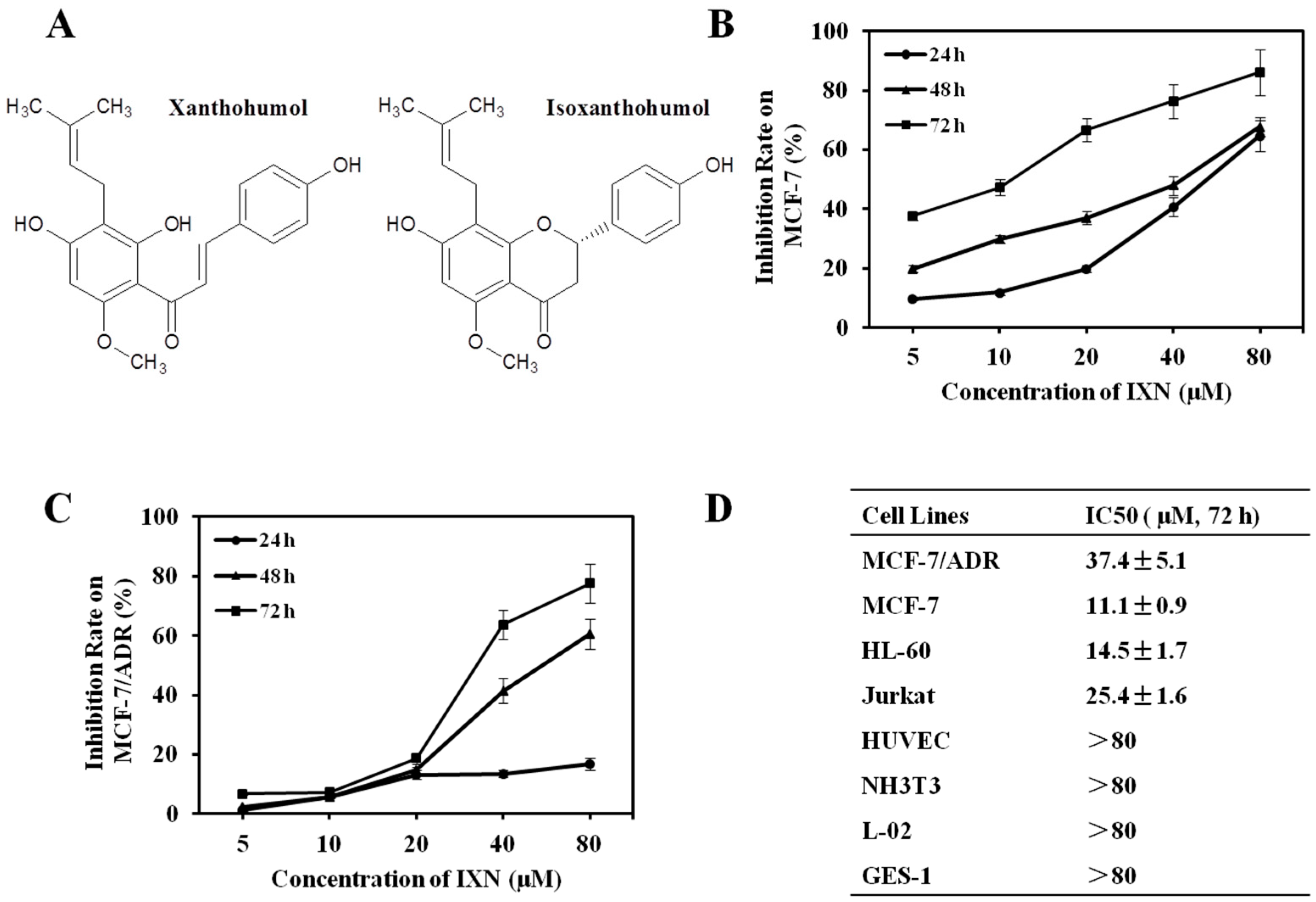
3.1. IXN Exhibited More Potent Cytotoxicity in MCF-7 Than in MCF-7/ADR Cells
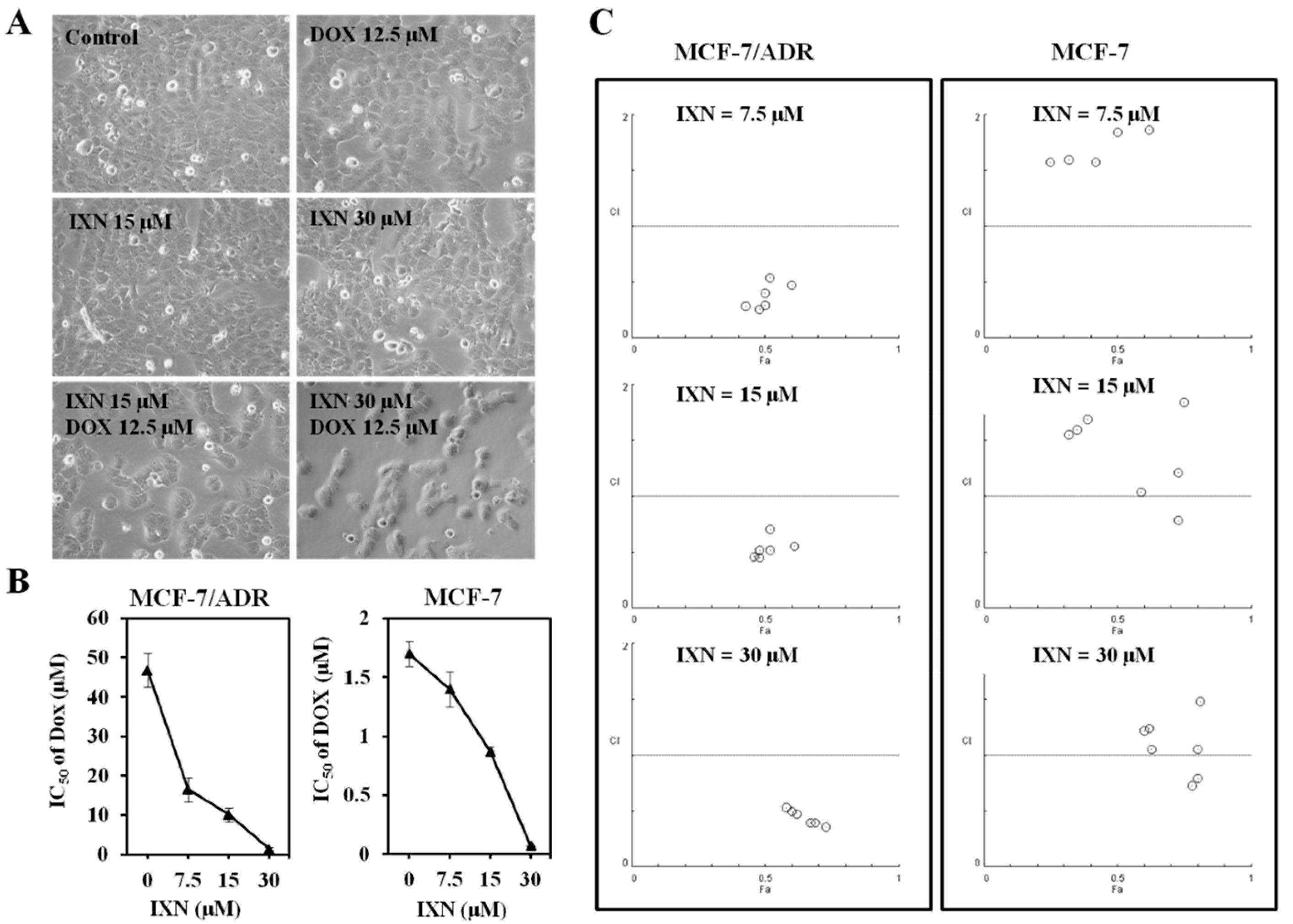
3.2. Synergic Effect of IXN in Combination with DOX
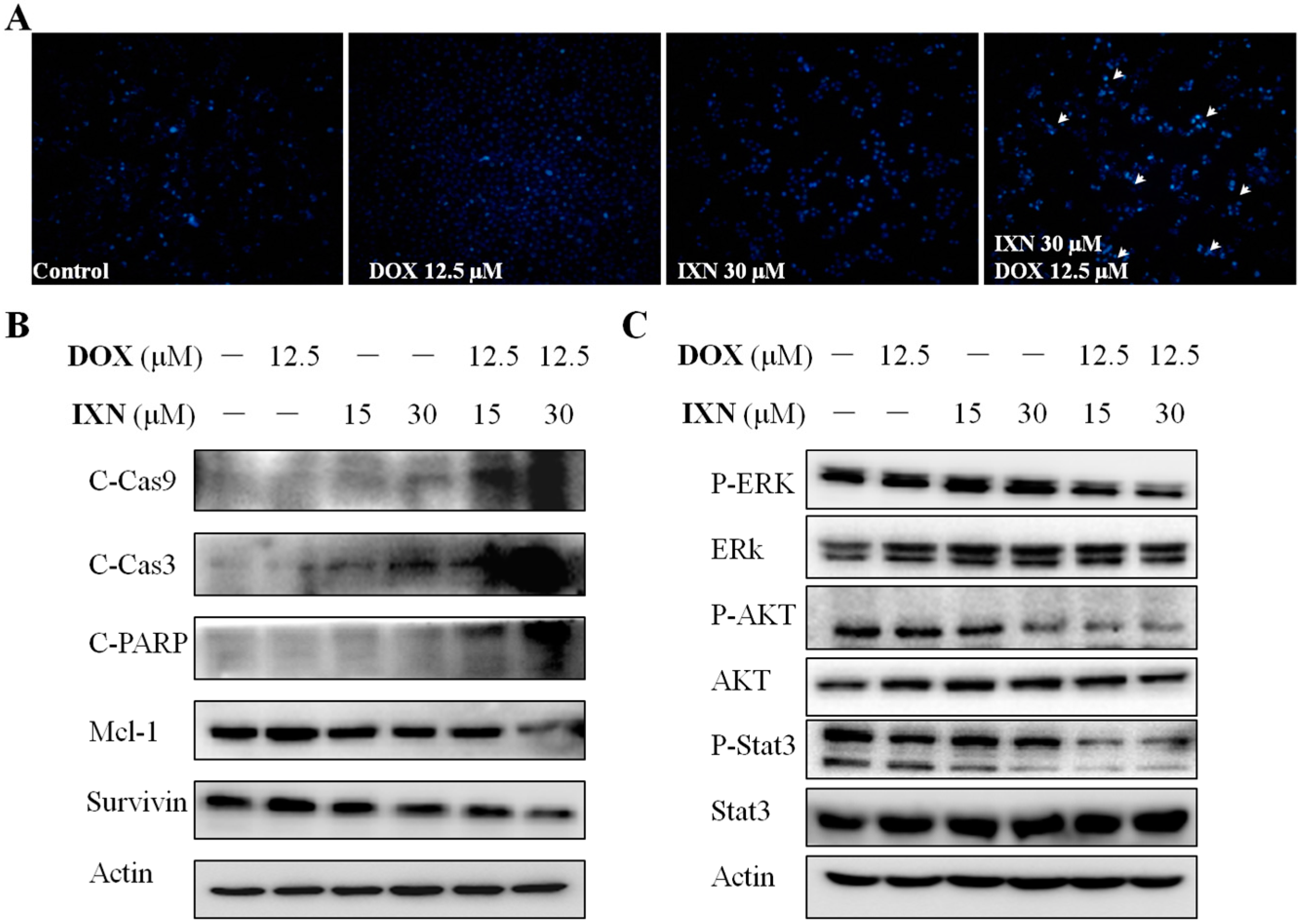
3.3. Combination of IXN and DOX Stimulated More Apoptosis in MCF-7/ADR Cells
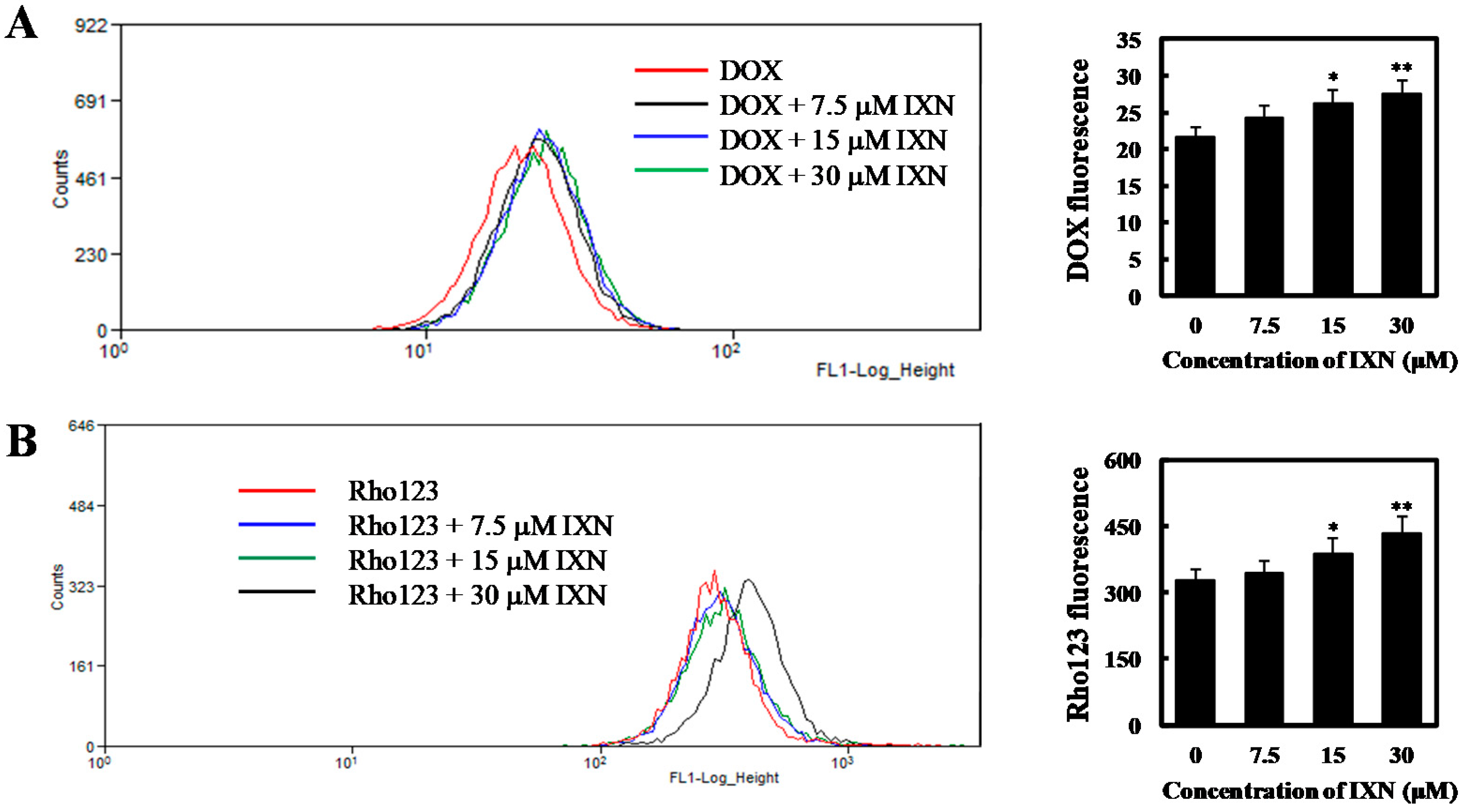
3.4. IXN Inhited the Transporting Functions of ABCB1 in MCF-7/ADR Cells
3.5. IXN Increased ABCB1 Expression and Stimulated the ATPase acTivity
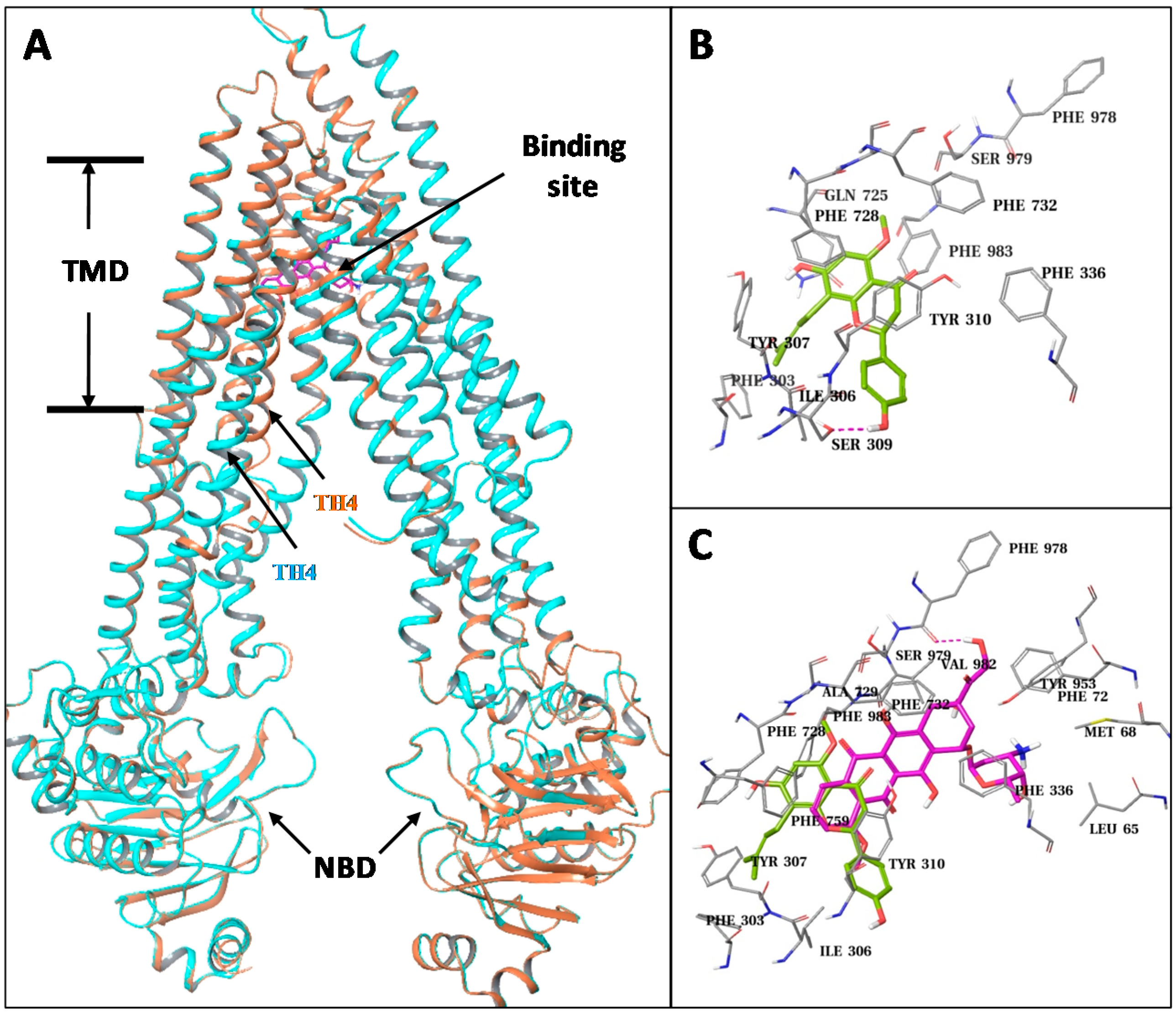
3.6. IXN Binds to the Central TMD Site Where DOX Binds
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, Y.; Wang, Y.; Kiani, M.F.; Wang, B. Classification, Treatment Strategy, and Associated Drug Resistance in Breast Cancer. Clin. Breast Cancer 2016, 16, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Xie, Y.; Baer, M.R.; Ross, D.D. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem. Pharmacol. 2012, 83, 1084–1103. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C. Introduction to the Toxins Special Issue on Dietary and Non-Dietary Phytochemicals and Cancer. Toxins 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, T.; Efferth, T. P-glycoprotein and its inhibition in tumors by phytochemicals derived from Chinese herbs. J. Ethnopharmacol. 2012, 141, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Żołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol—Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.; Alberto, V.; Pilar, M.; Domenico, M.; Giuliano Delle, M. Prenylated Flavonoids: Pharmacology and Biotechnology. Curr. Med. Chem. 2005, 12, 713–739. [Google Scholar]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef]
- Negrao, R.; Duarte, D.; Costa, R.; Soares, R. Isoxanthohumol modulates angiogenesis and inflammation via vascular endothelial growth factor receptor, tumor necrosis factor alpha and nuclear factor kappa B pathways. Biofactors 2013, 39, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Krajnović, T.; Kaluđerović, G.N.; Wessjohann, L.A.; Mijatović, S.; Maksimović-Ivanić, D. Versatile antitumor potential of isoxanthohumol: Enhancement of paclitaxel activity in vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Cooney, J.; Jensen, D.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Hop-derived prenylflavonoids are substrates and inhibitors of the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Mol. Nutr. Food Res. 2014, 58, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Qian, X.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, C.R.; Ivy, S.P.; Kao-Shan, C.S.; Whang-Peng, J.; Rosen, N.; Israel, M.A.; Melera, P.W.; Cowan, K.H.; Goldsmith, M.E. Isolation of amplified and overexpressed DNA sequences from adriamycin-resistant human breast cancer cells. Cancer Res. 1987, 47, 5141–5148. [Google Scholar] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Szewczyk, P.; Tao, H.; McGrath, A.P.; Villaluz, M.; Rees, S.D.; Lee, S.C.; Doshi, R.; Urbatsch, I.L.; Zhang, Q.; Chang, G. Snapshots of ligand entry, malleable binding and induced helical movement in P-glycoprotein. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, C.M.; Jiang, X.; Oldfield, T.; Waldman, M. LigandFit: A novel method for the shape-directed rapid docking of ligands to protein active sites. J. Mol. Graph. Model. 2003, 21, 289–307. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Park, M.A.; Heo, S.W.; Park, S.Y.; Kang, K.W.; Park, P.H.; Kim, J.A. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Lohner, K.; Schnäbele, K.; Daniel, H.; Oesterle, D.; Rechkemmer, G.; Göttlicher, M.; Wenzel, U. Flavonoids alter P-gp expression in intestinal epithelial cells in vitro and in vivo. Mol. Nutr. Food Res. 2007, 51, 293–300. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhang, W.; Zhang, W.; Zhou, X.; Li, M.; Miao, J. Prenylflavonoid Isoxanthohumol Sensitizes MCF-7/ADR Cells to Doxorubicin Cytotoxicity via Acting as a Substrate of ABCB1. Toxins 2017, 9, 208. https://doi.org/10.3390/toxins9070208
Liu M, Zhang W, Zhang W, Zhou X, Li M, Miao J. Prenylflavonoid Isoxanthohumol Sensitizes MCF-7/ADR Cells to Doxorubicin Cytotoxicity via Acting as a Substrate of ABCB1. Toxins. 2017; 9(7):208. https://doi.org/10.3390/toxins9070208
Chicago/Turabian StyleLiu, Ming, Weiyi Zhang, Wei Zhang, Xin Zhou, Ming Li, and Jinlai Miao. 2017. "Prenylflavonoid Isoxanthohumol Sensitizes MCF-7/ADR Cells to Doxorubicin Cytotoxicity via Acting as a Substrate of ABCB1" Toxins 9, no. 7: 208. https://doi.org/10.3390/toxins9070208





