Fusarium Mycotoxins in Swiss Wheat: A Survey of Growers’ Samples between 2007 and 2014 Shows Strong Year and Minor Geographic Effects
Abstract
:1. Introduction
2. Results
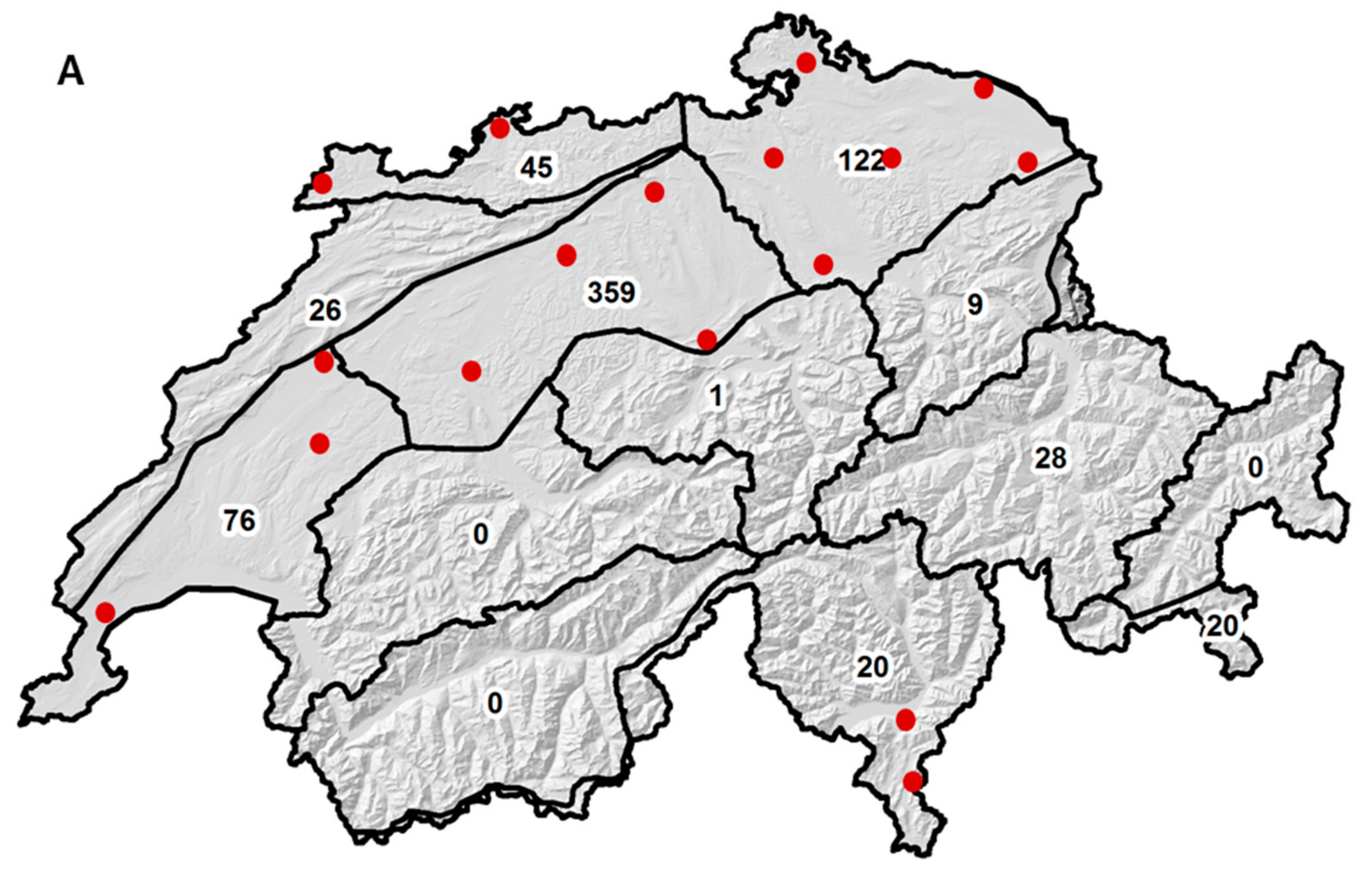
2.1. Sample Size and Origin
2.2. Detected Mycotoxins
2.3. Ratio of Samples Contaminated with DON, ZEA and NIV
2.4. Correlation between the Mycotoxins DON, ZEA and NIV
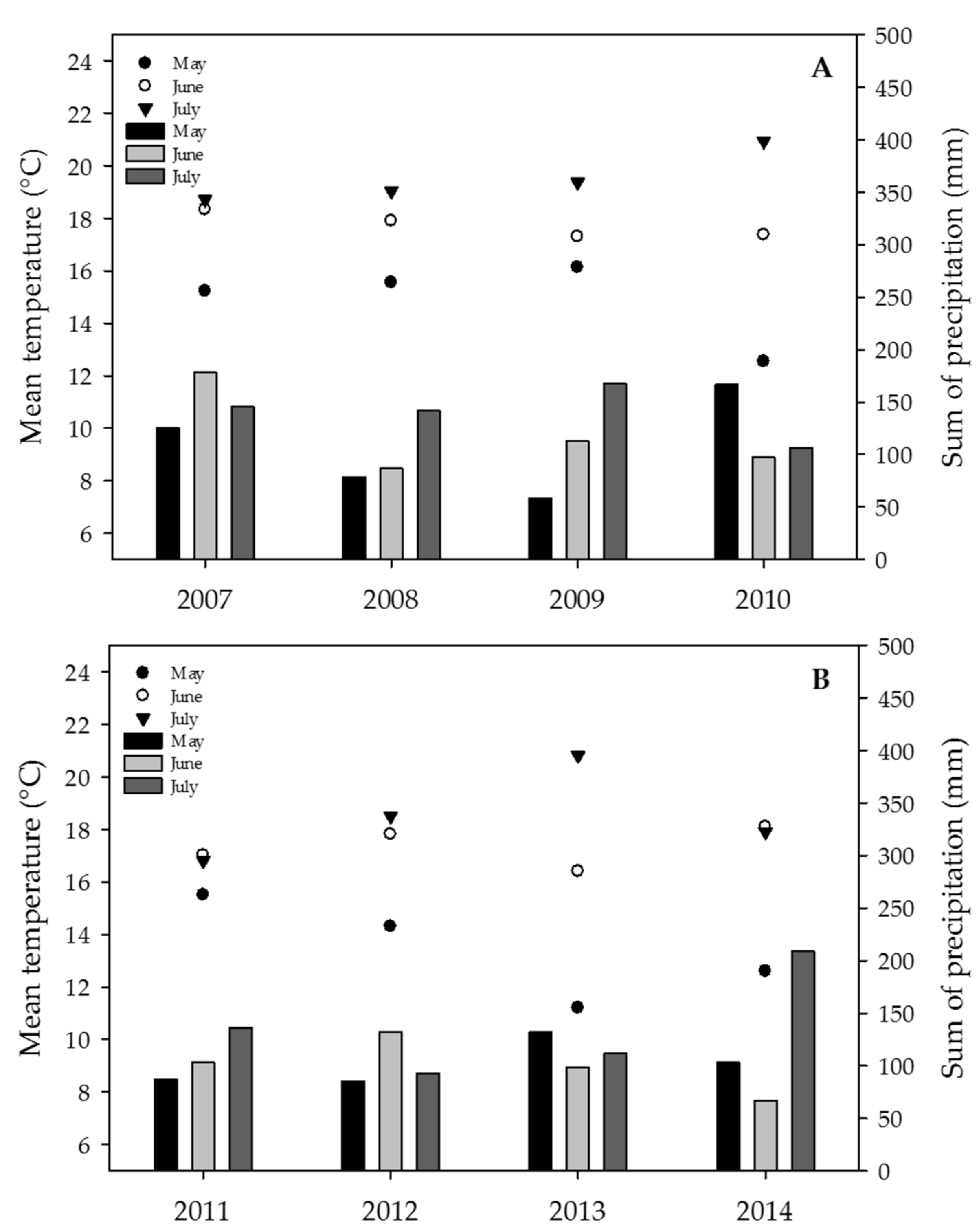
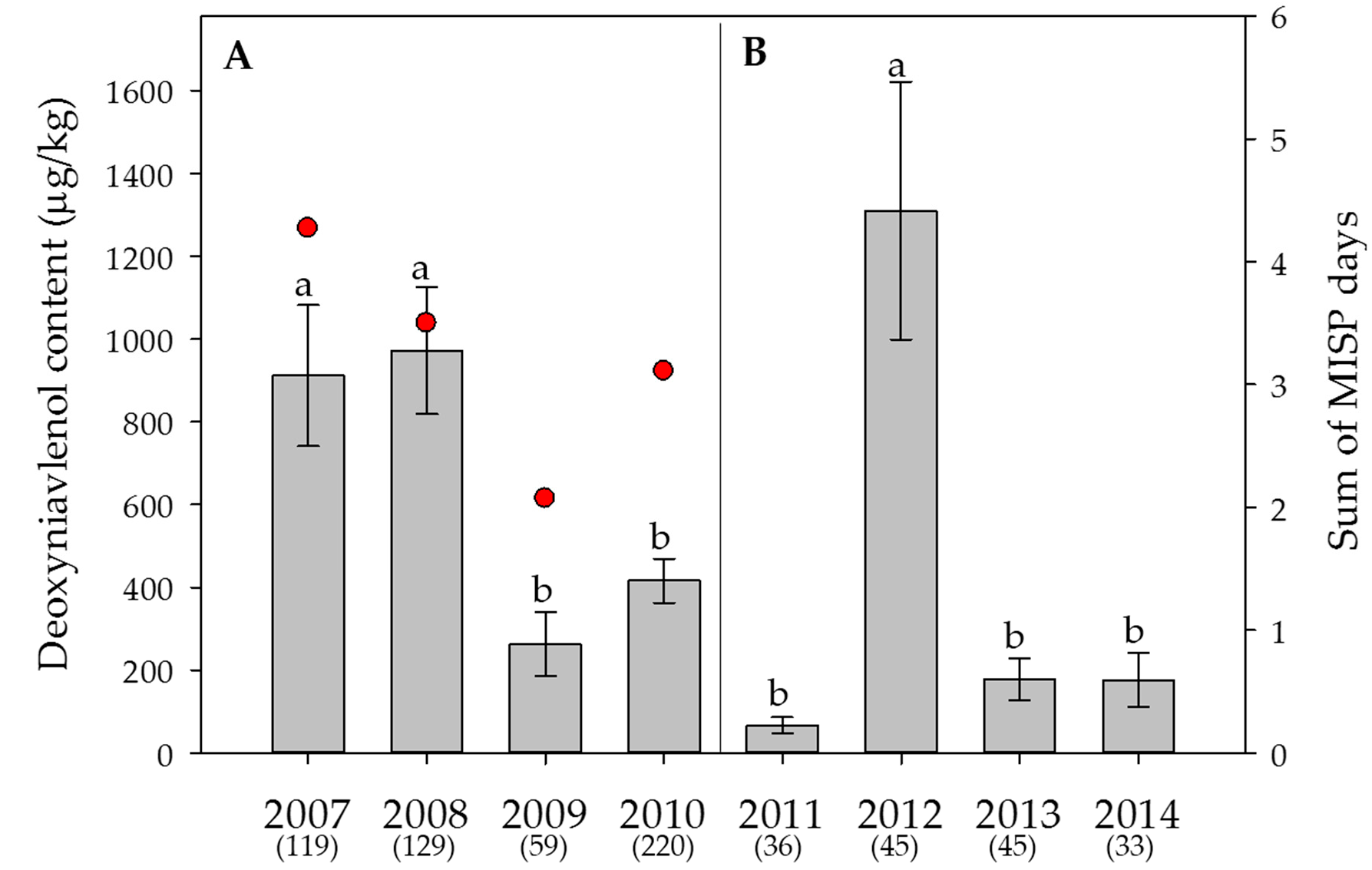
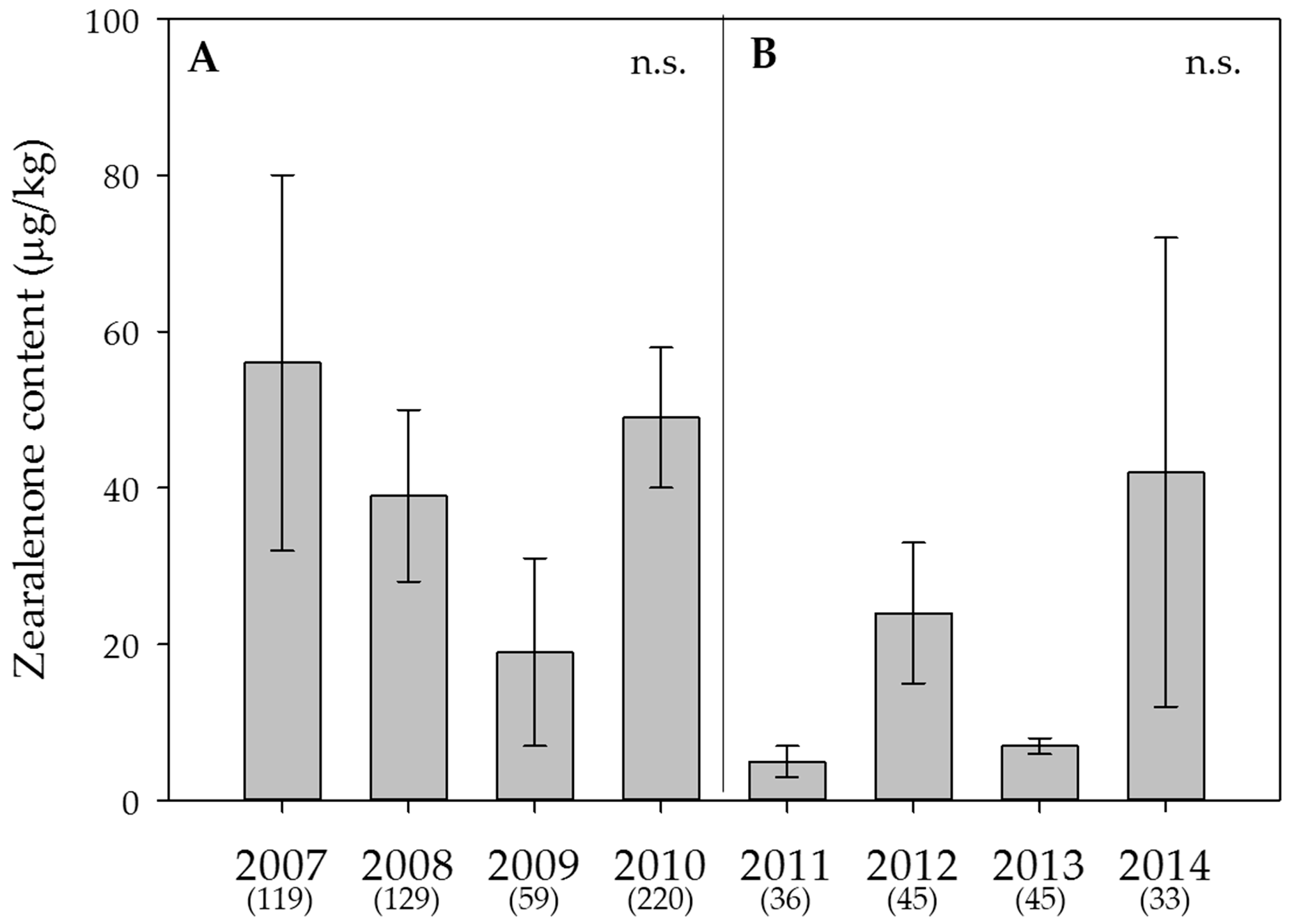
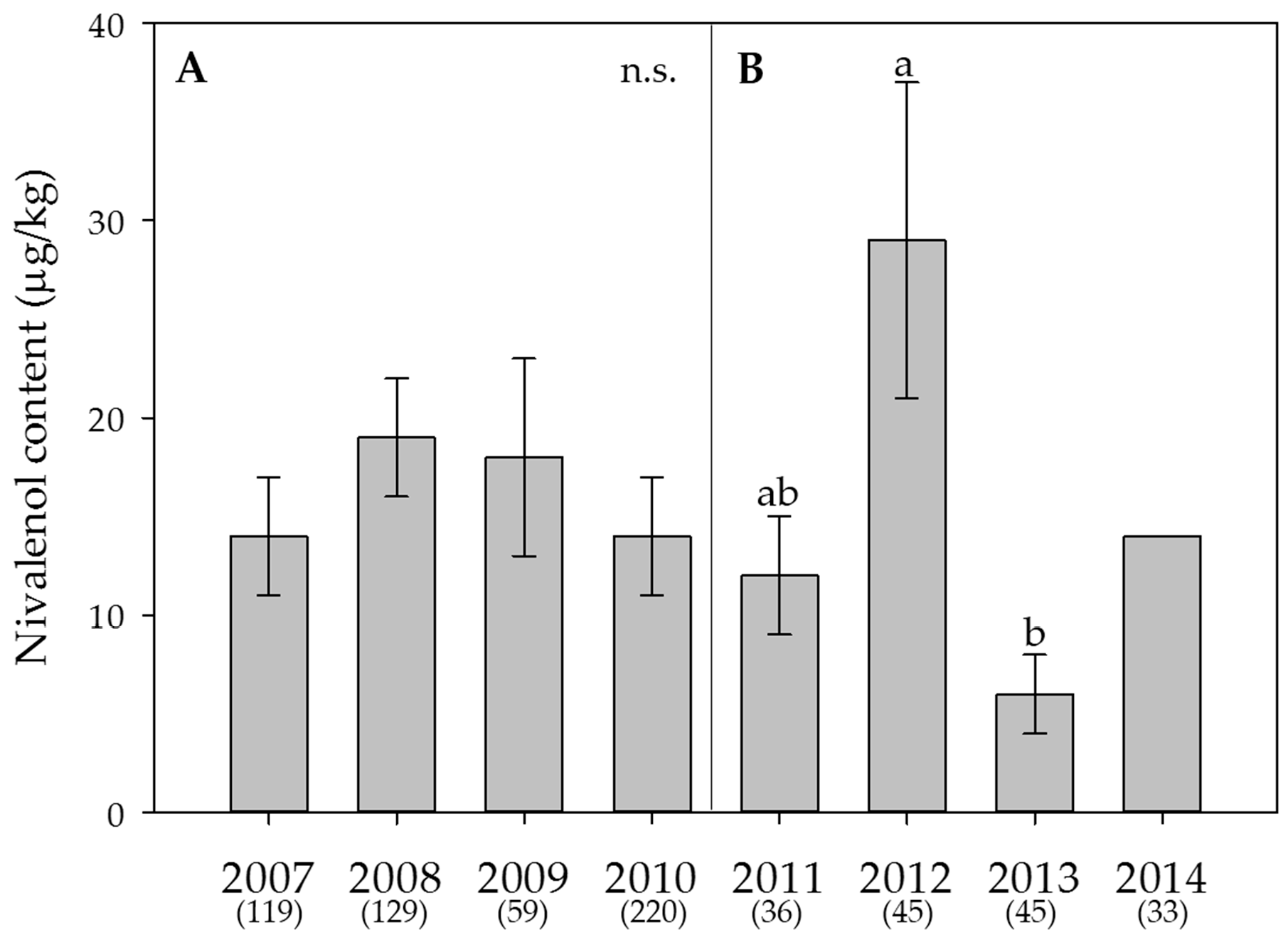
2.5. Weather Data, Effect of the Year on Mycotoxin Contents and Estimation of the DON Risk through FusaProg
2.6. Effect of Sample Origin on Mycotoxin Contents between 2007 and 2010
2.7. Ratio of Samples Exceeding Legal Limits for Food or Guidance Values for Feed
3. Discussion
3.1. Detected Mycotoxins and Correlations
3.2. Effect of the Year and the Sample Origin
3.3. Ratio of Samples Exceeding the Legal Limits for Food or Guidance Values for Feed
4. Conclusions
5. Materials and Methods
5.1. Sampling
5.2. Preparation of Subsamples
5.3. Quantification of Toxins
5.4. Estimation of the DON Risk through the Forecasting System FusaProg
5.5. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional economic impacts of Fusarium head blight in wheat and barley. Rev. Agric. Econ. 2004, 26, 332–347. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Selected Mycotoxins: Ochratoxins, Trichothecenes, Ergot; WHO, International Programme on Chemical Safety: Geneva, Switzerland, 1990; p. 263. [Google Scholar]
- Ueno, Y. Trichothecenes—Chemical, Biological and Toxicological Aspects; Kodansha: Tokyo, Japan; Elsevier: Amsterdam, The Netherlands, 1983; p. 313. [Google Scholar]
- Foroud, N.A.; Eudes, F. Tricothecenes in cereal grains. IJMS 2009, 10, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, Y. The toxicology of mycotoxins. CRC Crit. Rev. Toxicol. 1985, 14, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Baumgartner, S.; Josephs, R. The state-of-the-art in the analysis of type-A and -B trichothecene mycotoxins in cereals. Fresenius J. Anal. Chem. 2001, 371, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Kosiak, B.; Torp, M.; Skjerve, E.; Thrane, U. The prevalence and distribution of Fusarium species in Norwegian cereals: A survey. Acta Agric. Scand. Sect. B Soil Plant Sci. 2003, 53, 168–176. [Google Scholar]
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.T.; Ciasca, B.; Haidukowski, M.; Infantino, A.; Visconti, A.; Pascale, M. Mycotoxin profile of Fusarium langsethiae isolated from wheat in Italy: Production of type-A trichothecenes and relevant glucosyl derivatives. J. Mass Spectrom. 2013, 48, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”: Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Vogelgsang, S.; Sulyok, M.; Hecker, A.; Jenny, E.; Krska, R.; Schuhmacher, R.; Forrer, H.R. Toxigenicity and pathogenicity of Fusarium poae and Fusarium avenaceum on wheat. Eur. J. Plant Pathol. 2008, 122, 265–276. [Google Scholar] [CrossRef]
- Anonymous. Opinion of the scientific panel on contaminants in the food chain on a request from the Commission related to zearalenone as undesirable substance in animal feed. EFSA J. 2004, 89, 1–35. [Google Scholar]
- Anonymous. Commission Regulation (EC) Setting Maximum Levels for Certain Contaminants in Foodstuffs; No 1881/2006; The Commission of the European Communities: Brussels, Belgium, 2006; p. 20. [Google Scholar]
- Anonymous. Commission Recommendation on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products; No. 2013/165/EU; The Commission of the European Communities: Brussels, Belgium, 2013; p. 4. [Google Scholar]
- Anonymous. Commission Recommendation on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding; No. 2006/576/EC; The Commission of the European Communities: Brussels, Belgium, 2006; p. 3. [Google Scholar]
- Anonymous. BIOMIN World Mycotoxin Survey 2016; Annual Report No. 13; BIOMIN Holding GmbH: Getzersdorf, Austria, 2016; pp. 1–11. Available online: https://info.biomin.net/acton/attachment/14109/f-0463/1/-/-/l-0009/l-0009:6ee0/MAG_MTXsurveyReport_2016_EN_0117_PKO.pdf (accessed on 26 April 2007).
- Stanciu, O.; Banc, R.; Cozma, A.; Filip, L.; Miere, D.; Mañes, J.; Loghin, F. Occurence of Fusarium mycotoxins in wheat from Europe—A review. AUCFT 2015, 19, 35–60. [Google Scholar] [CrossRef]
- Chandelier, A.; Nimal, C.; André, F.; Planchon, V.; Oger, R. Fusarium species and DON contamination associated with head blight in winter wheat over a 7-year period (2003–2009) in Belgium. Eur. J. Plant Pathol. 2011, 130, 403–414. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. WMJ 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Cook, D.J.; Edwards, S.G.; Ray, R.V. The prevalence and impact of Fusarium head blight pathogens and mycotoxins on malting barley quality in UK. Int. J. Food Microbiol. 2014, 179, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. Part A 2016, 33, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Cowger, C.; Patton-Özkurt, J.; Brown-Guedira, G.; Perugini, L. Post-anthesis moisture increased Fusarium head blight and deoxynivalenol levels in North Carolina winter wheat. Phytopathology 2009, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Moschini, R.; Barreto, D.; Comerio, R. Effect of environment on Fusarium head blight intensity and deoxynivalenol content in wheat grains: Development of a forecasting system. Cereal Res. Commun. 2012, 40, 74–84. [Google Scholar] [CrossRef]
- Shah, D.A.; Molineros, J.E.; Paul, P.A.; Willyerd, K.T.; Madden, L.V.; De Wolf, E.D. Predicting Fusarium head blight epidemics with weather-driven pre- and post-anthesis logistic regression models. Phytopathology 2013, 103, 906–919. [Google Scholar] [CrossRef] [PubMed]
- MeteoSwiss, Swiss Climate. Available online: http://www.meteoswiss.admin.ch/home/climate/past/climate-of-switzerland.html (accessed on 26 April 2017).
- Schüepp, M.; Gensler, G. Klimaregionen der Schweiz. In Die Beobachtungsnetze der Schweizerischen Meteorologischen Anstalt: Konzept 1980; Arbeitsberichte der Schweizerischen Meteorologischen Anstalt: Zürich, Switzerland; Volume 93.
- Schaafsma, A.W.; Hooker, D.C. Climatic models to predict occurrence of Fusarium toxins in wheat and maize. Int. J. Food Microbiol. 2007, 119, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Musa, T.; Hecker, A.; Vogelgsang, S.; Forrer, H.R. Forecasting of Fusarium head blight and deoxynivalenol content in winter wheat with FusaProg. OEPP/EPPO Bull. 2007, 37, 283–289. [Google Scholar] [CrossRef]
- David, R.F.; BozorgMagham, A.E.; Schmale, D.G.; Ross, S.D.; Marr, L.C. Identification of meteorological predictors of Fusarium graminearum ascospore release using correlation and causality analyses. Eur. J. Plant Pathol. 2016, 145, 483–492. [Google Scholar] [CrossRef]
- Giroux, M.E.; Bourgeois, G.; Dion, Y.; Rioux, S.; Pageau, D.; Zoghlami, S.; Parent, C.; Vachon, E.; Vanasse, A. Evaluation of forecasting models for Fusarium head blight of wheat under growing conditions of Quebec, Canada. Plant Dis. 2016, 100, 1192–1201. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Hecker, A.; Musa, T.; Dorn, B.; Forrer, H.R. On-farm experiments over five years in a grain maize—Winter wheat rotation: Effect of maize residue treatments on Fusarium graminearum infection and deoxynivalenol contamination in wheat. Mycotoxin Res. 2011, 27, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Monaghan, S.; Moretti, A.; et al. Within-field variability of Fusarium head blight pathogens and their associated mycotoxins. Eur. J. Plant Pathol. 2008, 120, 21–34. [Google Scholar] [CrossRef]
- Audenaert, K.; Van Broeck, R.; Bekaert, B.; De Witte, F.; Heremans, B.; Messens, K.; Höfte, M.; Haesaert, G. Fusarium head blight (FHB) in Flanders: Population diversity, inter-species associations and DON contamination in commercial winter wheat varieties. Eur. J. Plant Pathol. 2009, 125, 445–458. [Google Scholar] [CrossRef]
- Suproniene, S.; Justesen, A.F.; Nicolaisen, M.; Mankeviciene, A.; Dabkevicius, Z.; Semaskiene, R.; Leistrumaite, A. Distribution of trichothecene and zearalenone producing Fusarium species in grain of different cereal species and cultivars grown under organic farming conidtions in Lithuania. Ann. Agric. Environ. Med. 2010, 17, 79–86. [Google Scholar] [PubMed]
- Waalwijk, C.; Kastelein, P.; De Vries, I.; Kerenyi, Z.; Van Der Lee, T.; Hesselink, T.; Köhl, J.; Kema, G. Major changes in Fusarium spp. in wheat in the Netherlands. Eur. J. Plant Pathol. 2003, 109, 743–754. [Google Scholar] [CrossRef]
- Ioos, R.; Belhadj, A.; Menez, M. Occurrence and distribution of Microdochium nivale and Fusarium species isolated from barley, durum and soft wheat grains in France from 2000 to 2002. Mycopathologia 2004, 158, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, C.A.; Dilkin, P.; Mallmann, A.O.; Oliveira, M.S.; Adaniya, Z.N.C.; Tonini, C. Prevalence and levels of deoxynivalenol and zearalenone in commercial barley and wheat grain produced in Southern Brazil: An eight-year (2008 to 2015) summary. Trop. Plant Pathol. 2017, 42, 146–152. [Google Scholar] [CrossRef]
- Fernandez Pinto, V.E.; Terminiello, L.A.; Basilico, J.C.; Ritieni, A. Natural occurrence of nivalenol and mycotoxigenic potential of Fusarium graminearum strains in wheat affected by head blight in Argentina. Braz. J. Microbiol. 2008, 39, 157–162. [Google Scholar] [CrossRef]
- Tan, D.; Flematti, G.; Ghisalberti, E.; Sivasithamparam, K.; Chakraborty, S.; Obanor, F.; Jayasena, K.; Barbetti, M. Mycotoxins produced by Fusarium spp. associated with Fusarium head blight of wheat in Western Australia. Mycotoxin Res. 2012, 28, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.; Choo, T.M.; Vigier, B.; Underhill, L. Mycotoxins in Barley and Oat Samples from Eastern Canada. Can. J. Plant Sci. 2000, 80, 977–980. [Google Scholar] [CrossRef]
- Walker, S.L.; Leath, S.; Hagler, W.M.; Murphy, J.P. Variation among isolates of Fusarium graminearum associated with Fusarium head blight in North Carolina. Plant Dis. 2001, 85, 404–410. [Google Scholar] [CrossRef]
- Pasquali, M.; Giraud, F.; Brochot, C.; Cocco, E.; Hoffmann, L.; Bohn, T. Genetic Fusarium chemotyping as a useful tool for predicting nivalenol contamination in winter wheat. Int. J. Food Microbiol. 2010, 137, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.-L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Hope, R.; Colleate, A.; Baxter, E.S. Relationship between growth and mycotoxin production by Fusarium species, biocides and environment. Eur. J. Plant Pathol. 2002, 108, 685–690. [Google Scholar] [CrossRef]
- Xu, X.M.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Nicholson, P.; Ritieni, A. Effects of fungal interactions among Fusarium head blight pathogens on disease development and mycotoxin accumulation. Int. J. Food Microbiol. 2007, 119, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Sass, V.; Milles, J.; Kramer, J.; Prange, A. Competitive interactions of Fusarium graminearum and Alternaria alternata in vitro in relation to deoxynivalenol and zearalenone production. J. Food Agric. Environ. 2007, 5, 257–261. [Google Scholar]
- Manstretta, V.; Rossi, V. Effects of weather variables on ascospore discharge from Fusarium graminearum perithecia. PLoS ONE 2015, 10, e0138860. [Google Scholar] [CrossRef] [PubMed]
- Manstretta, V.; Rossi, V. Effects of temperature and moisture on development of Fusarium graminearum perithecia in maize stalk residues. Appl. Environ. Microbiol. 2016, 82, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Monger, W.; Ritieni, A.; Nicholson, P. Effect of temperature and duration of wetness during initial infection periods on disease development, fungal biomass and mycotoxin concentrations on wheat inoculated with single, or combinations of Fusarium species. Plant Pathol. 2007, 56, 943–956. [Google Scholar] [CrossRef]
- Kharbikar, L.L.; Dickin, E.T.; Edwards, S.G. Impact of post-anthesis rainfall, fungicide and harvesting time on the concentration of deoxynivalenol and zearalenone in wheat. Food Addit. Contam. Part A 2015, 32, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S. Zearalenone risk in European wheat. WMJ 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Matthäus, K.; Dänicke, S.; Vahjen, W.; Simon, O.; Wang, J.; Valenta, H.; Meyer, K.; Strumpf, A.; Ziesenib, H.; Flachowsky, G. Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Arch. Anim. Nutr. 2004, 58, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Brinkmeyer, U.; Dänicke, S.; Valenta, H.; Flachowsky, G. Progression of deoxynivalenol and zearalenone concentrations in straw of wheat infected artificially with Fusarium culmorum. Mycotoxin Res. 2005, 21, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Pogoda, F.; Pallez, M.; Lazic, J.; Hoffmann, L.; Pasquali, M. Evidence for a reversible drought induced shift in the species composition of mycotoxin producing Fusarium head blight pathogens isolated from symptomatic wheat heads. Int. J. Food Microbiol. 2014, 182–183, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kriss, A.; Paul, P.; Xu, X.; Nicholson, P.; Doohan, F.; Hornok, L.; Rietini, A.; Edwards, S.; Madden, L. Quantification of the relationship between the environment and Fusarium head blight, Fusarium pathogen density, and mycotoxins in winter wheat in Europe. Eur. J. Plant Pathol. 2012, 133, 975–993. [Google Scholar] [CrossRef]
- Gourdain, E.; Piraux, F.; Barrier-Guillot, B. A model combining agronomic and weather factors to predict occurrence of deoxynivalenol in durum wheat kernels. WMJ 2011, 4, 129–139. [Google Scholar] [CrossRef]
- Müller, M.E.H.; Brenning, A.; Verch, G.; Koszinski, S.; Sommer, M. Multifactorial spatial analysis of mycotoxin contamination of winter wheat at the field and landscape scale. Agric. Ecosyst. Environ. 2010, 139, 245–254. [Google Scholar] [CrossRef]
- Eckard, S.; Wettstein, F.E.; Forrer, H.R.; Vogelgsang, S. Incidence of Fusarium species and mycotoxins in silage maize. Toxins 2011, 3, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Dorn, B.; Forrer, H.R.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Vogelgsang, S. Fusarium species complex and mycotoxins in grain maize from a multiyear maize hybrid trial and from grower’s fields. J. Appl. Microbiol. 2011, 111, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Oswald, I. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. WMJ 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Blandino, M.; Haidukowski, M.; Pascale, M.; Plizzari, L.; Scudellari, D.; Reyneri, A. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crops Res. 2012, 133, 139–149. [Google Scholar] [CrossRef]
- Anonymous. Kantonales Förderprogramm Boden—Speziell für Landwirte/Programme Cantonal de Promotion des Sols: Au Bénéfice des Agriculteurs. Available online: http://www.vol.be.ch/vol/de/index/landwirtschaft/landwirtschaft/bodenschutz/foerderprogramm_bodenkantonbern.html (accessed on 26 April 2017).
- Forrer, H.R.; Hecker, A.; Musa, T.; Schwab, F.; Bucheli, T.D.; Wettstein, F.E.; Vogelgsang, S. Fusarium head blight control and prevention of mycotoxin contamination in wheat with botanicals and tannic acid. Toxins 2014, 6, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals (maize, sorghum, forage grasses and dicotyledonous crops). Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Cohen, J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS; SAGE Publications: London, UK, 2009; p. 821. [Google Scholar]
| Mycotoxin Content (µg/kg) a | ||||||
|---|---|---|---|---|---|---|
| % >LOD | Mean | SEM b | Median | 90th Percentile | Maximum | |
| DON | 84 | 647 | 60 | 160 | 3686 | 10,600 |
| ZEA | 34 | 45 | 9 | 3 | 190 | 3070 |
| NIV | 19 | 15 | 2 | 4 | 78 | 470 |
| Mycotoxin Content (µg/kg) a | ||||||
|---|---|---|---|---|---|---|
| % >LOD | Mean | SEM b | Median | 90th Percentile | Maximum | |
| DON | 66 | 473 | 99 | 100 | 2510 | 9880 |
| ZEA | 22 | 18 | 7 | 5 | 47 | 1000 |
| NIV | 25 | 16 | 3 | 3 | 75 | 290 |
| Year (n) | ZEA | NIV | |
|---|---|---|---|
| 2007 (119) | DON | +0.575 ** | +0.588 ** |
| ZEA | - | +0.172 n.s. | |
| 2008 (129) | DON | +0.573 ** | +0.391 ** |
| ZEA | - | +0.211 * | |
| 2009 (59) | DON | +0.916 ** | +0.217 n.s. |
| ZEA | - | +0.067 n.s. | |
| 2010 (220) | DON | +0.523 ** | +0.189 ** |
| ZEA | - | -0.034 n.s. | |
| 2011 (36) | DON | +0.616 ** | +0.527 ** |
| ZEA | - | +0.162 n.s. | |
| 2012 (45) | DON | +0.767 ** | +0.604 ** |
| ZEA | - | +0.640 ** | |
| 2013 (45) | DON | +0.796 ** | +0.306 * |
| ZEA | - | +0.208 n.s. | |
| 2014 (33) | DON | +0.880 ** | n.a. |
| ZEA | - | n.a. |
| Climate Region a | N | DON b | ZEA (n.s.) | NIV (n.s.) |
|---|---|---|---|---|
| Eastern Jura | 45 | 1214 (203) | 27 (30) | 16 (6) |
| Western Jura | 26 | 876 (267) | 19 (40) | 13 (8) |
| N-E plateau | 122 | 404 (123) | 30 (18) | 14 (4) |
| Central plateau | 201 | 717 (96) | 81 (14) | 14 (3) |
| Western plateau | 75 | 598 (157) | 8 (23) | 30 (4) |
| E Alpine N-slope | 9 | 1211 (455) | 100 (67) | 12 (13) |
| N-C Grisons | 28 | 203 (258) | 11 (38) | 4 (7) |
| Alpine south side | 20 | 441 (305) | 12 (45) | 4 (9) |
| Year | n | % Samples DON >1250 µg/kg | % Samples ZEA >100 µg/kg |
|---|---|---|---|
| 2007 | 119 | 16.0 | 9.2 |
| 2008 | 129 | 19.4 | 10.1 |
| 2009 | 59 | 1.7 | 1.7 |
| 2010 | 220 | 7.7 | 12.3 |
| 2011 | 36 | 0.0 | 0.0 |
| 2012 | 45 | 26.7 | 6.7 |
| 2013 | 45 | 4.4 | 0.0 |
| 2014 | 33 | 3.0 | 6.1 |
| Overall | 686 | 11.2 | 7.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogelgsang, S.; Musa, T.; Bänziger, I.; Kägi, A.; Bucheli, T.D.; Wettstein, F.E.; Pasquali, M.; Forrer, H.-R. Fusarium Mycotoxins in Swiss Wheat: A Survey of Growers’ Samples between 2007 and 2014 Shows Strong Year and Minor Geographic Effects. Toxins 2017, 9, 246. https://doi.org/10.3390/toxins9080246
Vogelgsang S, Musa T, Bänziger I, Kägi A, Bucheli TD, Wettstein FE, Pasquali M, Forrer H-R. Fusarium Mycotoxins in Swiss Wheat: A Survey of Growers’ Samples between 2007 and 2014 Shows Strong Year and Minor Geographic Effects. Toxins. 2017; 9(8):246. https://doi.org/10.3390/toxins9080246
Chicago/Turabian StyleVogelgsang, Susanne, Tomke Musa, Irene Bänziger, Andreas Kägi, Thomas D. Bucheli, Felix E. Wettstein, Matias Pasquali, and Hans-Rudolf Forrer. 2017. "Fusarium Mycotoxins in Swiss Wheat: A Survey of Growers’ Samples between 2007 and 2014 Shows Strong Year and Minor Geographic Effects" Toxins 9, no. 8: 246. https://doi.org/10.3390/toxins9080246






