Differences in Genetic Background Contribute to Pseudomonas Exotoxin A-Induced Hepatotoxicity in Rats
Abstract
:1. Introduction
2. Results
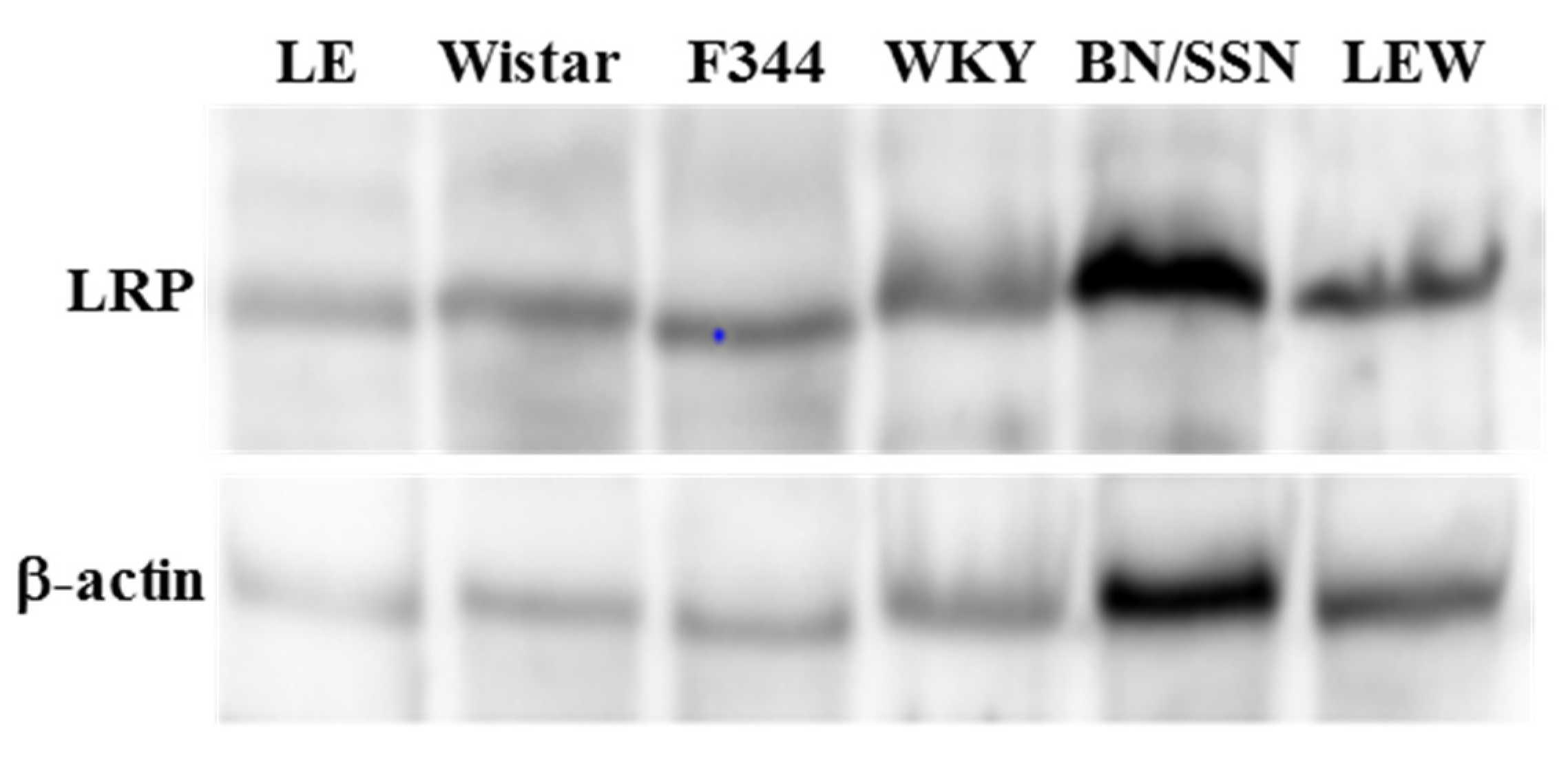
2.1. Hepatic Concentration of LRP in Different Rat Strains
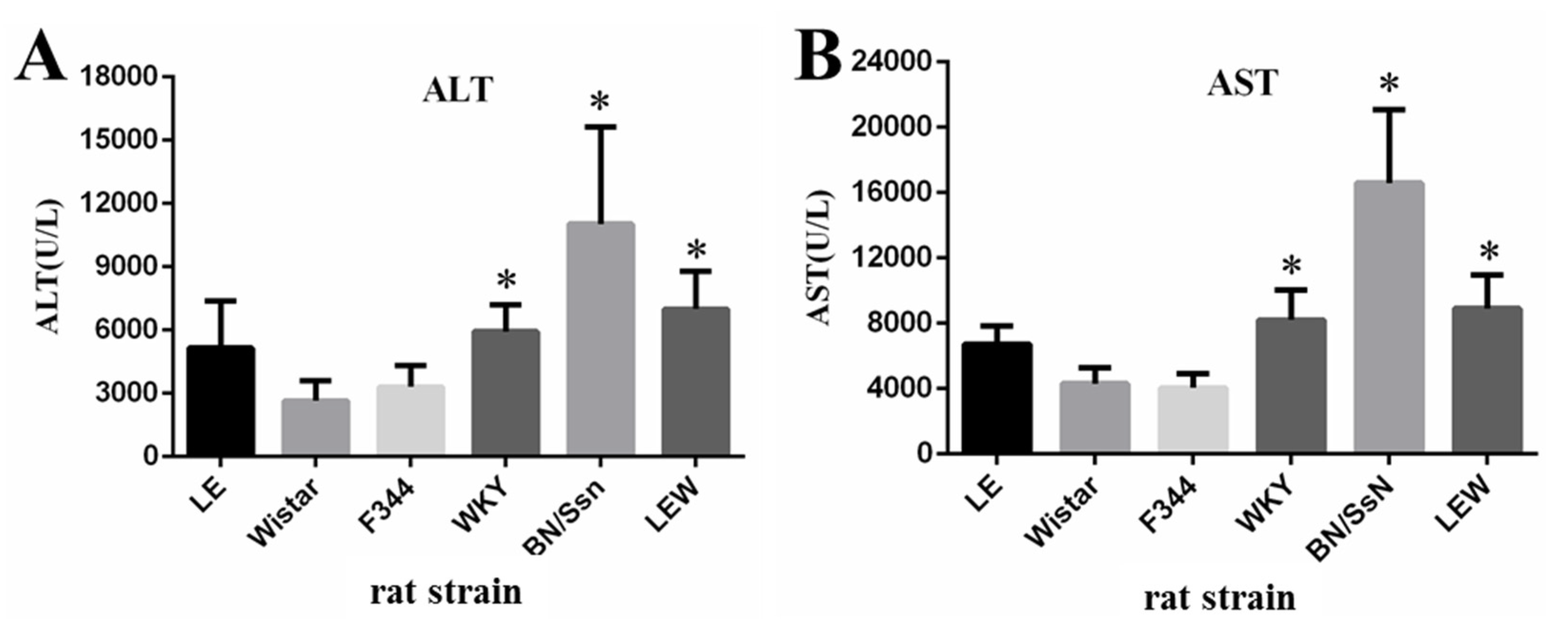
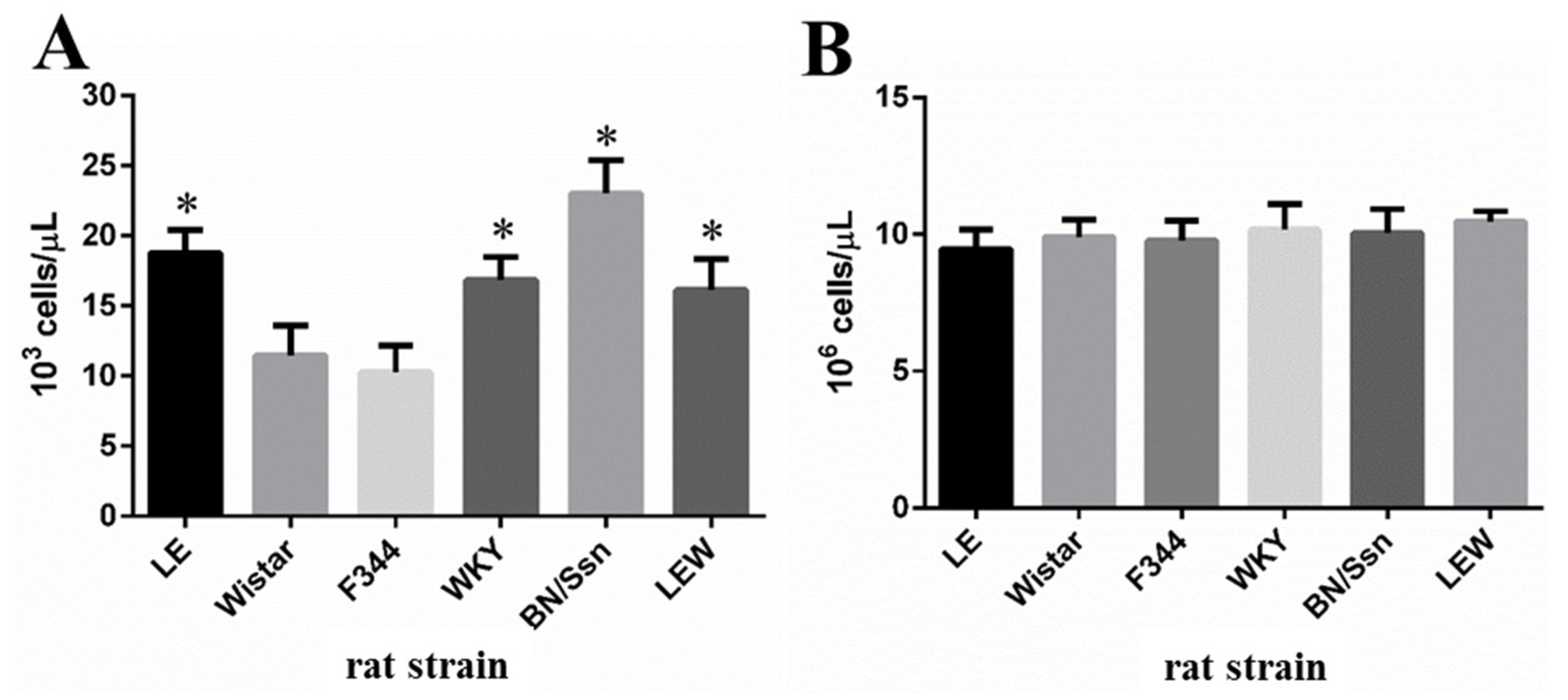
2.2. Clinical Chemistry and Complete Blood Count (CBC) Analysis
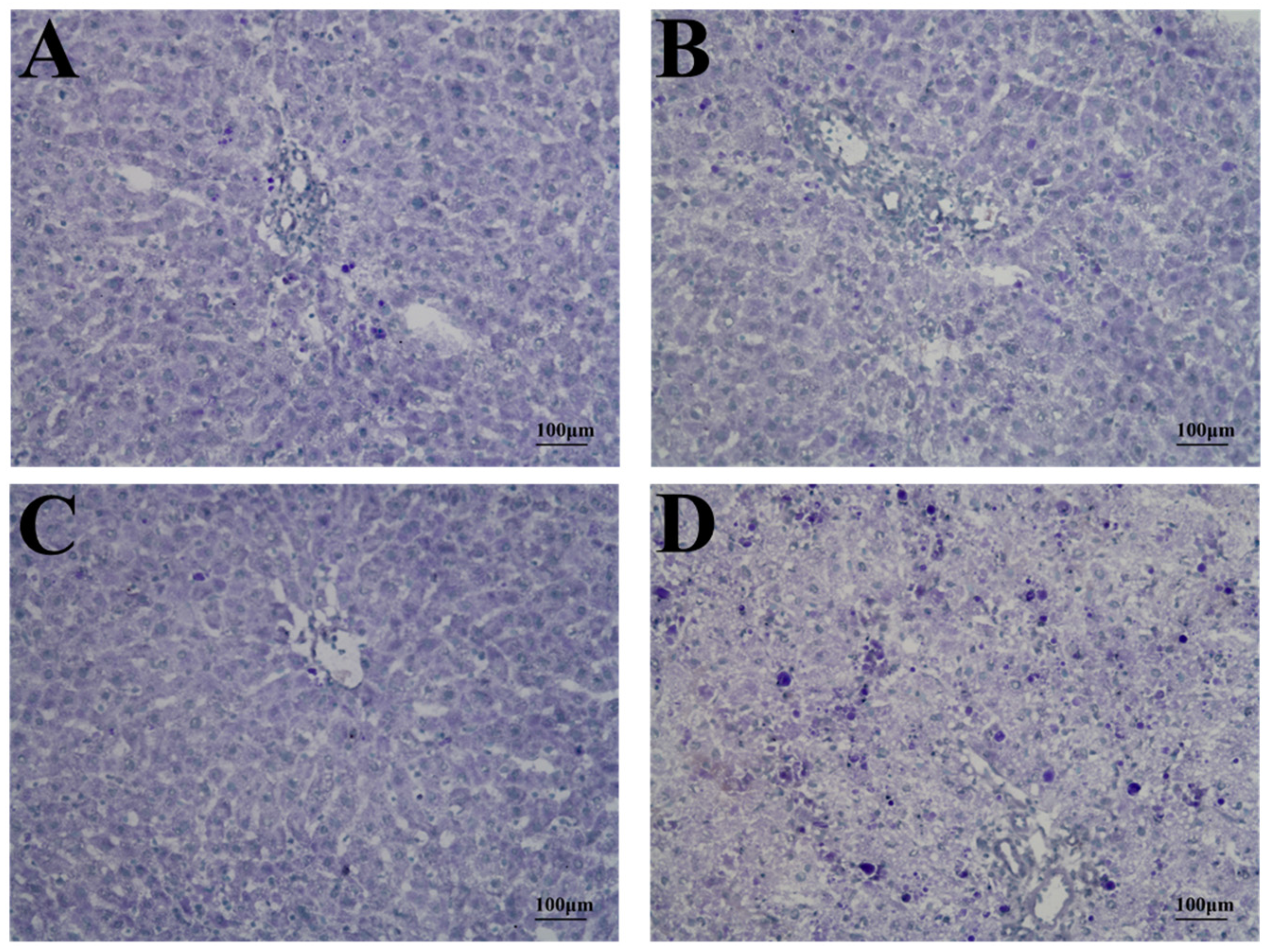
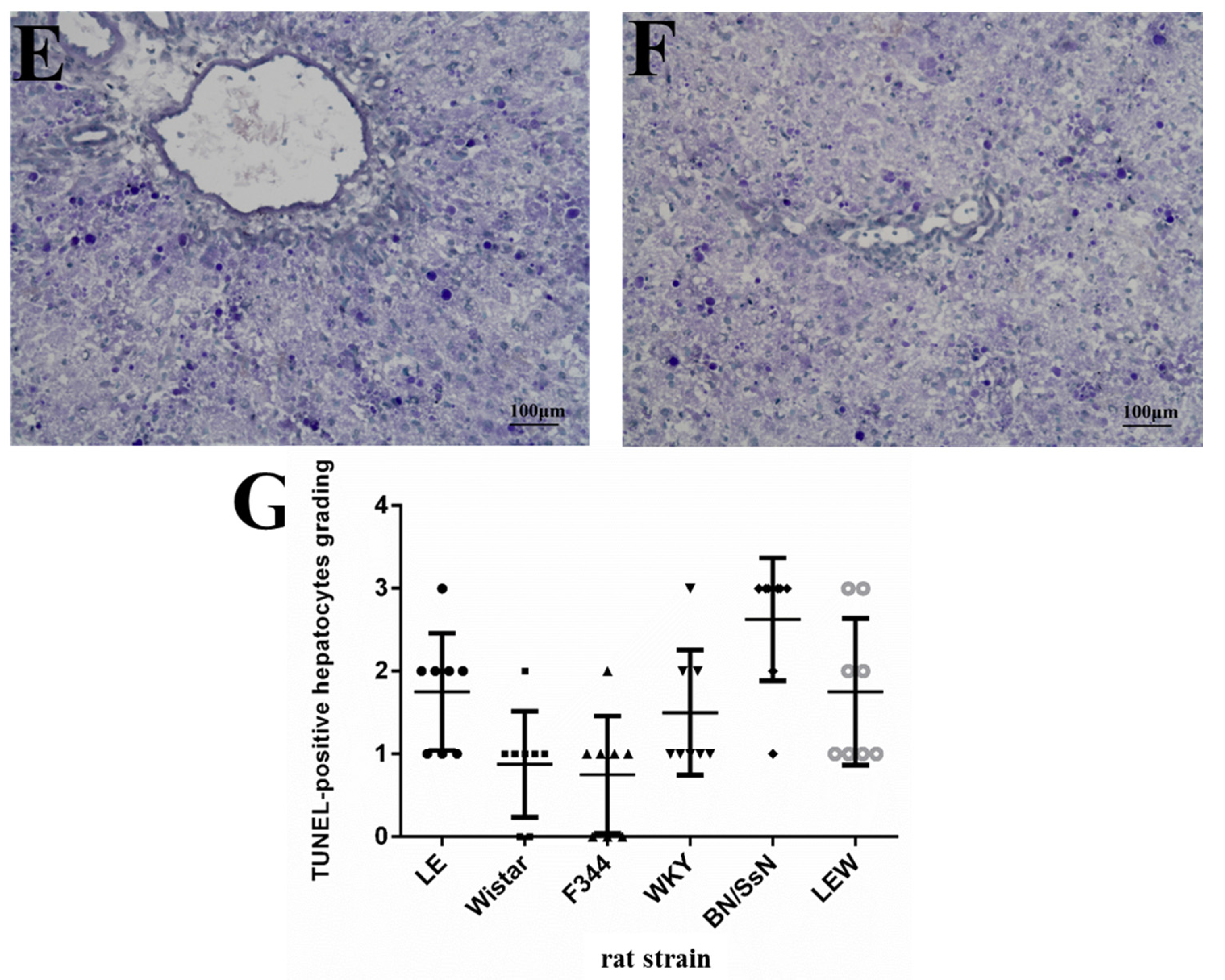
2.3. Histopathology and TUNEL Staining
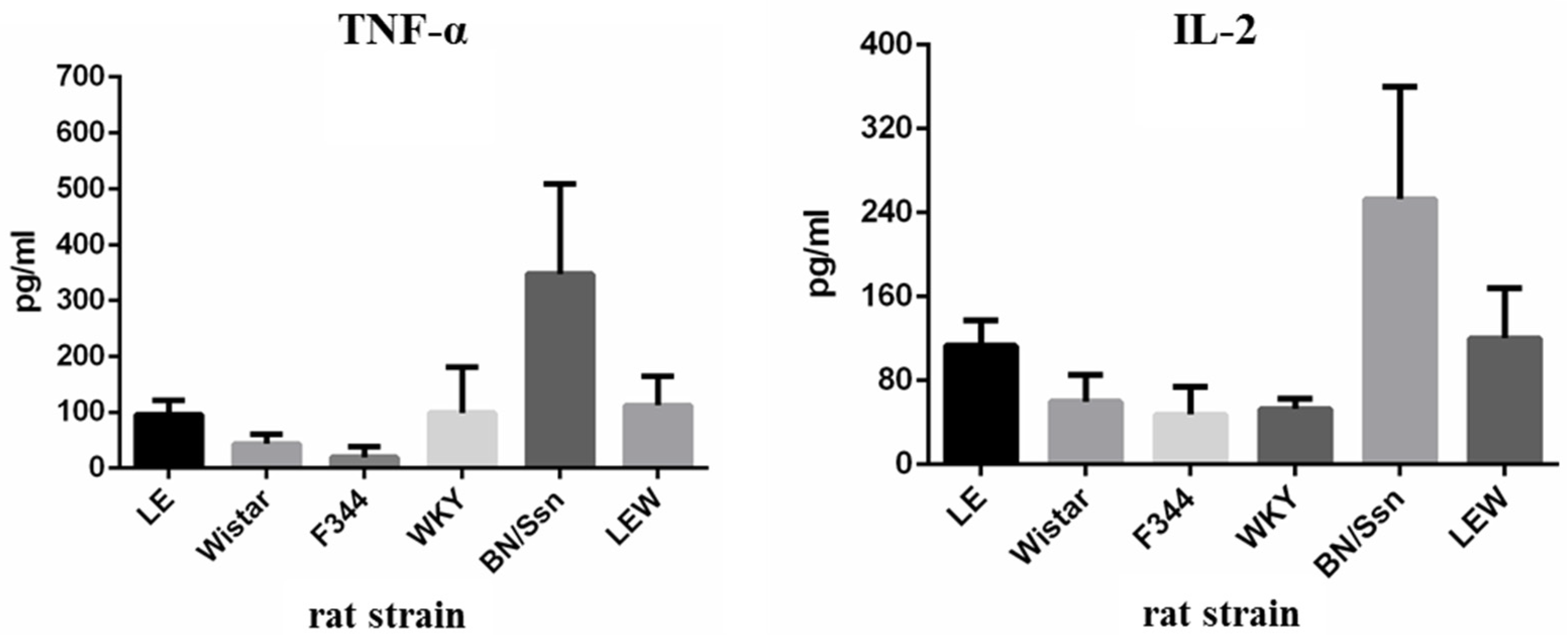
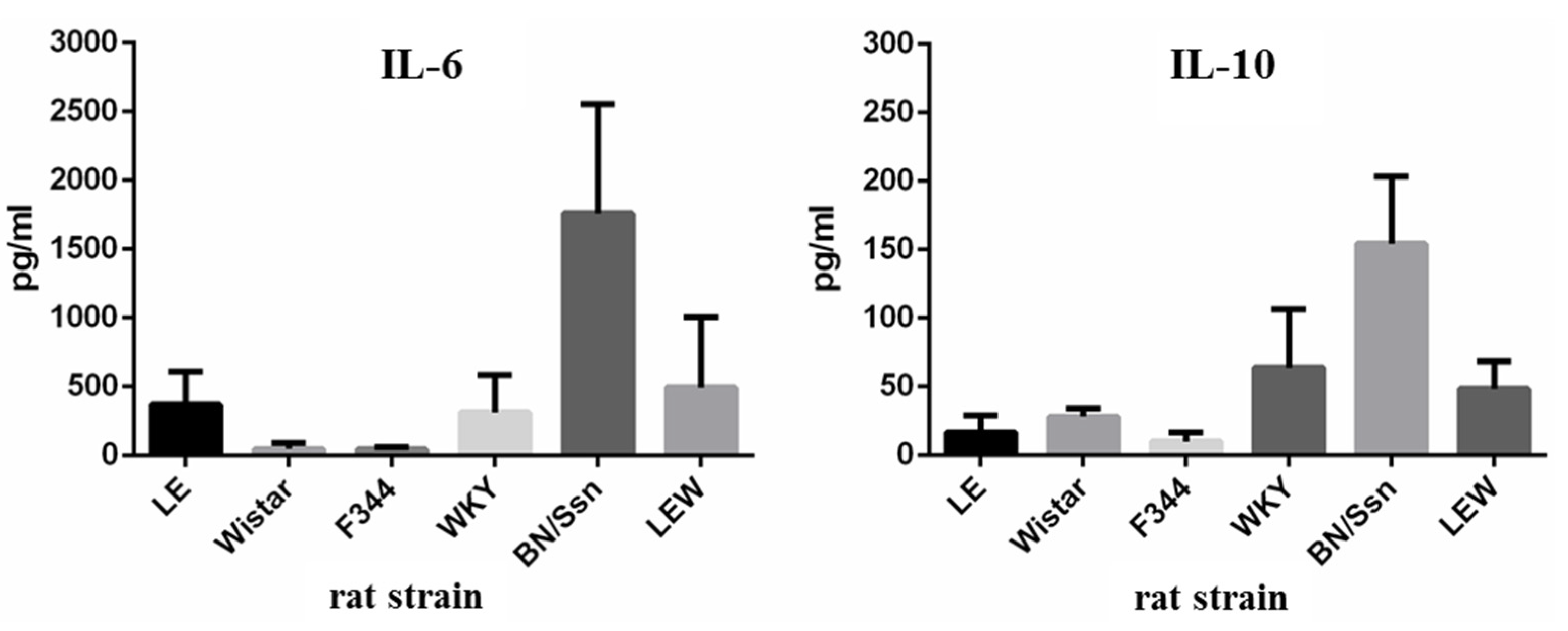
2.4. Serum Cytokine Levels
3. Discussion
4. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, H.B.; Breidenbach, W.C.; Callaghan, W.B.; Richards, G.K.; Prentis, J.J. Are burn wound biopsies obsolete? A comparative study of bacterial quantitation in burn patients using the absorbent disc and biopsy techniques. Ann. Plast. Surg. 1984, 13, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.L.; Emerson, J.; Stapp, J.R.; Yim, D.L.; Krzewinski, J.; Louden, L.; Ramsey, B.W.; Clausen, C.R. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 1998, 27, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.B.; DiMango, E.; Bryan, R.; Gambello, M.; Iglewski, B.H.; Goldberg, J.B.; Prince, A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect. Immun. 1996, 64, 37–43. [Google Scholar] [PubMed]
- Kounnas, M.Z.; Morris, R.E.; Thompson, M.R.; FitzGerald, D.J.; Strickland, D.K.; Saelinger, C.B. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J. Biol. Chem. 1992, 267, 12420–12423. [Google Scholar] [PubMed]
- Grimsley, P.G.; Quinn, K.A.; Chesterman, C.N.; Owensby, D.A. Low density lipoprotein receptor-related protein (LRP) expression varies among Hep G2 cell lines. Thromb. Res. 1997, 88, 485–498. [Google Scholar] [CrossRef]
- Laithwaite, J.E.; Benn, S.J.; Yamate, J.; FitzGerald, D.J.; LaMarre, J. Enhanced macrophage resistance to Pseudomonas exotoxin A is correlated with decreased expression of the low-density lipoprotein receptor-related protein. Infect. Immun. 1999, 67, 5827–5833. [Google Scholar] [PubMed]
- Laithwaite, J.E.; Benn, S.J.; Marshall, W.S.; FitzGerald, D.J.; LaMarre, J. Divergent Pseudomonas exotoxin A sensitivity in normal and transformed liver cells is correlated with low-density lipoprotein receptor-related protein expression. Toxicon 2001, 39, 1283–1290. [Google Scholar] [CrossRef]
- Chiu, C.C.; Chen, H.H.; Chuang, H.L.; Chung, T.C.; Chen, S.D.; Huang, Y.T. Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity: An animal model in rats. J. Vet. Med. Sci. 2009, 71, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.L.; Huang, Y.T.; Chiu, C.C.; Chen, H.H.; Chu, Y.Y.; Chen, T.H. Influence of age on susceptibility to Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in Long-Evans rats. J. Vet. Med. Sci. 2009, 71, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mizuhara, H.; Kuno, M.; Seki, N.; Yu, W.G.; Yamaoka, M.; Yamashita, M.; Ogawa, T.; Kaneda, K.; Fujii, T.; Senoh, H.; et al. Strain difference in the induction of T-cell activation-associated, interferon gamma-dependent hepatic injury in mice. Hepatology 1998, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Harrill, A.H.; Watkins, P.B.; Su, S.; Ross, P.K.; Harbourt, D.E.; Stylianou, I.M.; Boorman, G.A.; Russo, M.W.; Sackler, R.S.; Harris, S.C.; et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 2009, 19, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. 2005, 569, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Fengler, V.H.; Macheiner, T.; Kessler, S.M.; Czepukojc, B.; Gemperlein, K.; Müller, R.; Kiemer, A.K.; Magnes, C.; Haybaeck, J.; Lackner, C.; et al. Susceptibility of Different Mouse Wild Type Strains to Develop Diet-Induced NAFLD/AFLD-Associated Liver Disease. PLoS ONE 2016, 11, e0155163. [Google Scholar] [CrossRef] [PubMed]
- Mikus, L.D.; Rosenthal, L.A.; Sorkness, R.L.; Lemanske, R.F., Jr. Reduced interferon-gamma secretion by natural killer cells from rats susceptible to postviral chronic airway dysfunction. Am. J. Respir. Cell Mol. Biol. 2001, 24, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.A.; Szakaly, R.J.; Leng, N.; Webster, A.T.; Verheyden, J.M.; Lashua, A.J.; Kendziorski, C.; Rosenthal, L.A.; Gern, J.E.; Sorkness, R.L.; et al. Comparison of temporal transcriptomic profiles from immature lungs of two rat strains reveals a viral response signature associated with chronic lung dysfunction. PLoS ONE 2014, 9, e112997. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sendelbach, L.E.; Parkinson, A.; Klaassen, C.D. Endotoxin pretreatment protects against the hepatotoxicity of acetaminophen and carbon tetrachloride: Role of cytochrome P450 suppression. Toxicology 2000, 147, 167–176. [Google Scholar] [CrossRef]
- Chiu, C.C.; Huang, Y.T.; Wang, Y.C.; Chang, Y.C.; Ching, Y.H.; Chen, H.H.; Chuang, H.L. Pretreatment with lipopolysaccharide ameliorates Pseudomonas exotoxin A-induced hepatotoxicity in rats. Immunopharmacol. Immunotoxicol. 2013, 35, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, R.; Jones-Carson, J.; Reed, D.A.; Dinarello, C.A.; Feingold, K.R.; Grunfeld, C.; Fantuzzi, G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: Role of tumor necrosis factor alpha and IL-18. Proc. Natl. Acad. Sci. USA 2000, 97, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Mühlen, K.A.; Schümann, J.; Wittke, F.; Stenger, S.; Van Rooijen, N.; Van Kaer, L.; Tiegs, G. NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J. Immunol. 2004, 172, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Schümann, J.; Mühlen, K.; Kiemer, A.K.; Vollmar, A.M.; Tiegs, G. Parenchymal, but not leukocyte, TNF receptor 2 mediates T cell-dependent hepatitis in mice. J. Immunol. 2003, 170, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Huang, Y.T.; Chuang, H.L.; Chen, H.H.; Chung, T.C. Co-exposure of lipopolysaccharide and Pseudomonas aeruginosa exotoxin A-induced multiple organ injury in rats. Immunopharmacol. Immunotoxicol. 2009, 31, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Beltrami, B.; Raschi, E.; Di Marco, R.; Magro, G.; Grasso, S.; Bendtzen, K.; Fiorelli, G.; Meroni, P.L. Protection from concanavalin A (Con A)-induced T cell-dependent hepatic lesions and modulation of cytokine release in mice by sodium fusidate. Clin. Exp. Immunol. 1997, 110, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Gantner, F.; Leist, M.; Lohse, A.W.; Germann, P.G.; Tiegs, G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: The role of tumor necrosis factor. Hepatology 1995, 21, 190–198. [Google Scholar] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Barkhausen, T.; Tschernig, T.; Rosenstiel, P.; van Griensven, M.; Vonberg, R.P.; Dorsch, M.; Mueller-Heine, A.; Chalaris, A.; Scheller, J.; Rose-John, S.; et al. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 2011, 39, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E.; Jackson, J.V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect. Immun. 1993, 61, 1496–1499. [Google Scholar] [PubMed]
- Thompson, K.; Maltby, J.; Fallowfield, J.; McAulay, M.; Millward-Sadler, H.; Sheron, N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology 1998, 28, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Zhou, Y.; Xu, Y.M.; Qiu, X.C.; Zhou, B.G.; Wang, F.; Long, H.; Chen, X.; Yang, T.T.; Ma, B.A.; et al. A caspase-6 and anti-HER2 antibody chimeric tumor-targeted proapoptotic molecule decreased metastasis of human osteosarcoma. Cancer Investig. 2009, 27, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, M.; Gorzkiewicz, M.; Grzesik, J.; Walczak-Drzewiecka, A.; Salkowska, A.; Rodakowska, E.; Steczkiewicz, K.; Rychlewski, L.; Dastych, J.; Ginalski, K. Towards Engineering Novel PE-Based Immunotoxins by Targeting Them to the Nucleus. Toxins (Basel) 2016, 8. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-C.; Wang, Y.-C.; Huang, W.-C.; Chen, Y.-H.; Hung, S.-W.; Huang, Y.-T.; Chuang, H.-L.; Chang, Y.-C. Differences in Genetic Background Contribute to Pseudomonas Exotoxin A-Induced Hepatotoxicity in Rats. Toxins 2017, 9, 224. https://doi.org/10.3390/toxins9070224
Chiu C-C, Wang Y-C, Huang W-C, Chen Y-H, Hung S-W, Huang Y-T, Chuang H-L, Chang Y-C. Differences in Genetic Background Contribute to Pseudomonas Exotoxin A-Induced Hepatotoxicity in Rats. Toxins. 2017; 9(7):224. https://doi.org/10.3390/toxins9070224
Chicago/Turabian StyleChiu, Chien-Chao, Yu-Chih Wang, Wen-Ching Huang, Yi-Hsun Chen, Shao-Wen Hung, Yen-Te Huang, Hsiao-Li Chuang, and Yi-Chih Chang. 2017. "Differences in Genetic Background Contribute to Pseudomonas Exotoxin A-Induced Hepatotoxicity in Rats" Toxins 9, no. 7: 224. https://doi.org/10.3390/toxins9070224






