The Preparation and Identification of a Monoclonal Antibody against Domoic Acid and Establishment of Detection by Indirect Competitive ELISA
Abstract
:1. Introduction
2. Results
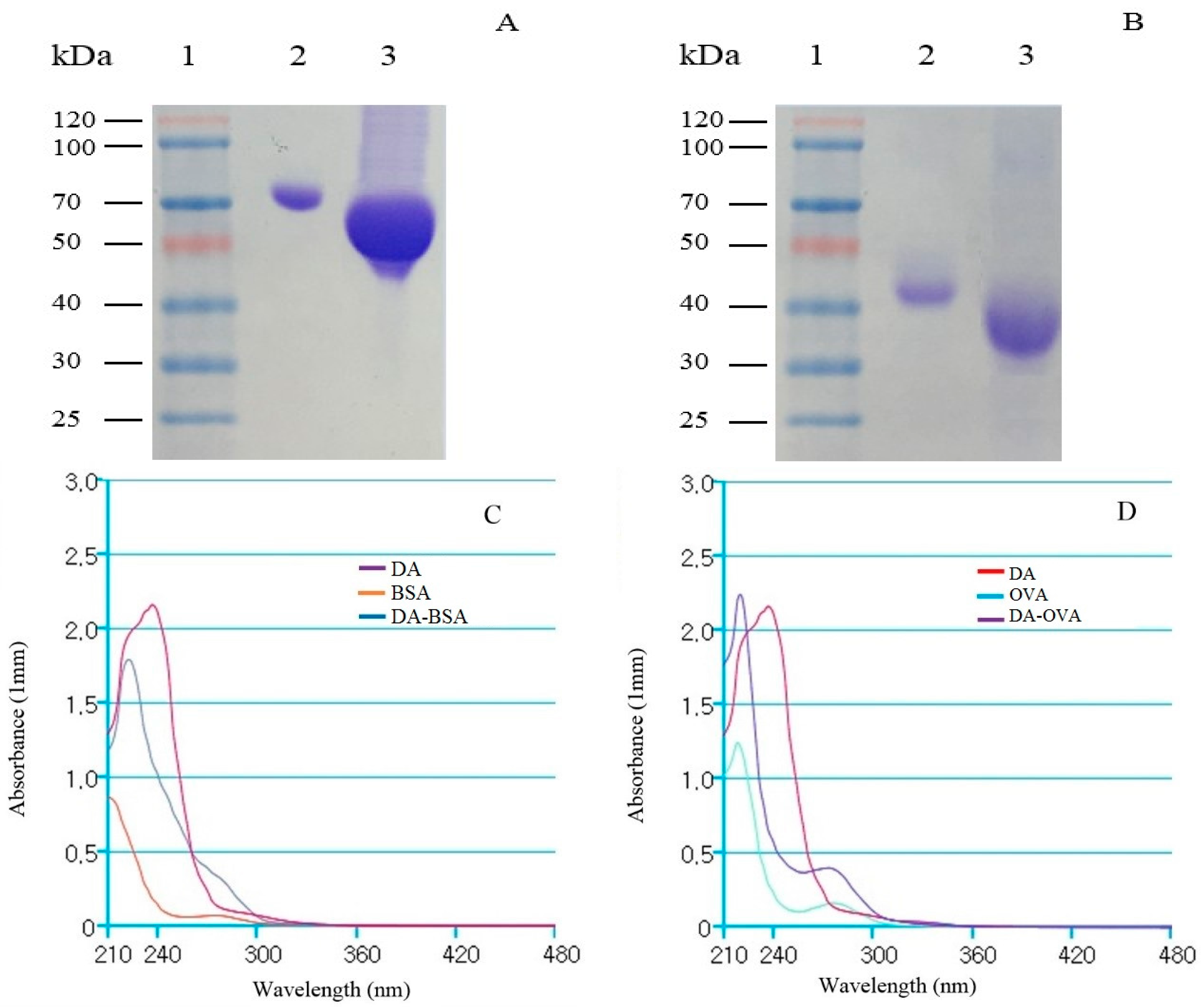
2.1. Analysis of DA-Protein Conjugates
2.2. Anti-Serum Analysis by Indirect Non-Competitive ELISA from Administered Mice
2.3. Cell Fusion and Screening of the Positive Clone
2.4. Subclass and Chromosome Count of 1C3 Hybridoma Cell Line
2.5. Production and Purification Analysis of the 1C3 mAb
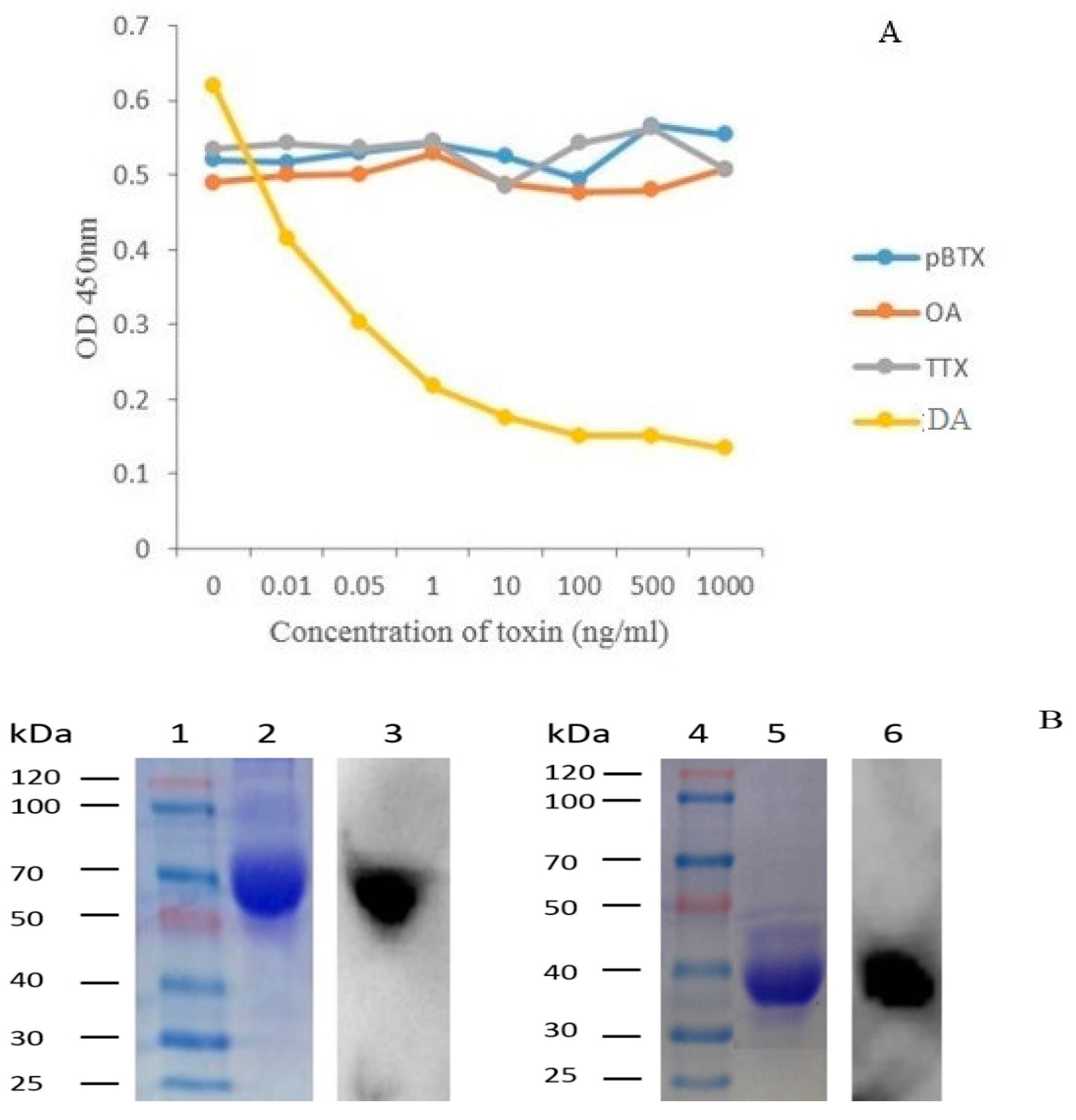
2.6. Affinity Analysis of 1C3 mAb
2.7. Specificity of 1C3 mAb
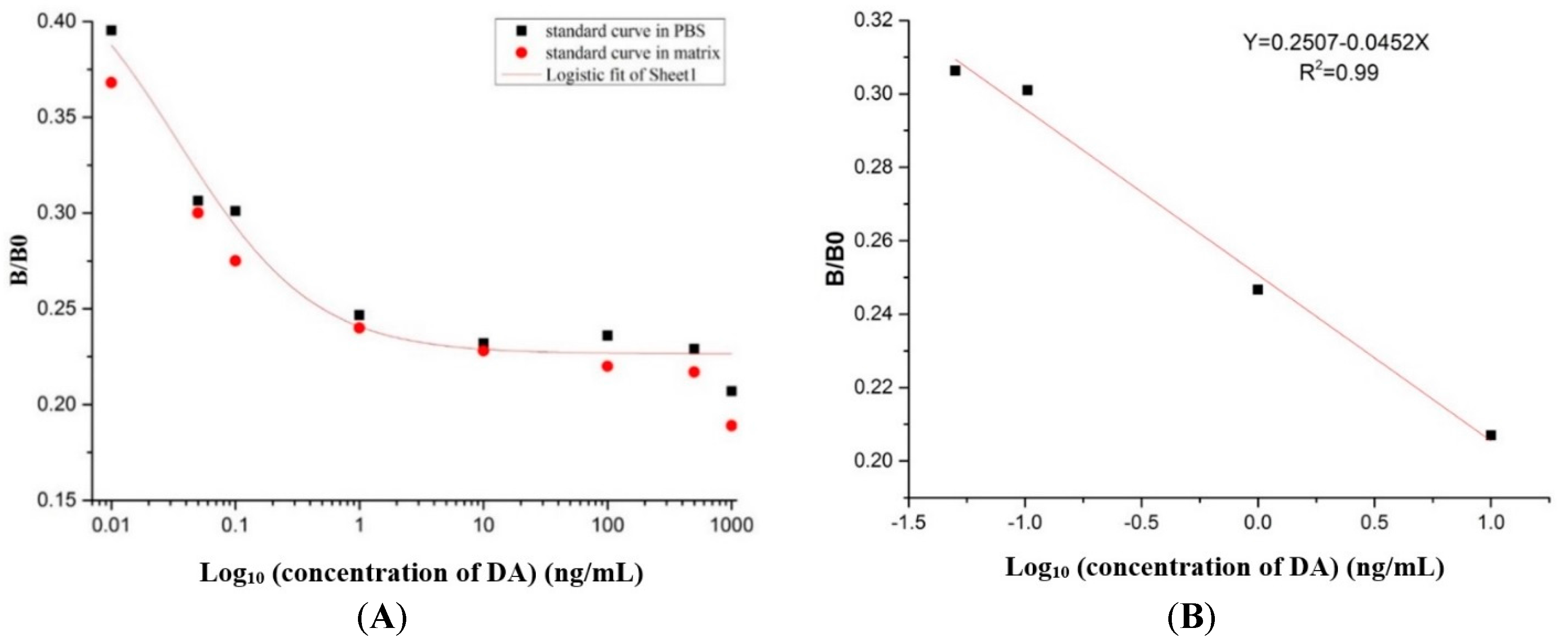
2.8. Preparation of the Standard Curve
2.9. Analysis of Recovery Test and Sample Detection by ic-ELISA Using 1C3 mAb
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials, Animals and Cell Line
5.2. Ethical Statement and Animal Care
5.3. Synthesis of DA-Protein Conjugates and Analysis
5.4. Mice Administration and iELISA Assay of Anti-Serum
5.5. Cell Fusion and Screening of Anti-DA mAb
5.6. Subclass and Determination of Chromosome Count of 1C3 mAb Producing Hybridoma
5.7. Ascitic Production, Purification and Analysis of Purified 1C3 mAb
5.8. Affinity Analysis and Specificity of 1C3 mAb
5.9. Development of ic-ELISA
5.10. Preparation of the Standard Curve and the Recovery Test
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weirich, C.A.; Miller, T.R. Freshwater harmful algal blooms: Toxins and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.R.; Wenthold, R. A kainic acid receptor from frog brain purified using domoic acid affinity chromatography. J. Biol. Chem. 1988, 263, 2500–2505. [Google Scholar] [PubMed]
- Saeed, A.F.; Awan, S.A.; Ling, S.; Wang, R.; Wang, S. Domoic acid: Attributes, exposure risks, innovative detection techniques and therapeutics. Algal Res. 2017, 24, 97–110. [Google Scholar] [CrossRef]
- Jin, N.; Ling, S.; Yang, C.; Wang, S. Preparation and identification of monoclonal antibody against citreoviridin and development of detection by ic-elisa. Toxicon 2014, 90, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Awan, S. Production of monoclonal antibody against domoic acid (da) by murine hybridoma using conditioned cell culture medium in vitro. JAPS J. Anim. Plant Sci. 2017, 27, 106–111. [Google Scholar]
- Litaker, R.W.; Stewart, T.N.; Eberhart, B.T.L.; Wekell, J.C.; Trainer, V.L.; Kudela, R.M.; Miller, P.E.; Roberts, A.; Hertz, C.; Johnson, T.A. Rapid enzyme-linked immunosorbent assay for detection of the algal toxin domoic acid. J. Shellfish Res. 2008, 27, 1301–1310. [Google Scholar] [CrossRef]
- Dakshinamurti, K.; Sharma, S.; Sundaram, M.; Watanabe, T. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. J. Neurosci. 1993, 13, 4486–4495. [Google Scholar] [PubMed]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fekete, A.; Chen, G.; Harir, M.; Zhang, L.; Tong, P.; Schmitt-Kopplin, P. Analytical approaches for an important shellfish poisoning agent: Domoic acid. J. Agric. Food Chem. 2010, 58, 11525–11533. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, M.A.; Xie, M.; Hardstaff, W. Rapid extraction and cleanup for liquid chromatographic determination of domoic acid in unsalted seafood. J. AOAC Int. 1995, 78, 543. [Google Scholar]
- Pardo, O.; Yusà, V.; León, N.; Pastor, A. Development of a pressurised liquid extraction and liquid chromatography with electrospray ionization-tandem mass spectrometry method for the determination of domoic acid in shellfish. J. Chromatogr. A 2007, 1154, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Coltro, W.K.; Mora, M.F.; Garcia, C.D.; Escarpa, A.; González, M.C.; López, M.Á. Instrumental aspects of food analysis by electrochemical methods. In Agricultural and Food Electroanalysis; John Wiley & Sons Ltd.: Chichester, UK, 2015; pp. 443–477. [Google Scholar]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Paulie, S.; Perlmann, H. Enzyme-Linked Immunosorbent Assay; John Wiley & Sons Ltd.: Chichester, UK, 2016. [Google Scholar]
- Saeed, A.F.; Wang, R.; Ling, S.; Wang, S. Antibody engineering for pursuing a healthier future. Front. Microbiol. 2017, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Finlay, W.J.; Shaw, I.; Reilly, J.P.; Kane, M. Generation of high-affinity chicken single-chain fv antibody fragments for measurement of the pseudonitzschia pungens toxin domoic acid. Appl. Environ. Microbiol. 2006, 72, 3343–3349. [Google Scholar] [CrossRef] [PubMed]
- Kania, M.; Hock, B. Development of monoclonal antibodies to domoic acid for the detection of domoic acid in blue mussel (mytilus edulis) tissue by elisa. Anal. Lett. 2002, 35, 855–868. [Google Scholar] [CrossRef]
- Liu, R.; Yu, Z.; He, Q.; Xu, Y. Preparation and identification of a monoclonal antibody against citrinin. Wei Sheng Yan Jiu = J. Hyg. Res. 2007, 36, 190–193. [Google Scholar]
- Tsao, Z.J.; Liao, Y.C.; Liu, B.H.; Su, C.C.; Yu, F.Y. Development of a monoclonal antibody against domoic acid and its application in enzyme-linked immunosorbent assay and colloidal gold immunostrip. J. Agric. Food Chem. 2007, 55, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Biswas, A.; Choi, K.; Pal, U. Methods for rapid detection of foodborne pathogens: An overview. Am. J. Food Technol. 2011, 6, 87–102. [Google Scholar] [CrossRef]
- Yousefi, M.; Khosravi-Eghbal, R.; Reza Mahmoudi, A.; Jeddi-Tehrani, M.; Rabbani, H.; Shokri, F. Comparative in vitro and in vivo assessment of toxin neutralization by anti-tetanus toxin monoclonal antibodies. Hum. Vaccines Immunother. 2014, 10, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Pan, F.; Liu, Z.; Wang, Z. Identification of tetrodotoxin antigens and a monoclonal antibody. Food Chem. 2009, 112, 582–586. [Google Scholar] [CrossRef]
- Lei, H.; He, Z.; Yuan, H.; Wu, J.; Wen, L.; Li, R.; Zhang, M.; Yuan, L.; Yuan, Z. Generation and characterization of a monoclonal antibody to penicillic acid from penicillium cyclopium. Afr. J. Biotechnol. 2010, 9, 3026–3031. [Google Scholar]
- Branaa, P.; Naar, J.; Chinain, M.; Pauillac, S. Preparation and characterization of domoic acid−protein conjugates using small amount of toxin in a reversed micellar medium: Application in a competitive enzyme-linked immunosorbent assay. Bioconjug. Chem. 1999, 10, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zeng, L.; Yang, H.; Zhong, Y.; Wang, J.; Ling, S.; Farhan Saeed, A.; Yuan, J.; Wang, S. Detection of okadaic acid (oa) using elisa and colloidal gold immunoassay based on monoclonal antibody. J. Hazard. Mater. 2017, 339, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Quilliam, M. Methods for domoic acid, the amnesic shellfish poisons. In Manual on Harmful Marine Microalgae. IOC Manuals and Guides; UNESCO Publishing: Paris, France, 1995; Volume 33, pp. 113–127. [Google Scholar]
- Wang, Y.; He, C.H.; Zheng, H.; Zhang, H. Characterization and comparison of fumonisin b(1)-protein conjugates by six methods. Int. J. Mol. Sci. 2012, 13, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Kalayu Yirga, S.; Ling, S.; Yang, Y.; Yuan, J.; Wang, S. The preparation and identification of a monoclonal antibody against citrinin and the development of detection via indirect competitive elisa. Toxins 2017, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Pang, J.; Yu, J.; Wang, R.; Liu, L.; Ma, Y.; Zhang, Y.; Jin, N.; Wang, S. Preparation and identification of monoclonal antibody against fumonisin b 1 and development of detection by ic-elisa. Toxicon 2014, 80, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, K.C.; Lira, M.S.; Malta, M.B.; Soares, S.L.; Neto, D.G.; Cardoso, J.L.; Santoro, M.L.; Junior, V.H. Comparative study on extracts from the tissue covering the stingers of freshwater (potamotrygon falkneri) and marine (dasyatis guttata) stingrays. Toxicon 2007, 50, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.; Busman, M.; Plattner, R. Development of monoclonal antibodies for the fusarin mycotoxins. Food Addit. Contam. 2008, 25, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kleivdal, H.; Kristiansen, S.I.; Nilsen, M.V.; Briggs, L. Single-laboratory validation of the biosense direct competitive enzyme-linked immunosorbent assay (elisa) for determination of domoic acid toxins in shellfish. J. AOAC Int. 2007, 90, 1000–1010. [Google Scholar] [PubMed]
- Garthwaite, I.; Ross, K.M.; Miles, C.O.; Hansen, R.P.; Foster, D.; Wilkins, A.L.; Towers, N.R. Polyclonal antibodies to domoic acid, and their use in immunoassays for domoic acid in sea water and shellfish. Nat. Toxins 1998, 6, 93–104. [Google Scholar] [CrossRef]
- Kawatsu, K.; Hamano, Y.; Noguchi, T. Production and characterization of a monoclonal antibody against domoic acid and its application to enzyme immunoassay. Toxicon 1999, 37, 1579–1589. [Google Scholar] [CrossRef]
- Stevens, R.C.; Soelberg, S.D.; Eberhart, B.-T.L.; Spencer, S.; Wekell, J.C.; Chinowsky, T.; Trainer, V.L.; Furlong, C.E. Detection of the toxin domoic acid from clam extracts using a portable surface plasmon resonance biosensor. Harmful Algae 2007, 6, 166–174. [Google Scholar] [CrossRef]
- Hummert, C.; Rühl, A.; Reinhardt, K.; Gerdts, G.; Luckas, B. Simultaneous analysis of different algal toxins by lc-ms. Chromatographia 2002, 55, 673–680. [Google Scholar] [CrossRef]
- Barbaro, E.; Zangrando, R.; Barbante, C.; Gambaro, A. Fast and sensitive method for determination of domoic acid in mussel tissue. Sci. World J. 2016, 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- James, K.J.; Gillman, M.; Lehane, M.; Gago-Martinez, A. New fluorimetric method of liquid chromatography for the determination of the neurotoxin domoic acid in seafood and marine phytoplankton. J. Chromatogr. A 2000, 871, 1–6. [Google Scholar] [CrossRef]
- Di Girolamo, F.; Ponzi, M.; Crescenzi, M.; Alessandroni, J.; Guadagni, F. A simple and effective method to analyze membrane proteins by sds-page and maldi mass spectrometry. Anticancer Res. 2010, 30, 1121–1129. [Google Scholar] [PubMed]
- Yap, W.T.; Song, W.K.; Chauhan, N.; Scalise, P.N.; Agarwal, R.; Shea, L.D. Quantification of particle-conjugated or-encapsulated peptides on interfering reagent backgrounds. Biotechniques 2014, 57, 39. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Kennebunk, M. Selecting the detection system—colorimetric, fluorescent, luminescent methods. Corning Life Sci. ELISA Tech. Bull. 2001, 14. [Google Scholar]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Lee, D.H.; Kim, D.O.; Min, W.K.; Bong, K.T.; Lee, G.G.; Seo, J.H. Production of a monoclonal antibody against ochratoxin a and its application to immunochromatographic assay. J. Agric. Food Chem. 2005, 53, 8447–8451. [Google Scholar] [CrossRef] [PubMed]
- Kozak, C.; Lawrence, J.; Ruddle, F. A sequential staining technique for the chromosomal analysis of interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp. Cell Res. 1977, 105, 109–117. [Google Scholar] [CrossRef]
- Bai, L.; Qian, J.H.; Wang, J. Purification of mouse igg from ascites fluid and serum by caprylic acid and ammonium sulfate. J. Dali Med. Coll. 2000, 4, 001. [Google Scholar]
- Liu, G.; Liang, M.; Zuo, X.; Zhao, X.; Guo, F.; Yang, S.; Zhu, R. Monoclonal antibodies directed against the outer membrane protein of bordetella avium. Monoclon. Antib. Immunodiagn. Immunother. 2013, 32, 295–300. [Google Scholar] [PubMed]
- Beatty, J.D.; Beatty, B.G.; Vlahos, W.G. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J. Immunol. Methods 1987, 100, 173–179. [Google Scholar] [CrossRef]
- Ling, S.; Chen, Q.A.; Zhang, Y.; Wang, R.; Jin, N.; Pang, J.; Wang, S. Development of elisa and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody. Biosens. Bioelectron. 2015, 71, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, M.A.; Thomas, K.; Wright, J.L. Analysis of domoic acid in shellfish by thin-layer chromatography. Nat. Toxins 1998, 6, 147–152. [Google Scholar] [CrossRef]
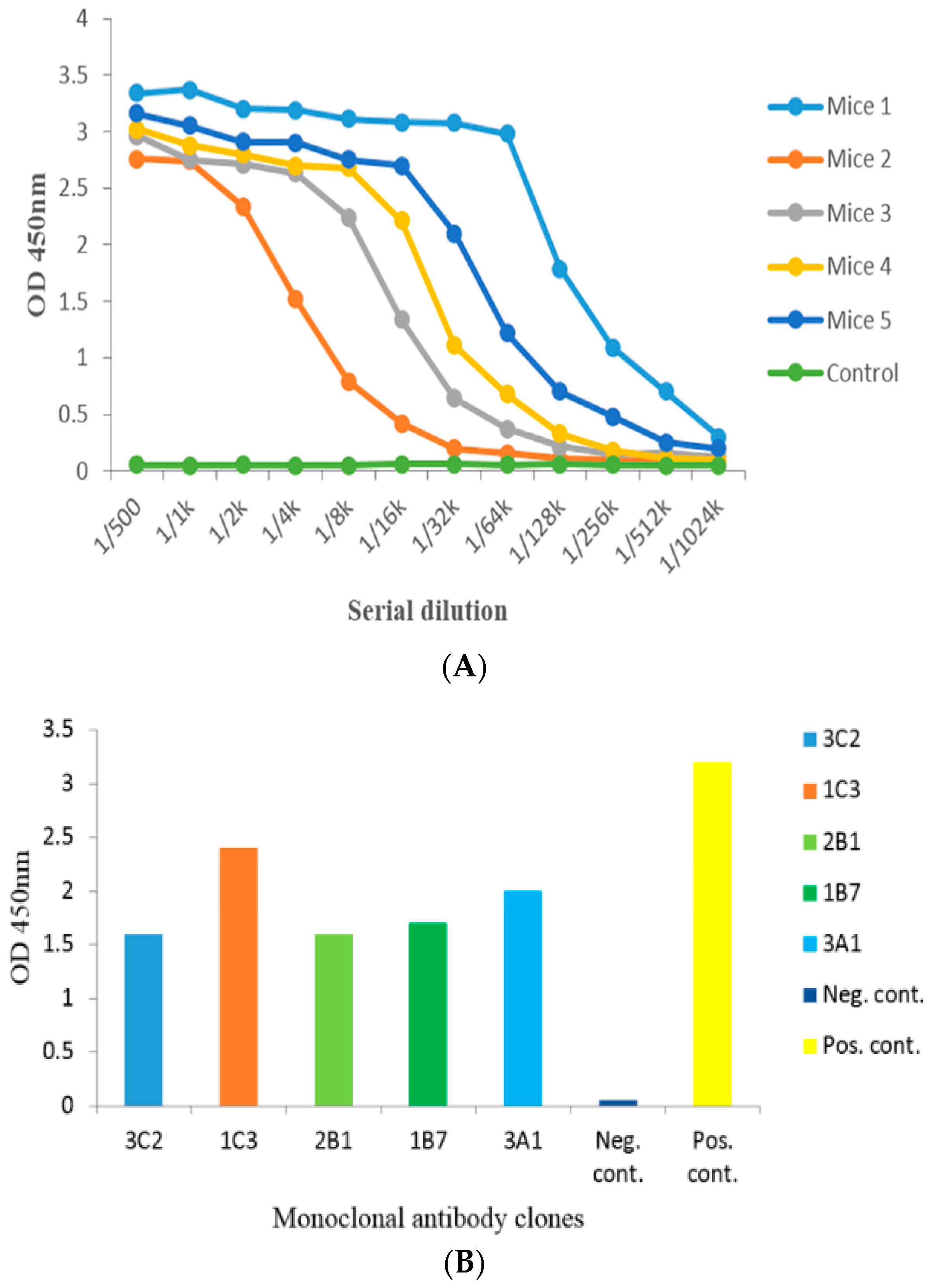


| Plates No. | Fusion Rate (%) | Positive Rate (%) | Positive Hybridoma Clones * |
|---|---|---|---|
| 1 | 90.63% (87/96) | 14.58% (14/96) | 1B7, 1C3 |
| 2 | 88.54% (85/96 | 17.71% (17/96) | 2B1 |
| 3 | 83.33% (80/96) | 12.50% (12/96) | 3A1, 3C2 |
| Average | 87.50% | 14.93% |
| Spiked Level (ng/mL) | Measured Concentration (ng/mL) | Recovery (%) | CV (%) |
|---|---|---|---|
| 0.5 | 0.53 ± 0.01 | 106.18 ± 1.7 | 0.02 |
| 5 | 5.25 ± 0.2 | 104.99 ± 3.4 | 0.03 |
| 100 | 95.03 ± 5.4 | 95.03 ± 5.4 | 0.1 |
| 1000 | 960.52 ± 7.8 | 96.05 ± 0.8 | 0.01 |
| Average | 100.56 ± 2.8 | 0.03 |
| Shellfish Samples | OD 450 nm | Detection Results (ng/mL) a |
|---|---|---|
| Control PBS (B0) | 0.44 ± 0.03 | ND b |
| Abalone | 0.28 ± 0.003 | 0.76 ± 0.5 |
| Sea mussel | 0.44 ± 0.01 | ND |
| Fresh oyster | 0.32 ± 0.01 | 0.02 ± 0.01 |
| Giant clam | 0.26 ± 0.01 | 2.89 ± 2.5 |
| King scallop | 0.41 ± 0.1 | ND |
| Razor clam | 0.25 ± 0.01 | 6.7 ± 6.4 |
| Fresh water mussel | 0.44 ± 0.04 | ND |
| Triangular clam | 0.41 ± 0.01 | ND |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, A.F.U.H.; Ling, S.; Yuan, J.; Wang, S. The Preparation and Identification of a Monoclonal Antibody against Domoic Acid and Establishment of Detection by Indirect Competitive ELISA. Toxins 2017, 9, 250. https://doi.org/10.3390/toxins9080250
Saeed AFUH, Ling S, Yuan J, Wang S. The Preparation and Identification of a Monoclonal Antibody against Domoic Acid and Establishment of Detection by Indirect Competitive ELISA. Toxins. 2017; 9(8):250. https://doi.org/10.3390/toxins9080250
Chicago/Turabian StyleSaeed, Abdullah F. U. H., Sumei Ling, Jun Yuan, and Shihua Wang. 2017. "The Preparation and Identification of a Monoclonal Antibody against Domoic Acid and Establishment of Detection by Indirect Competitive ELISA" Toxins 9, no. 8: 250. https://doi.org/10.3390/toxins9080250







