Rehabilitation plus OnabotulinumtoxinA Improves Motor Function over OnabotulinumtoxinA Alone in Post-Stroke Upper Limb Spasticity: A Single-Blind, Randomized Trial
Abstract
:1. Introduction
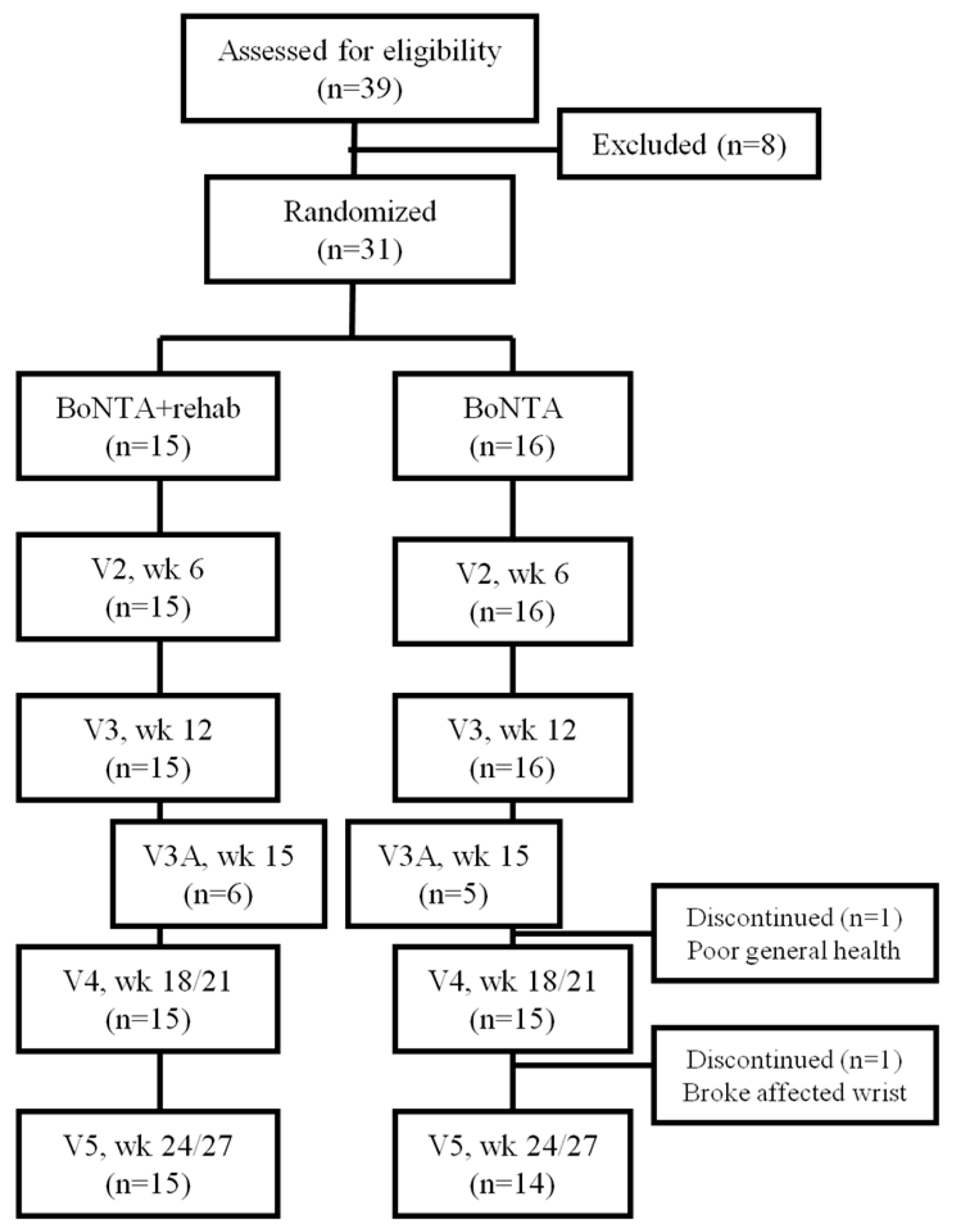
2. Results
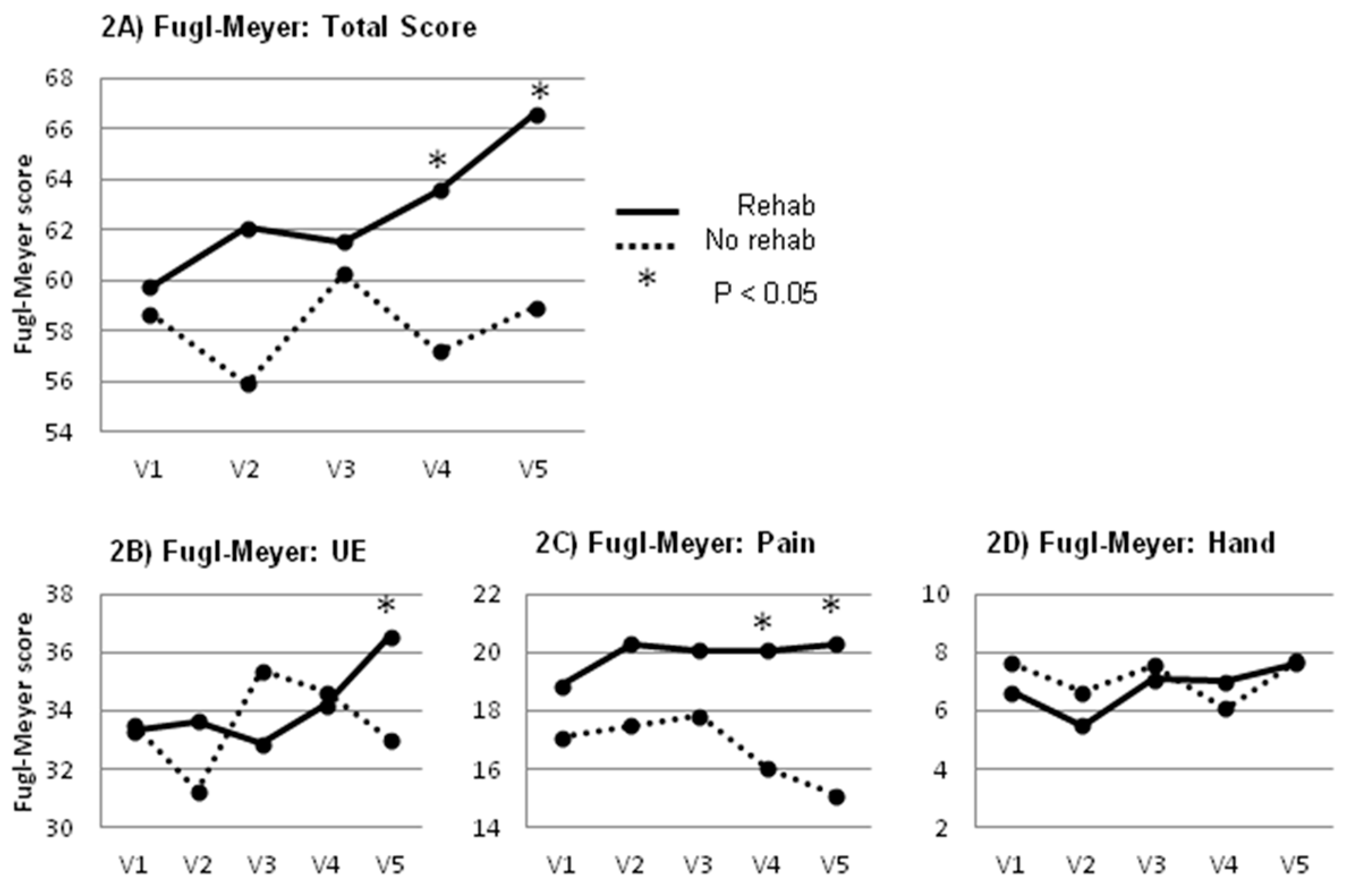
2.1. Primary Outcome Measure
2.2. Secondary Outcome Measures
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Recruitment and Selection
5.2. Baseline
5.3. Random Allocation
5.4. Additional Visits
5.5. Assessment Tools
5.6. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Sampaio, C.; Ferreira, J.J.; Pinto, A.A.; Crespo, M.; Ferro, J.M.; Castro-Caldas, A. Botulinum toxin type a for the treatment of arm and hand spasticity in stroke patients. Clin. Rehabil. 1997, 11, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.M.; Pittock, S.; Moore, A.P.; Wurker, M.; Otto, S.; Erbguth, F.; Coxon, L. A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type a in upper limb spasticity in patients with stroke. Eur. J. Neurol. 2001, 8, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Brashear, A.; Gordon, M.F.; Elovic, E.; Kassicieh, V.D.; Marciniak, C.; Do, M.; Lee, C.H.; Jenkins, S.; Turkel, C.; Botox Post-Stroke Spasticity Study Group. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N. Engl. J. Med. 2002, 347, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Pandyan, A.D.; Vuadens, P.; van Wijck, F.M.; Stark, S.; Johnson, G.R.; Barnes, M.P. Are we underestimating the clinical efficacy of botulinum toxin (type a)? Quantifying changes in spasticity, strength and upper limb function after injections of botox to the elbow flexors in a unilateral stroke population. Clin. Rehabil. 2002, 16, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, M.; Kozlowski, O.; Froger, J. Efficacy of botulinum toxin a in upper limb function of hemiplegic patients. J. Neurol. 2002, 249, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.F.; Brashear, A.; Elovic, E.; Kassicieh, D.; Marciniak, C.; Liu, J.; Turkel, C.; Group, B.P.S.S. Repeated dosing of botulinum toxin type a for upper limb spasticity following stroke. Neurology 2004, 63, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Slawek, J.; Bogucki, A.; Reclawowicz, D. Botulinum toxin type a for upper limb spasticity following stroke: An open-label study with individualised, flexible injection regimens. Neurol. Sci. 2005, 26, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, B.B.; Cozens, J.A.; Bamford, J.M.; Chamberlain, M.A. Use of botulinum toxin in stroke patients with severe upper limb spasticity. J. Neurol. Neurosurg. Psychiatry 1996, 61, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Foley, N.; Pereira, S.; Salter, K.; Fernandez, M.M.; Speechley, M.; Sequeira, K.; Miller, T.; Teasell, R. Treatment with botulinum toxin improves upper-extremity function post stroke: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.A.; Pereira, G. The efficacy of botulinum toxin a for limb spasticity on improving activity restriction and quality of life: A systematic review and meta-analysis using the grade approach. Clin. Rehabil. 2015, 30, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Brashear, A.; Zafonte, R.; Corcoran, M.; Galvez-Jimenez, N.; Gracies, J.M.; Gordon, M.F.; McAfee, A.; Ruffing, K.; Thompson, B.; Williams, M.; et al. Inter- and intrarater reliability of the ashworth scale and the disability assessment scale in patients with upper-limb poststroke spasticity. Arch. Phys. Med. Rehabil. 2002, 83, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; Foley, N.; Pereira, S.; Sequeira, K.; Miller, T. Evidence to practice: Botulinum toxin in the treatment of spasticity post stroke. Top. Stroke Rehabil. 2012, 19, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Demetrios, M.; Gorelik, A.; Louie, J.; Brand, C.; Baguley, I.J.; Khan, F. Outcomes of ambulatory rehabilitation programmes following botulinum toxin for spasticity in adults with stroke. J. Rehabil. Med. 2014, 46, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Demetrios, M.; Khan, F.; Turner-Stokes, L.; Brand, C.; McSweeney, S. Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity. Cochrane Database Syst. Rev. 2013, 6, CD009689. [Google Scholar] [CrossRef]
- Wolf, S.L.; Milton, S.B.; Reiss, A.; Easley, K.A.; Shenvi, N.V.; Clark, P.C. Further assessment to determine the additive effect of botulinum toxin type a on an upper extremity exercise program to enhance function among individuals with chronic stroke but extensor capability. Arch. Phys. Med. Rehabil. 2012, 93, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L.; Bo, T.I. Botuls: A multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type a. Health Technol. Assess. 2010, 14, 1–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.F.; Hsu, C.W.; Sun, H.P.; Hwang, C.W.; Yang, C.L.; Wang, J.L. Combined botulinum toxin type a with modified constraint-induced movement therapy for chronic stroke patients with upper extremity spasticity: A randomized controlled study. Neurore. Neural Repair 2010, 24, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Abogunrin, S.; Hortobagyi, L.; Remak, E.; Dinet, J.; Gabriel, S.; Bakheit, A.M. Budget impact analysis of botulinum toxin a therapy for upper limb spasticity in the united kingdom. Clin. Outcomes Res. 2015, 7, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- De Boer, K.S.; Arwert, H.J.; de Groot, J.H.; Meskers, C.G.; Mishre, A.D.; Arendzen, J.H. Shoulder pain and external rotation in spastic hemiplegia do not improve by injection of botulinum toxin a into the subscapular muscle. J. Neurol. Neurosurg. Psychiatry 2008, 79, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Koh, J.H.; Paik, N.J. Intramuscular botulinum toxin-a reduces hemiplegic shoulder pain: A randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke 2008, 39, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Mayer, N.H.; Whyte, J.; Wannstedt, G.; Ellis, C.A. Comparative impact of 2 botulinum toxin injection techniques for elbow flexor hypertonia. Arch. Phys. Med. Rehabil. 2008, 89, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.P.; Wade, D.T.; Turner-Stokes, L.; Kingswell, R.S.; Dott, C.S.; Coxon, E.A. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964, 192, 540–542. [Google Scholar] [PubMed]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the fugl-meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurore. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Lai, S.M.; Keighley, J. Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology 2000, 39, 835–841. [Google Scholar] [CrossRef]
- Hsu, J.C. Multiple Comparisons: Theory and Methods, 1st ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 1996. [Google Scholar]

| Visit | Screen/Physical | Fugl–Meyer | Ashworth | Self-Reports | FIM | Inject |
|---|---|---|---|---|---|---|
| Screen | X | X | X | |||
| V1, Wk0 | X | X | X | X | X | |
| V2, Wk6 | X | X | X | |||
| V3/3A, Wk12/15 | X | X | X | X | ||
| V4, Wk18/20 | X | X | X | |||
| V5, Wk24/27 | X | X | X | X |
| Demographic and Clinical Variables | BoNT-A + Rehab (n = 15) | 95% CI | BoNT-A no Rehab (n = 16) | 95% CI | p Value |
|---|---|---|---|---|---|
| Age, year | 58.0 ± 6.6 | 54.40–61.67 | 60.9 ± 11.0 | 55.07–66.81 | 0.384 |
| Sex, M/F | 11/4 | 10/6 | 0.704 | ||
| Stroke in dominant Hemisphere * n/% | 5/33% | 8/50% | 0.473 | ||
| Race (Caucasian, African American, Hispanic) | 10/3/2 | 11/4/1 | 0.782 | ||
| Fugl–Meyer | 58.5 ± 12.9 | 51.39–65.67 | 58.1 ± 15.6 | 49.75–66.38 | 0.928 |
| FIM, motor subscale | 70.9 ± 15.2 | 62.43–79.3 | 73.5 ± 17.5 | 64.16–82.84 | 0.659 |
| Ashworth | 9.3 ± 2.8 | 7.79–10.88 | 10.1 ± 2.7 | 8.67–11.58 | 0.431 |
| DAS | 6.9 ± 2.9 | 5.32–8.55 | 6.5 ± 2.2 | 5.33–7.67 | 0.642 |
| PDS | 18.2 ± 6.4 | 14.68–21.72 | 18.0 ± 5.1 | 15.26–20.74 | 0.924 |
| VAS | 1.9 ± 2.8 | 0.29–3.44 | 1.3 ± 2.1 | 0.19–2.41 | 0.530 |
| Average Modified Ashworth Scores | Rehab | No Rehab |
|---|---|---|
| Baseline | 10.6 | 10.8 |
| V1 | 9.3 | 10.1 |
| V2 | 5.4 | 5.7 |
| V3 | 8.6 | 6.0 |
| V4 | 5.0 | 6.3 |
| V5 | 6.1 | 8.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devier, D.; Harnar, J.; Lopez, L.; Brashear, A.; Graham, G. Rehabilitation plus OnabotulinumtoxinA Improves Motor Function over OnabotulinumtoxinA Alone in Post-Stroke Upper Limb Spasticity: A Single-Blind, Randomized Trial. Toxins 2017, 9, 216. https://doi.org/10.3390/toxins9070216
Devier D, Harnar J, Lopez L, Brashear A, Graham G. Rehabilitation plus OnabotulinumtoxinA Improves Motor Function over OnabotulinumtoxinA Alone in Post-Stroke Upper Limb Spasticity: A Single-Blind, Randomized Trial. Toxins. 2017; 9(7):216. https://doi.org/10.3390/toxins9070216
Chicago/Turabian StyleDevier, Deidre, JoAnn Harnar, Leandro Lopez, Allison Brashear, and Glenn Graham. 2017. "Rehabilitation plus OnabotulinumtoxinA Improves Motor Function over OnabotulinumtoxinA Alone in Post-Stroke Upper Limb Spasticity: A Single-Blind, Randomized Trial" Toxins 9, no. 7: 216. https://doi.org/10.3390/toxins9070216




