Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase
Abstract
:1. Introduction
2. Results
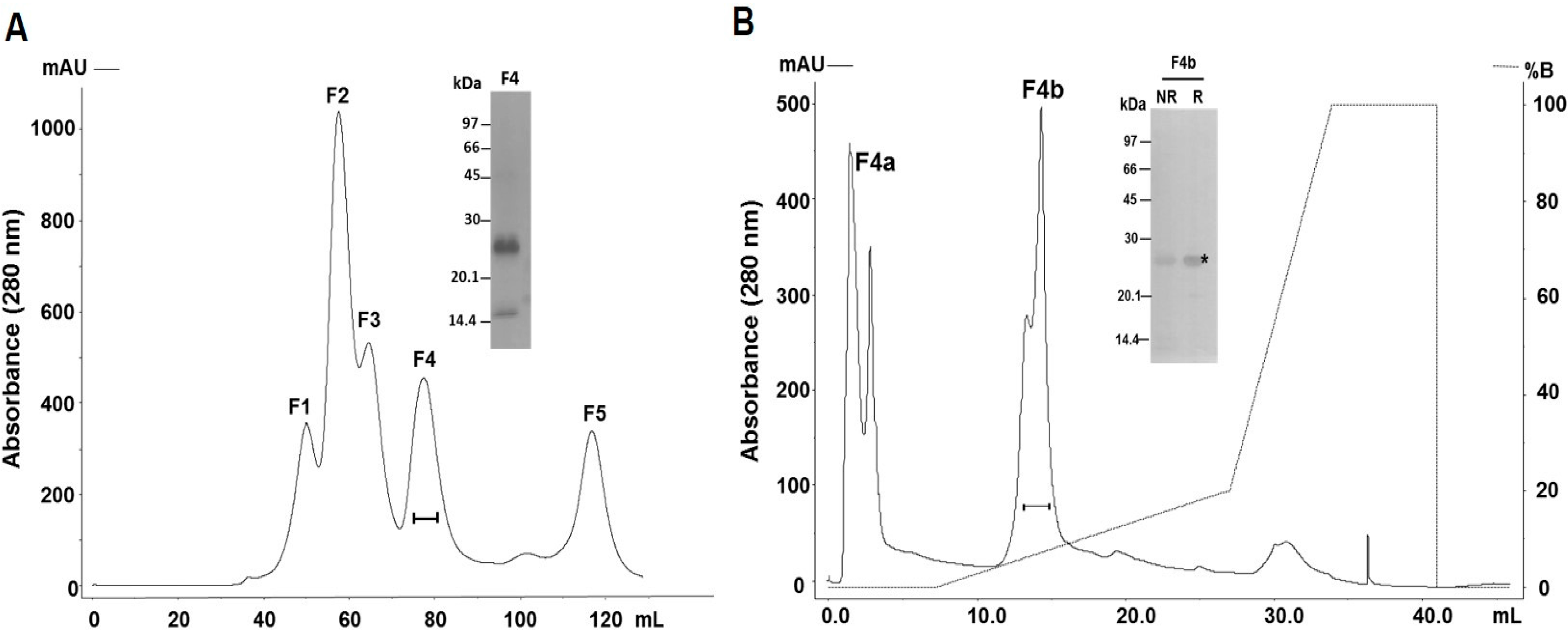
2.1. Purification and Identification of the Major PI-Class SVMP of B. atrox Venom
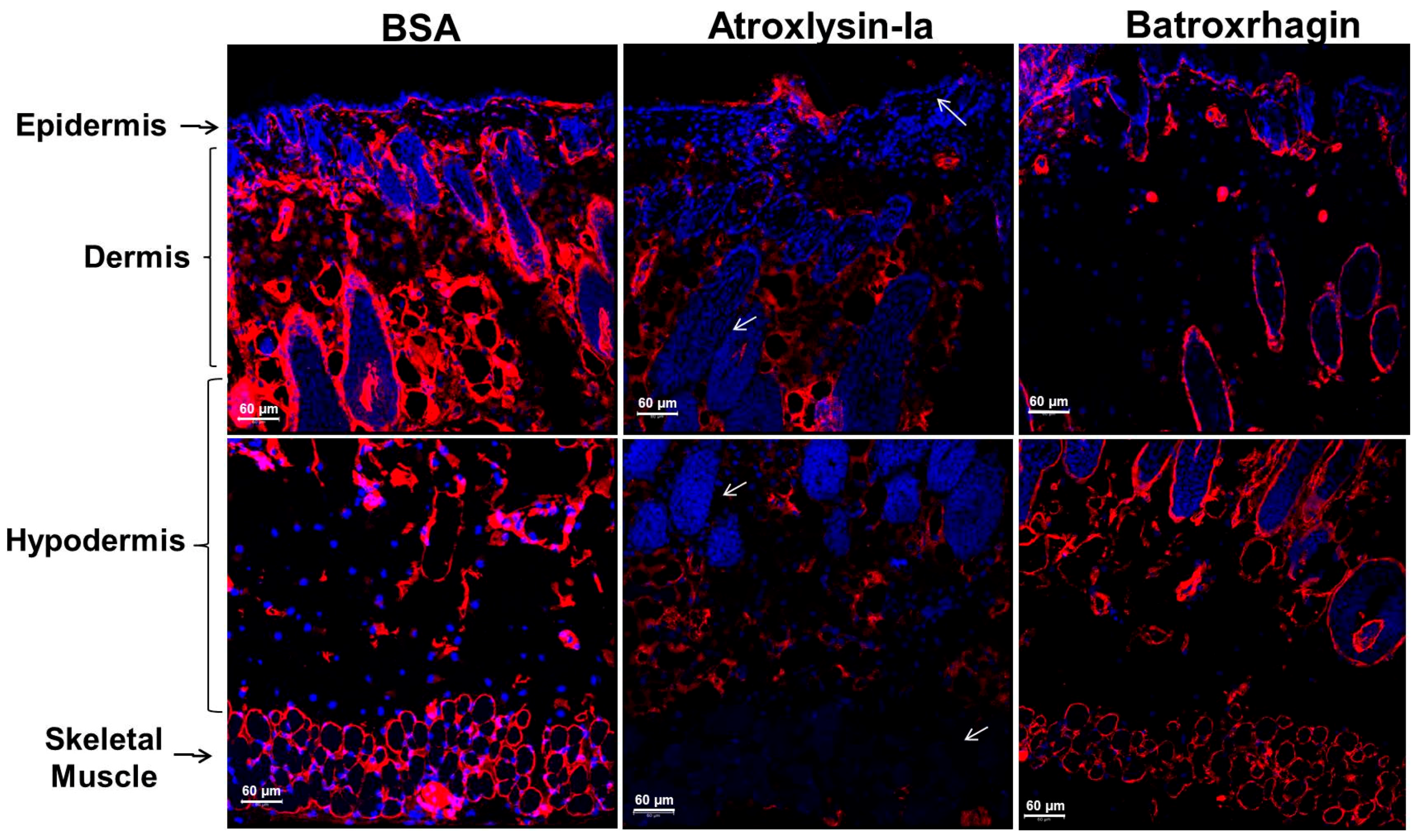
2.2. Hemorrhagic and Dermonecrotic Activities of Atroxlysin-Ia
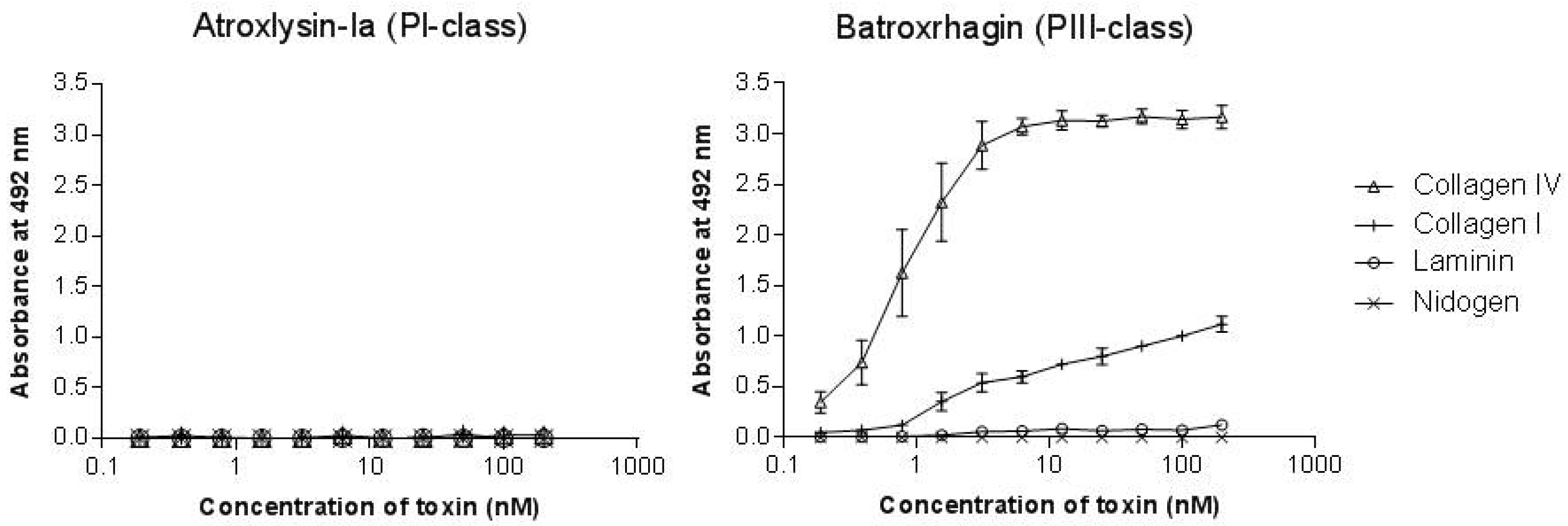
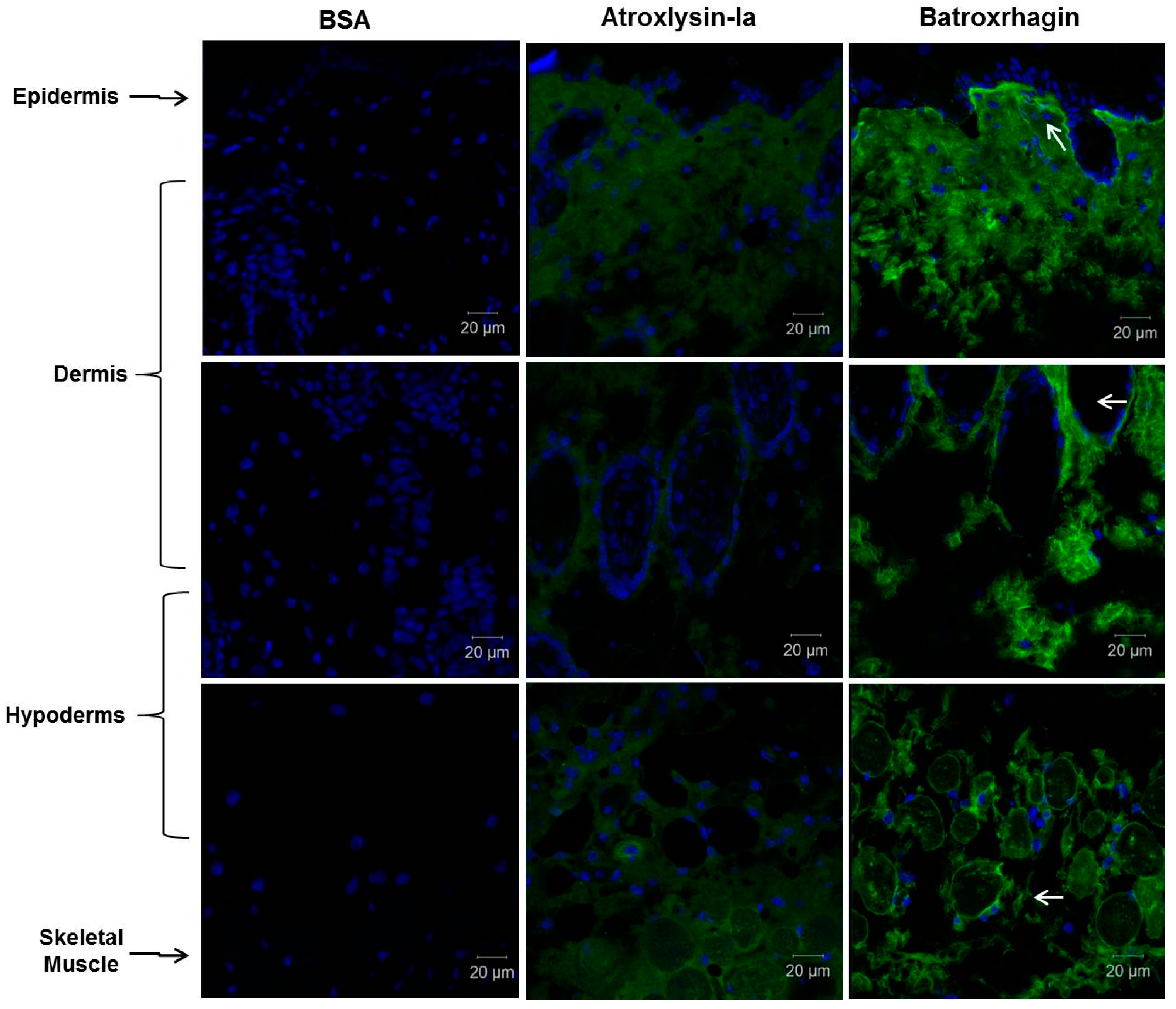
2.3. Binding of SVMPs to Plasma and Extracellular Matrix Proteins In Vitro
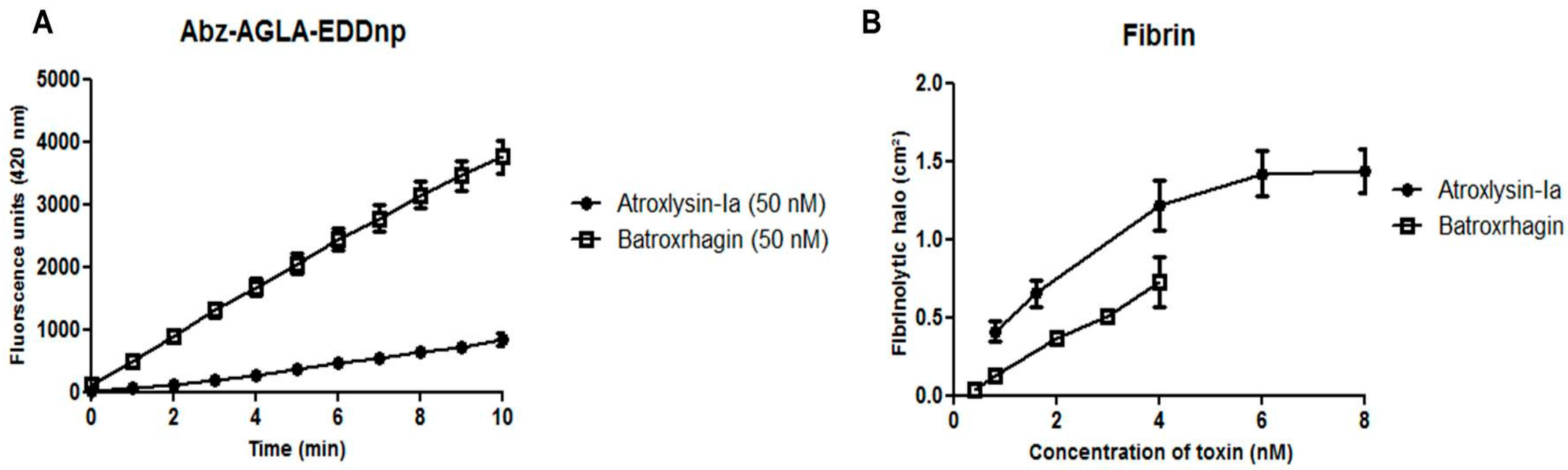
2.4. Hydrolytic Activity of Hemorrhagic SVMPs In Vitro
3. Discussion
4. Materials and Methods
4.1. Venom
4.2. Animals
4.3. Purification of SVMPs
4.4. Protein Identification
4.5. Proteolytic Activity Assays In Vitro
4.5.1. Fluorimetric Assays
4.5.2. Hydrolysis of Fibrin
4.5.3. Hydrolysis of Proteins of the Basement Membrane
4.6. Binding of SVMPs to Plasma and Extracellular Matrix Proteins In Vitro
4.7. Biological Activities In Vivo
Hemorrhagic and Dermonecrotic Activities
4.8. Histological Analysis and Detection of ECM Components
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hati, R.; Mitra, P.; Sarker, S.; Bhattacharyya, K.K. Snake venom hemorrhagins. Crit. Rev. Toxicol. 1999, 29, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Romero, M.; Núñez, J.; Chaves, F.; Borkow, G.; Ovadia, M. Skeletal muscle necrosis and regeneration after injection of bah1, a hemorrhagic metalloproteinase isolated from the venom of the snake Bothrops asper (terciopelo). Exp. Mol. Pathol. 1995, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.; Escalante, T.; Gutiérrez, J.M.; Rucavado, A. Skin pathology induced by snake venom metalloproteinase: Acute damage, revascularization, and re-epithelization in a mouse ear model. J. Investig. Dermatol. 2008, 128, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Laing, G.D.; Paine, M.J.; Dennison, J.M.; Politi, V.; Crampton, J.M.; Theakston, R.D. Processing of pro-tumor necrosis factor-alpha by venom metalloproteinases: A hypothesis explaining local tissue damage following snake bite. Eur. J. Immunol. 1996, 26, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Laing, G.D.; Clissa, P.B.; Theakston, R.D.; Moura-da-Silva, A.M.; Taylor, M.J. Inflammatory pathogenesis of snake venom metalloproteinase-induced skin necrosis. Eur. J. Immunol. 2003, 33, 3458–3463. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.S.; Baldo, C.; Oliveira, C.E.F.; de Alcântara, T.M.; Oliveira, J.D.; Gourlart, L.R.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Moura-da-Silva, A.M.; Clissa, P.B.; et al. Characterization of inflammatory reaction induced by neuwiedase, a p-i metalloproteinase isolated from Bothrops neuwiedi venom. Toxicon 2009, 54, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M. Structural considerations of the snake venom metalloproteinases, key members of the m12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Baldo, C.; Jamora, C.; Yamanouye, N.; Zorn, T.M.; Moura-da-Silva, A.M. Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: Tissue distribution and in situ hydrolysis. PLoS Negl. Trop. Dis. 2010, 4, e727. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Ortiz, N.; Rucavado, A.; Sanchez, E.F.; Richardson, M.; Fox, J.W.; Gutiérrez, J.M. Role of collagens and perlecan in microvascular stability: Exploring the mechanism of capillary vessel damage by snake venom metalloproteinases. PLoS ONE 2011, 6, e28017. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.D.; Baramova, E.N.; Bjarnason, J.B.; Fox, J.W. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type iv collagen and gelatin. J. Biol. Chem. 1989, 264, 11575–11583. [Google Scholar] [PubMed]
- Escalante, T.; Shannon, J.; Moura-da-Silva, A.M.; Gutiérrez, J.M.; Fox, J.W. Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: A biochemical and immunohistochemical study. Arch. Biochem. Biophys. 2006, 455, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Escalante, T.; Voisin, M.B.; Rucavado, A.; Morazán, D.; Macêdo, J.K.; Calvete, J.J.; Sanz, L.; Nourshargh, S.; Gutiérrez, J.M.; et al. Tissue localization and extracellular matrix degradation by pi, pii and piii snake venom metalloproteinases: Clues on the mechanisms of venom-induced hemorrhage. PLoS Negl. Trop. Dis. 2015, 9, e0003731. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.M.; Soares, A.M.; Andrião-Escarso, S.H.; Franceschi, A.M.; Rucavado, A.; Gutiérrez, J.M.; Giglio, J.R. Pathological alterations induced by neuwiedase, a metalloproteinase isolated from Bothrops neuwiedi snake venom. Biochimie 2001, 83, 471–479. [Google Scholar] [CrossRef]
- Marcussi, S.; Bernardes, C.P.; Santos-Filho, N.A.; Mazzi, M.V.; Oliveira, C.Z.; Izidoro, L.F.; Fuly, A.L.; Magro, A.J.; Braz, A.S.; Fontes, M.R.; et al. Molecular and functional characterization of a new non-hemorrhagic metalloprotease from Bothrops jararacussu snake venom with antiplatelet activity. Peptides 2007, 28, 2328–2339. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.P.; Santos-Filho, N.A.; Costa, T.R.; Gomes, M.S.; Torres, F.S.; Costa, J.; Borges, M.H.; Richardson, M.; dos Santos, D.M.; de Castro Pimenta, A.M.; et al. Isolation and structural characterization of a new fibrin(ogen)olytic metalloproteinase from Bothrops moojeni snake venom. Toxicon 2008, 51, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.A.; Hermogenes, A.L.; Magalhaes, A.; Veiga, S.S.; Gremski, L.H.; Richardson, M.; Sanchez, E.F. Isolation and biochemical characterization of a fibrinolytic proteinase from Bothrops leucurus (white-tailed jararaca) snake venom. Biochimie 2006, 88, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.P.; Menaldo, D.L.; Camacho, E.; Rosa, J.C.; Escalante, T.; Rucavado, A.; Lomonte, B.; Gutiérrez, J.M.; Sampaio, S.V. Proteomic analysis of Bothrops pirajai snake venom and characterization of bpirmp, a new p-i metalloproteinase. J. Proteom. 2013, 80, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Torres-Huaco, F.D.; Ponce-Soto, L.A.; Martins-de-Souza, D.; Marangoni, S. Purification and characterization of a new weak hemorrhagic metalloproteinase bmhf-1 from Bothrops marajoensis snake venom. Protein J. 2010, 29, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Romero, M.; Díaz, C.; Borkow, G.; Ovadia, M. Isolation and characterization of a metalloproteinase with weak hemorrhagic activity from the venom of the snake Bothrops asper (terciopelo). Toxicon 1995, 33, 19–29. [Google Scholar] [CrossRef]
- Cintra, A.C.; De Toni, L.G.; Sartim, M.A.; Franco, J.J.; Caetano, R.C.; Murakami, M.T.; Sampaio, S.V. Batroxase, a new metalloproteinase from B. atrox snake venom with strong fibrinolytic activity. Toxicon 2012, 60, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Freitas-de-Sousa, L.A.; Amazonas, D.R.; Sousa, L.F.; Sant’Anna, S.S.; Nishiyama, M.Y.; Serrano, S.M.; Junqueira-de-Azevedo, I.L.; Chalkidis, H.M.; Moura-da-Silva, A.M.; Mourão, R.H. Comparison of venoms from wild and long-term captive Bothrops atrox snakes and characterization of batroxrhagin, the predominant class piii metalloproteinase from the venom of this species. Biochimie 2015, 118, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, A.R. Serpentes peçonhentas do Brasil. In Animais Peçonhentos no Brasil, 2nd ed.; Cardoso, J.L.C., de Siqueira França, F.O., Wen, F.H., Málaque, C.M.S.A., Haddad, V., Jr., Eds.; Sarvier: São Paulo, Brazil, 2009; p. 56. [Google Scholar]
- Pardal, P.P.; Souza, S.M.; Monteiro, M.R.; Fan, H.W.; Cardoso, J.L.; França, F.O.; Tomy, S.C.; Sano-Martins, I.S.; de Sousa-e-Silva, M.C.; Colombini, M.; et al. Clinical trial of two antivenoms for the treatment of bothrops and lachesis bites in the north eastern amazon region of brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 28–42. [Google Scholar] [CrossRef]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourão, R.H.; Lima-dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of phylogeny, venom composition and neutralization by antivenom in diverse species of Bothrops complex. PLoS Negl. Trop. Dis. 2013, 7, e2442. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Schneider, F.S.; Yarleque, A.; Borges, M.H.; Richardson, M.; Figueiredo, S.G.; Evangelista, K.S.; Eble, J.A. The novel metalloproteinase atroxlysin-i from peruvian Bothrops atrox (jergón) snake venom acts both on blood vessel ecm and platelets. Arch. Biochem. Biophys. 2010, 496, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L.; Shannon, J.D.; Valente, R.H.; Rucavado, A.; Alape-Girón, A.; Kamiguti, A.S.; Theakston, R.D.; Fox, J.W.; Gutiérrez, J.M.; Arni, R.K. Amino acid sequence and crystal structure of bap1, a metalloproteinase from Bothrops asper snake venom that exerts multiple tissue-damaging activities. Protein Sci. 2003, 12, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- ClustalW. Available online: https://npsa-prabi.ibcp.fr (accessed on 17 March 2017).
- Petretski, J.H.; Kanashiro, M.M.; Rodrigues, F.G.; Alves, E.W.; Machado, O.L.T.; Kipnis, T.L. Purification and identification of a 25 kda hemorrhagin from B. Atrox venom. Protein Pept. Lett. 2001, 8, 187–192. [Google Scholar] [CrossRef]
- Patiño, A.C.; Pereañez, J.A.; Núñez, V.; Benjumea, D.M.; Fernandez, M.; Rucavado, A.; Sanz, L.; Calvete, J.J. Isolation and biological characterization of batx-i, a weak hemorrhagic and fibrinogenolytic pi metalloproteinase from colombian Bothrops atrox venom. Toxicon 2010, 56, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Jacob-Ferreira, A.L.; Bernardes, C.P.; Cintra, A.C.; Sampaio, S.V. Purification procedure for the isolation of a p-i metalloprotease and an acidic phospholipase a2 from Bothrops atrox snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M. Insights into and speculations about snake venom metalloproteinase (svmp) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.K.; Paes Leme, A.F.; Asega, A.F.; Camargo, A.C.; Fox, J.W.; Serrano, S.M. New insights into the structural elements involved in the skin haemorrhage induced by snake venom metalloproteinases. Thromb. Haemost. 2010, 104, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Baldo, C. Jararhagin, a hemorrhagic snake venom metalloproteinase from bothrops jararaca. Toxicon 2012, 60, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Koh, C.Y. Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins 2016, 8, E284. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Ramos, O.H.; Baldo, C.; Niland, S.; Hansen, U.; Ventura, J.S.; Furlan, S.; Butera, D.; Della-Casa, M.S.; Tanjoni, I.; et al. Collagen binding is a key factor for the hemorrhagic activity of snake venom metalloproteinases. Biochimie 2008, 90, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Tanjoni, I.; Butera, D.; Bento, L.; Della-Casa, M.S.; Marques-Porto, R.; Takehara, H.A.; Gutiérrez, J.M.; Fernandes, I.; Moura-da-Silva, A.M. Snake venom metalloproteinases: Structure/function relationships studies using monoclonal antibodies. Toxicon 2003, 42, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Macêdo, J.K.; Feoli, A.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M.; Fox, J.W. Muscle tissue damage induced by the venom of Bothrops asper: Identification of early and late pathological events through proteomic analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004599. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen iv is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miner, J.H.; Li, C.; Mudd, J.L.; Go, G.; Sutherland, A.E. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 2004, 131, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Macdonald, B.; Kalluri, R. Structure and function of basement membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Wallnoefer, H.G.; Lingott, T.; Gutiérrez, J.M.; Merfort, I.; Liedl, K.R. Backbone flexibility controls the activity and specificity of a protein-protein interface: Specificity in snake venom metalloproteases. J. Am. Chem. Soc. 2010, 132, 10330–10337. [Google Scholar] [CrossRef] [PubMed]
- Akao, P.K.; Tonoli, C.C.; Navarro, M.S.; Cintra, A.C.; Neto, J.R.; Arni, R.K.; Murakami, M.T. Structural studies of bmoompalpha-i, a non-hemorrhagic metalloproteinase from Bothrops moojeni venom. Toxicon 2010, 55, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Dagda, R.K.; Gasanov, S.E.; Zhang, B.; Welch, W.; Rael, E.D. Molecular models of the mojave rattlesnake (Crotalus scutulatus scutulatus) venom metalloproteinases reveal a structural basis for differences in hemorrhagic activities. J. Biol. Phys. 2014, 40, 193–216. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.A.; Díaz, N.; Nagem, R.A.; Ferreira, R.S.; Suárez, D. Unraveling the distinctive features of hemorrhagic and non-hemorrhagic snake venom metalloproteinases using molecular simulations. J. Comput. Aided Mol. Des. 2016, 30, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Adair-Kirk, T.L.; Griffin, G.L.; Meyer, M.J.; Kelley, D.G.; Miner, J.H.; Keene, D.R.; Marinkovich, M.P.; Ruppert, J.M.; Uitto, J.; Senior, R.M. Keratinocyte-targeted expression of human laminin γ2 rescues skin blistering and early lethality of laminin γ2 deficient mice. PLoS ONE 2012, 7, e45546. [Google Scholar] [CrossRef] [PubMed]
- Santos Barreto, G.N.; de Oliveira, S.S.; Dos Anjos, I.V.; Chalkidis, H.M.; Mourão, R.H.; Moura-da-Silva, A.M.; Sano-Martins, I.S.; Gonçalves, L.R. Experimental Bothrops atrox envenomation: Efficacy of antivenom therapy and the combination of Bothrops antivenom with dexamethasone. PLoS Negl. Trop. Dis. 2017, 11, e0005458. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.L.; Sherman, N.E.; Kinter, M.T.; Goldberg, J.B. Comparison of proteins expressed by Pseudomonas aeruginosa strains representing initial and chronic isolates from a cystic fibrosis patient: An analysis by 2-d gel electrophoresis and capillary column liquid chromatography-tandem mass spectrometry. Microbiology 2000, 146, 2495–2508. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, A.K.; Rocha, M.; Cajado Carvalho, D.; Juliano, M.A.; Juliano Neto, L.; Tambourgi, D.V.; Portaro, F.C. Angiotensin-degrading serine peptidase: A new chymotrypsin-like activity in the venom of Bothrops jararaca partially blocked by the commercial antivenom. Toxicon 2012, 59, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Knittel, P.S.; Long, P.F.; Brammall, L.; Marques, A.C.; Almeida, M.T.; Padilla, G.; Moura-da-Silva, A.M. Characterising the enzymatic profile of crude tentacle extracts from the south atlantic jellyfish olindias sambaquiensis (cnidaria: Hydrozoa). Toxicon 2016, 119, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jespersen, J.; Astrup, T. A study of the fibrin plate assay of fibrinolytic agents. Optimal conditions, reproducibility and precision. Haemostasis 1983, 13, 301–315. [Google Scholar] [PubMed]
- Baldo, C.; Tanjoni, I.; León, I.R.; Batista, I.F.; Della-Casa, M.S.; Clissa, P.B.; Weinlich, R.; Lopes-Ferreira, M.; Lebrun, I.; Amarante-Mendes, G.P.; et al. Bnp1, a novel p-i metalloproteinase from Bothrops neuwiedi venom: Biological effects benchmarking relatively to jararhagin, a p-iii svmp. Toxicon 2008, 51, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar] [PubMed]
- Gonzalez, R.C.; Woods, R.E.; Eddins, S.L. Digital Image Processing Using Matlab, 2nd ed.; Gatesmark Publishing: Knoxville, TN, USA, 2009. [Google Scholar]
- Dougherty, E.R. Foundations of morphological image processing. In Encyclopedia of Imaging Science and Technology; Hornak, J.P., Ed.; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Guerra, C.E.; Xavier, A.R.; Andrade, A.J.N. Watershed threshold and gray level morfology applied to object detecting in remote sensing and petrographics images. In Proceedings of the 12th International Congress of the Brazilian Geophysical Society & EXPOGEF, Rio de Janeiro, Brazil, 15–18 August 2011. [Google Scholar]
- Dougherty, E.R.; Lotufo, R.A. Hands-On Morphological Image Processing; SPIE—The International Society for Optical Engineering: Bellingham, WA, USA, 2003. [Google Scholar]






| Identified Protein # | Mascot Protein Score | Expect | Identified Peptides |
|---|---|---|---|
| BATXSVMPI5 | 4966 | 1.4 ×10−3 | TDLLNR |
| 9.8 × 10−4 | KTDLLNR | ||
| 1.3 × 10−1 | TDLLNRK | ||
| 3.5 × 10−2 | KTDLLNRK | ||
| 1.5 × 10−4 | IHQMVNIMK | ||
| 1.4 × 10−4 | ENPQCILNK | ||
| 2.0 × 10−4 | RIHQMVNIMK | ||
| 1.7 × 10−5 | ENPQCILNKR | ||
| 5 × 10−8 | STGVIQDHSEQDLK | ||
| 9.2 × 10−5 | RSTGVIQDHSEQDLK | ||
| 8.3 × 10−5 | YVELLIVVDHGMFMK | ||
| 5.9 × 10−6 | YFSDCSYIQCWDFIMK | ||
| 4.9 × 10−13 | DLINVQPAAPQTLDSFGEWR | ||
| 9.6 × 10−7 | DLINVQPAAPQTLDSFGEWRK | ||
| 9.3 × 10−6 | EAYSTMYIDILLTGVEIWSNK | ||
| 1.3 × 10−6 | SHDNAQLLTSTDFNGPTIGLAYVGSMCDPK | ||
| 9.2 × 10−6 | KSHDNAQLLTSTDFNGPTIGLAYVGSMCDPK | ||
| 2.8 × 10−11 | SHDNAQLLTSTDFNGPTIGLAYVGSMCDPKR | ||
| 3.3 × 10−6 | KSHDNAQLLTSTDFNGPTIGLAYVGSMCDPKR | ||
| 1.0 × 10−3 | VAITMAHELGHNLGISHDTGSCSCGGYSCIMSPVLSHEPSK | ||
| 4.0 × 10−2 | EAYSTMYIDILLTGVEIWSNKDLINVQPAAPQTLDSFGEWR |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas-de-Sousa, L.A.; Colombini, M.; Lopes-Ferreira, M.; Serrano, S.M.T.; Moura-da-Silva, A.M. Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase. Toxins 2017, 9, 239. https://doi.org/10.3390/toxins9080239
Freitas-de-Sousa LA, Colombini M, Lopes-Ferreira M, Serrano SMT, Moura-da-Silva AM. Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase. Toxins. 2017; 9(8):239. https://doi.org/10.3390/toxins9080239
Chicago/Turabian StyleFreitas-de-Sousa, Luciana Aparecida, Mônica Colombini, Mônica Lopes-Ferreira, Solange M. T. Serrano, and Ana Maria Moura-da-Silva. 2017. "Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase" Toxins 9, no. 8: 239. https://doi.org/10.3390/toxins9080239






