Structural Features of Apicomplexan Pore-Forming Proteins and Their Roles in Parasite Cell Traversal and Egress
Abstract
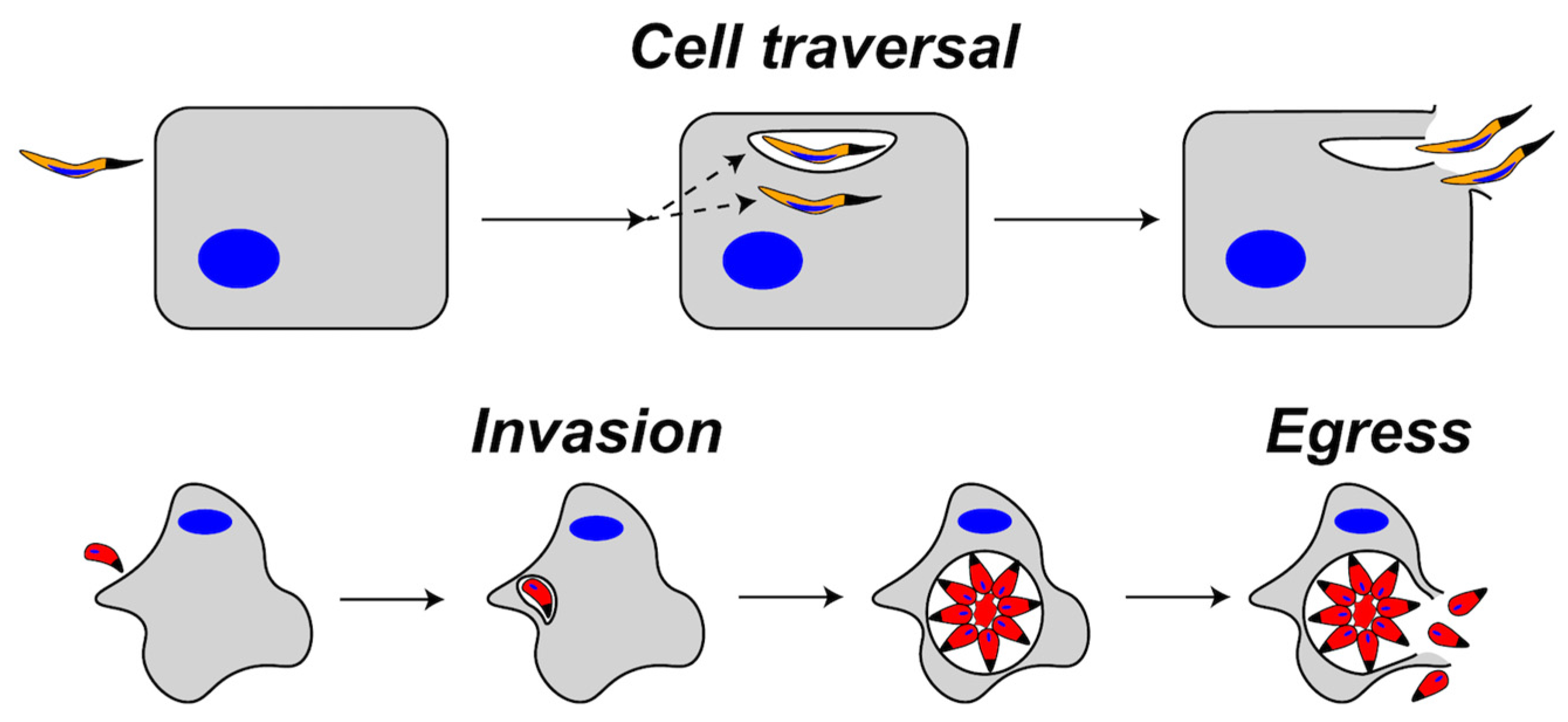
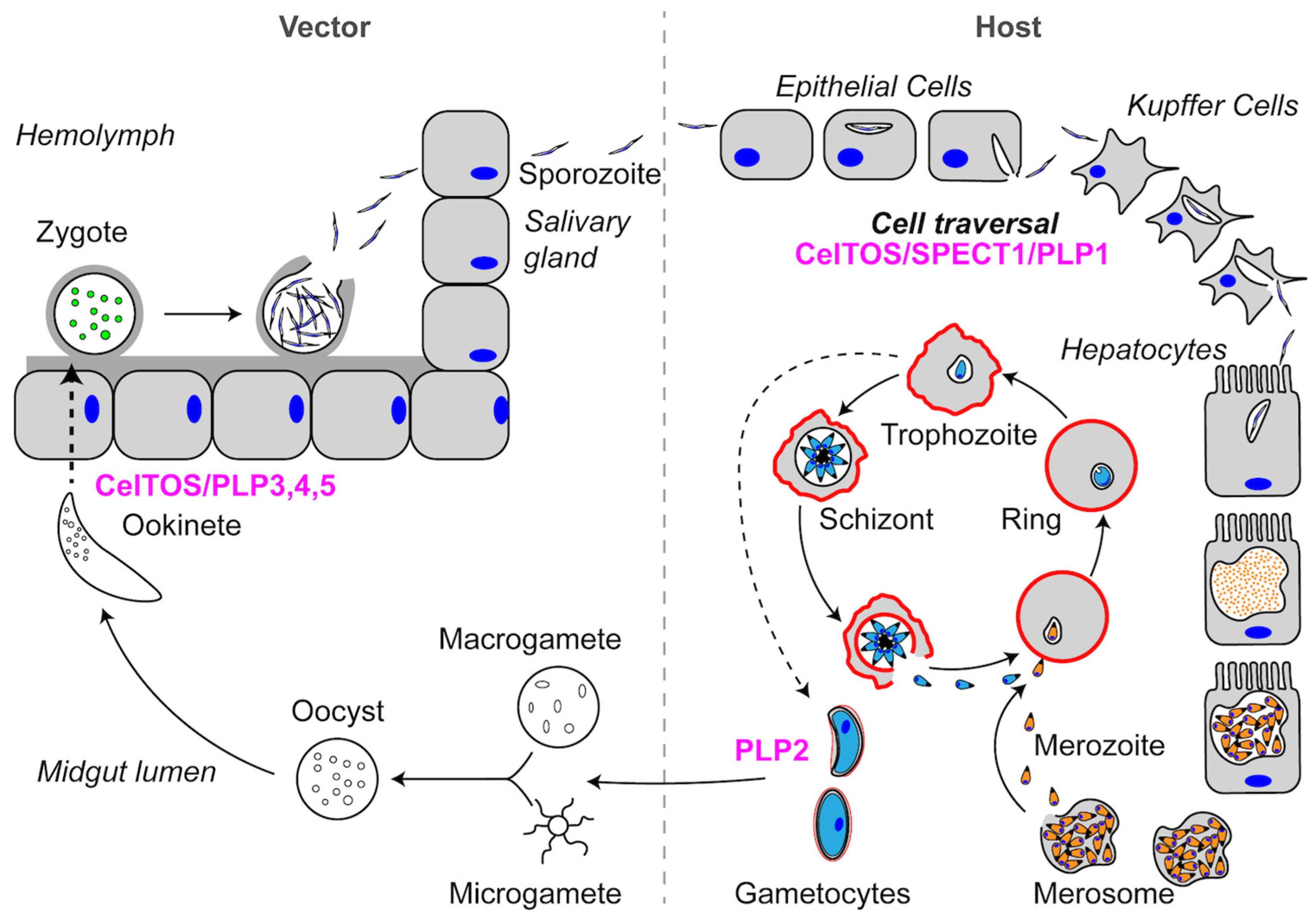
:1. Introduction
2. Malaria Encodes Two PFPs That Are Unique among Apicomplexans
2.1. SPECT1: A Unique Plasmodium Protein for Cell Traversal
2.1.1. SPECT1 and Cell Traversal
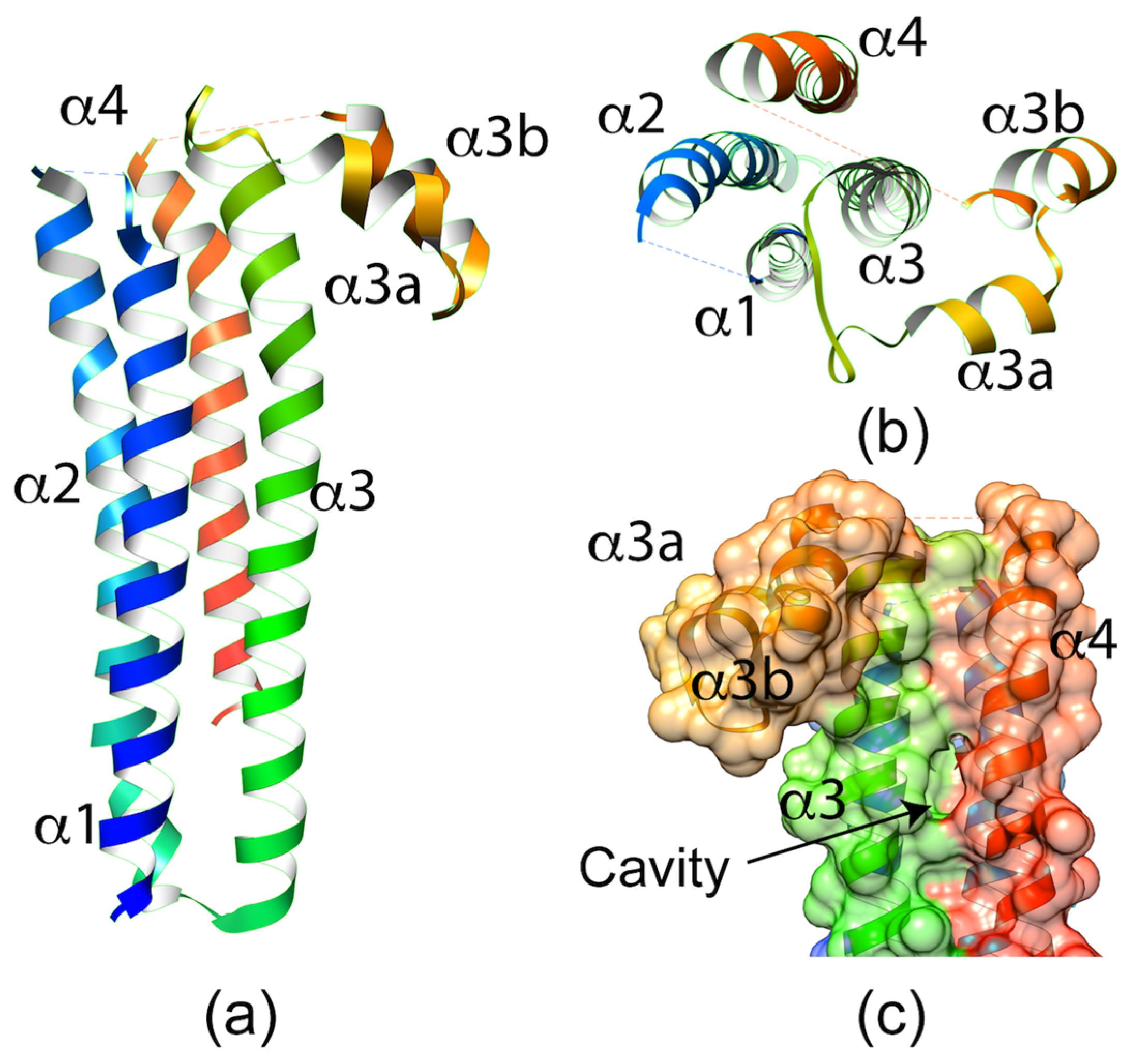
2.1.2. P. berghei SPECT1 Crystal Structure: A Common Structural Fold Associated with Membrane Proteins
2.2. CelTOS: A Conserved Protein in the Aconoidasida Is Required for Cell Traversal in Both the Mammalian Host and Vector
2.2.1. CelTOS Dictates Cell Traversal by Sporozoites and Ookinetes
2.2.2. CelTOS Is a PFP with a Preference for Phosphatidic Acid
3. Apicomplexans Share a Family of MACPF PFPs that Function at Multiple Steps in Their Life Cycles
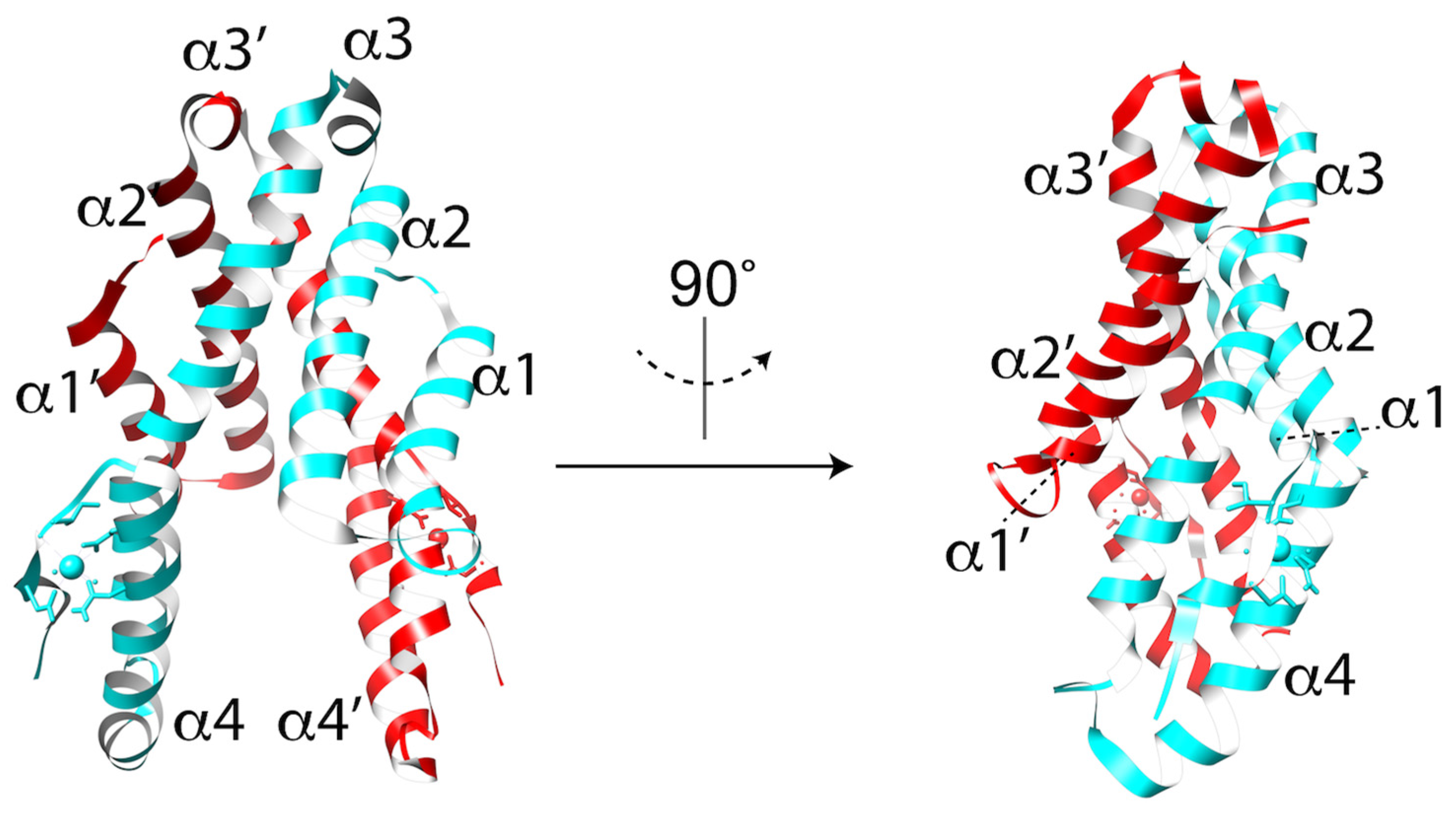
3.1. MACPF/CDC Proteins Have a Variable C-Terminal Domain
3.2. Plasmodium MACPF Proteins Are Important for All Stages of the Parasite Life Cycle
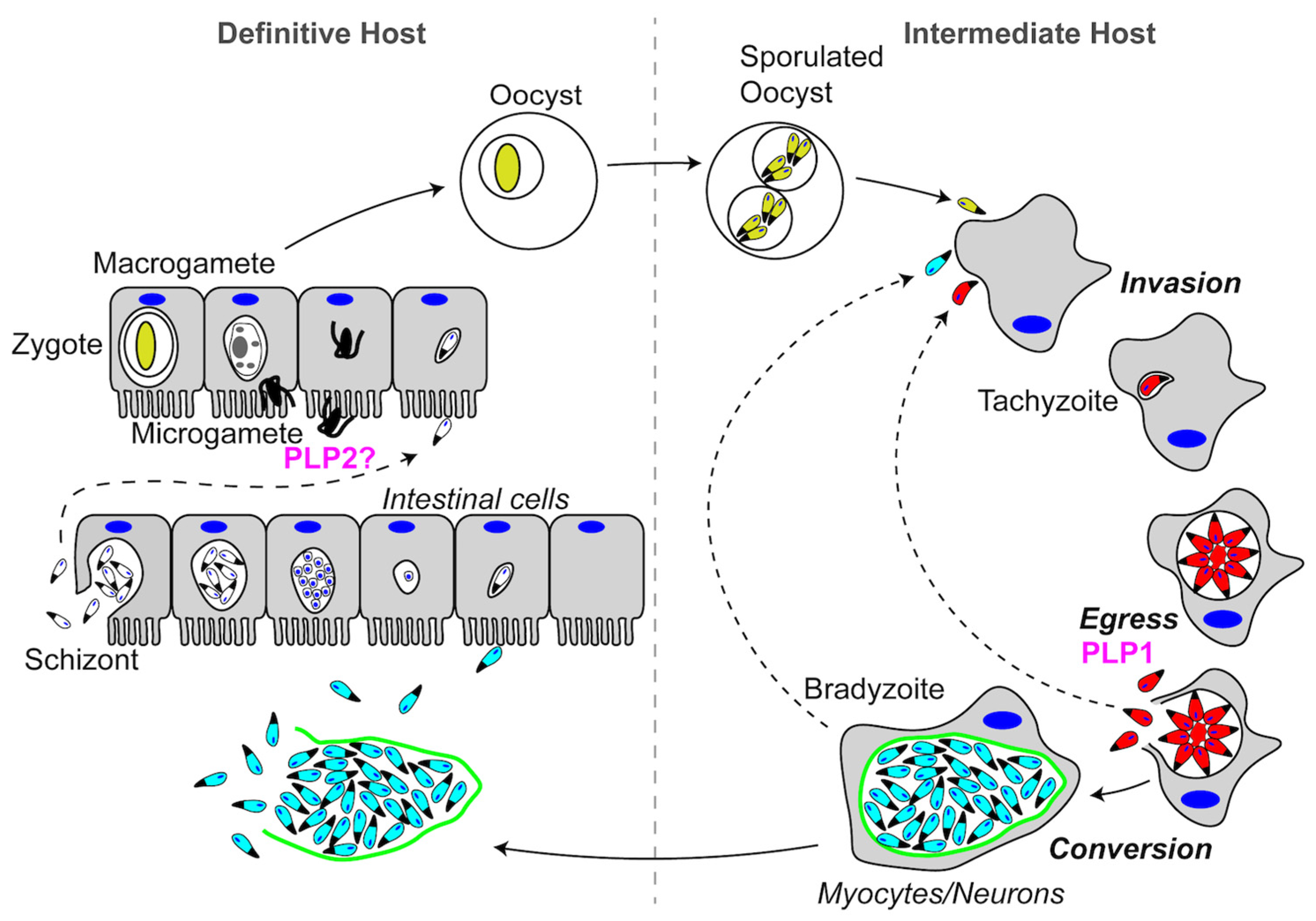
3.2.1. Role of Plasmodium PLP1 in Cell Traversal
3.2.2. Plasmodium PLP2 Functions in Gametocyte Egress
3.2.3. Plasmodium PLP3,4,5 and Cell Traversal in the Mosquito Midgut
3.3. Toxoplasma MACPF Proteins
3.3.1. Toxoplasma Infection of Its Hosts
3.3.2. Toxoplasma PLP1 & PLP2
4. Regulation of Apicomplexan PFPs
5. Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davies, A.P.; Chalmers, R.M. Cryptosporidiosis. BMJ 2009, 339, b4168. [Google Scholar] [CrossRef] [PubMed]
- Rich, S.M.; Leendertz, F.H.; Xu, G.; LeBreton, M.; Djoko, C.F.; Aminake, M.N.; Takang, E.E.; Diffo, J.L.; Pike, B.L.; Rosenthal, B.M.; et al. The Origin of Malignant Malaria. Proc. Natl. Acad. Sci. USA 2009, 106, 14902–14907. [Google Scholar] [CrossRef] [PubMed]
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [PubMed]
- Foreyt, W.J. Coccidiosis and Cryptosporidiosis in Sheep and Goats. Vet. Clin. N. Am. Food Anim. Pract. 1990, 6, 655–670. [Google Scholar] [CrossRef]
- Anderson, M.; Barr, B.; Rowe, J.; Conrad, P. Neosporosis in Dairy Cattle. Jpn. J. Vet. Res. 2012, 60, S51–S54. [Google Scholar] [PubMed]
- Kim, K.; Weiss, L.M. Toxoplasma gondii: The Model Apicomplexan. Int. J. Parasitol. 2004, 34, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Dal Peraro, M.; van der Goot, F.G. Pore-Forming Toxins: Ancient, but Never really Out of Fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Dunstone, M.A.; Tweten, R.K. Packing a Punch: The Mechanism of Pore Formation by Cholesterol Dependent Cytolysins and Membrane Attack complex/perforin-Like Proteins. Curr. Opin. Struct. Biol. 2012, 22, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.K.; Henstridge, M.A.; Warr, C.G. MACPF/CDC Proteins in Development: Insights from Drosophila Torso-Like. Semin. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Gilbert, R.J.C. Repurposing a Pore: Highly Conserved Perforin-Like Proteins with Alternative Mechanisms. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.M.; Pradel, G.; Vanderberg, J.P.; Hafalla, J.C.; Frevert, U.; Nussenzweig, R.S.; Nussenzweig, V.; Rodriguez, A. Migration of Plasmodium Sporozoites through Cells before Infection. Science 2001, 291, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Amino, R.; Giovannini, D.; Thiberge, S.; Gueirard, P.; Boisson, B.; Dubremetz, J.F.; Prevost, M.C.; Ishino, T.; Yuda, M.; Menard, R. Host Cell Traversal is Important for Progression of the Malaria Parasite through the Dermis to the Liver. Cell Host Microbe 2008, 3, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risco-Castillo, V.; Topcu, S.; Marinach, C.; Manzoni, G.; Bigorgne, A.E.; Briquet, S.; Baudin, X.; Lebrun, M.; Dubremetz, J.F.; Silvie, O. Malaria Sporozoites Traverse Host Cells within Transient Vacuoles. Cell Host Microbe 2015, 18, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Soldati, D.; Meissner, M. Toxoplasma as a Novel System for Motility. Curr. Opin. Cell Biol. 2004, 16, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kafsack, B.F.; Pena, J.D.; Coppens, I.; Ravindran, S.; Boothroyd, J.C.; Carruthers, V.B. Rapid Membrane Disruption by a Perforin-Like Protein Facilitates Parasite Exit from Host Cells. Science 2009, 323, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Garg, M.; Kumar, P.; Munjal, A.; Raja, K.D. Host-Parasite Interactions in Human Malaria: Clinical Implications of Basic Research. Front. Microbiol. 2017, 8, 889. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Darde, M.L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meis, J.F.; Verhave, J.P.; Jap, P.H.; Meuwissen, J.H. An Ultrastructural Study on the Role of Kupffer Cells in the Process of Infection by Plasmodium berghei Sporozoites in Rats. Parasitology 1983, 86, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kafsack, B.F.C.; Carruthers, V.B. Apicomplexan Perforin-Like Proteins. Commun. Integr. Biol. 2010, 3, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ishino, T.; Yano, K.; Chinzei, Y.; Yuda, M. Cell-Passage Activity is Required for the Malarial Parasite to Cross the Liver Sinusoidal Cell Layer. PLoS Biol. 2004, 2, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.S.; O’Neill, M.T.; Jennison, C.; Lopaticki, S.; Allison, C.C.; Armistead, J.S.; Erickson, S.M.; Rogers, K.L.; Ellisdon, A.M.; Whisstock, J.C.; et al. Cell Traversal Activity is Important for Plasmodium falciparum Liver Infection in Humanized Mice. Cell Rep. 2017, 18, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, B.Y.; Ghosh, P. Structure of the Essential Plasmodium Host Cell Traversal Protein SPECT1. PLoS ONE 2014, 9, e114685. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; Rosenstrom, P. Dali Server: Conservation Mapping in 3D. Nucleic Acids Res. 2010, 38, W545–W549. [Google Scholar] [CrossRef] [PubMed]
- Brett, T.J.; Legendre-Guillemin, V.; McPherson, P.S.; Fremont, D.H. Structural Definition of the F-Actin-Binding THATCH Domain from HIP1R. Nat. Struct. Mol. Biol. 2006, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Collier, S.M.; Moffett, P.; Chai, J. Structural Basis for the Interaction between the Potato Virus X Resistance Protein (Rx) and its Cofactor Ran GTPase-Activating Protein 2 (RanGAP2). J. Biol. Chem. 2013, 288, 35868–35876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Beyrakhova, K.; Liu, Y.; Alvarez, C.P.; Bueler, S.A.; Xu, L.; Xu, C.; Boniecki, M.T.; Kanelis, V.; Luo, Z.Q.; et al. Molecular Basis for the Binding and Modulation of V-ATPase by a Bacterial Effector Protein. PLoS Pathog. 2017, 13, e1006394. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Zhang, Y.; Mei, K.; Wang, S.; Lesigang, J.; Zhu, Y.; Dong, G.; Guo, W. Sec3 Promotes the Initial Binary t-SNARE Complex Assembly and Membrane Fusion. Nat. Commun. 2017, 8, 14236. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Han, L.; Bianchi, E.; Wright, G.J.; de Sanctis, D.; Jovine, L. The Structure of Sperm Izumo1 Reveals Unexpected Similarities with Plasmodium Invasion Proteins. Curr. Biol. 2016, 26, R661–R662. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Inoue, N.; Shimizu, T. Structure of IZUMO1-JUNO Reveals Sperm-Oocyte Recognition during Mammalian Fertilization. Nature 2016, 534, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Kariu, T.; Ishino, T.; Yano, K.; Chinzei, Y.; Yuda, M. CelTOS, a Novel Malarial Protein that Mediates Transmission to Mosquito and Vertebrate Hosts. Mol. Microbiol. 2006, 59, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Salman, A.M.; Leoratti, F.; Lopez-Camacho, C.; Viveros-Sandoval, M.E.; Lall, A.; El-Turabi, A.; Bachmann, M.F.; Hill, A.V.; Janse, C.J.; et al. Evaluation of Plasmodium Vivax Cell-Traversal Protein for Ookinetes and Sporozoites as a Preerythrocytic P. Vivax Vaccine. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.A.; Vega-Rodriguez, J.; Flores-Garcia, Y.; Noe, A.R.; Munoz, C.; Coleman, R.; Bruck, T.; Haney, K.; Stevens, A.; Retallack, D.; et al. The Plasmodium falciparum Cell-Traversal Protein for Ookinetes and Sporozoites as a Candidate for Preerythrocytic and Transmission-Blocking Vaccines. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Jimah, J.R.; Salinas, N.D.; Sala-Rabanal, M.; Jones, N.G.; Sibley, L.D.; Nichols, C.G.; Schlesinger, P.H.; Tolia, N.H. Malaria Parasite CelTOS Targets the Inner Leaflet of Cell Membranes for Pore-Dependent Disruption. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Amino, R.; Menard, R. The Role of MACPF Proteins in the Biology of Malaria and Other Apicomplexan Parasites. Subcell. Biochem. 2014, 80, 241–253. [Google Scholar] [PubMed]
- Lukoyanova, N.; Hoogenboom, B.W.; Saibil, H.R. The Membrane Attack Complex, Perforin and Cholesterol-Dependent Cytolysin Superfamily of Pore-Forming Proteins. J. Cell. Sci. 2016, 129, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sharma, V.; Ramu, D.; Singh, S. In Silico Analysis of Calcium Binding Pocket of Perforin Like Protein 1: Insights into the Regulation of Pore Formation. Syst. Synth. Biol. 2015, 9, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Roiko, M.S.; Carruthers, V.B. Functional Dissection of Toxoplasma gondii Perforin-Like Protein 1 Reveals a Dual Domain Mode of Membrane Binding for Cytolysis and Parasite Egress. J. Biol. Chem. 2013, 288, 8712–8725. [Google Scholar] [CrossRef] [PubMed]
- Roiko, M.S.; Svezhova, N.; Carruthers, V.B. Acidification Activates Toxoplasma gondii Motility and Egress by Enhancing Protein Secretion and Cytolytic Activity. PLoS Pathog. 2014, 10, e1004488. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Agarwal, S.; Kumar, S.; Shams Yazdani, S.; Chitnis, C.E.; Singh, S. Calcium-Dependent Permeabilization of Erythrocytes by a Perforin-Like Protein during Egress of Malaria Parasites. Nat. Commun. 2013, 4, 1736. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.; Lukoyanova, N.; Voskoboinik, I.; Caradoc-Davies, T.T.; Baran, K.; Dunstone, M.A.; D’Angelo, M.E.; Orlova, E.V.; Coulibaly, F.; Verschoor, S.; et al. The Structural Basis for Membrane Binding and Pore Formation by Lymphocyte Perforin. Nature 2010, 468, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Boddey, J.A. Molecular Mechanisms of Host Cell Traversal by Malaria Sporozoites. Int. J. Parasitol. 2017, 47, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ishino, T.; Chinzei, Y.; Yuda, M. A Plasmodium Sporozoite Protein with a Membrane Attack Complex Domain is Required for Breaching the Liver Sinusoidal Cell Layer Prior to Hepatocyte Infection. Cell. Microbiol. 2005, 7, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Formaglio, P.; Thiberge, S.; Mordelet, E.; Van Rooijen, N.; Medvinsky, A.; Menard, R.; Amino, R. Role of Host Cell Traversal by the Malaria Sporozoite during Liver Infection. J. Exp. Med. 2013, 210, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Pradel, G.; Frevert, U. Malaria Sporozoites Actively Enter and Pass through Rat Kupffer Cells Prior to Hepatocyte Invasion. Hepatology 2001, 33, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, E.; Morgan, R.N.; Bertuccini, L.; Wirth, C.C.; Silmon de Monerri, N.C.; Spanos, L.; Blackman, M.J.; Louis, C.; Pradel, G.; Siden-Kiamos, I. A Perforin-Like Protein Mediates Disruption of the Erythrocyte Membrane during Egress of Plasmodium berghei Male Gametocytes. Cell. Microbiol. 2013, 15, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Ishino, T.; Matsuyama, T.; Chinzei, Y.; Yuda, M. Essential Role of Membrane-Attack Protein in Malarial Transmission to Mosquito Host. Proc. Natl. Acad. Sci. USA 2004, 101, 16310–16315. [Google Scholar] [CrossRef] [PubMed]
- Ecker, A.; Bushell, E.S.; Tewari, R.; Sinden, R.E. Reverse Genetics Screen Identifies Six Proteins Important for Malaria Development in the Mosquito. Mol. Microbiol. 2008, 70, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Wade, K.R.; Tweten, R.K. The Apicomplexan CDC/MACPF-Like Pore-Forming Proteins. Curr. Opin. Microbiol. 2015, 26, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.; Brennan, A.J.; Whisstock, J.C.; Voskoboinik, I.; Trapani, J.A. Protecting a Serial Killer: Pathways for Perforin Trafficking and Self-Defense Ensure Sequential Target Cell Death. Trends Immunol. 2012, 33, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.J.; Chia, J.; Browne, K.A.; Ciccone, A.; Ellis, S.; Lopez, J.A.; Susanto, O.; Verschoor, S.; Yagita, H.; Whisstock, J.C.; et al. Protection from Endogenous Perforin: Glycans and the C Terminus Regulate Exocytic Trafficking in Cytotoxic Lymphocytes. Immunity 2011, 34, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Pszenny, V.; Ehrenman, K.; Romano, J.D.; Kennard, A.; Schultz, A.; Roos, D.S.; Grigg, M.E.; Carruthers, V.B.; Coppens, I. A Lipolytic Lecithin:Cholesterol Acyltransferase Secreted by Toxoplasma Facilitates Parasite Replication and Egress. J. Biol. Chem. 2016, 291, 3725–3746. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, P.; Schauer, K.; Coppens, I.; Nussenzweig, V. A Surface Phospholipase is Involved in the Migration of Plasmodium Sporozoites through Cells. J. Biol. Chem. 2005, 280, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Burda, P.C.; Roelli, M.A.; Schaffner, M.; Khan, S.M.; Janse, C.J.; Heussler, V.T. A Plasmodium Phospholipase is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane. PLoS Pathog. 2015, 11, e1004760. [Google Scholar] [CrossRef] [PubMed]
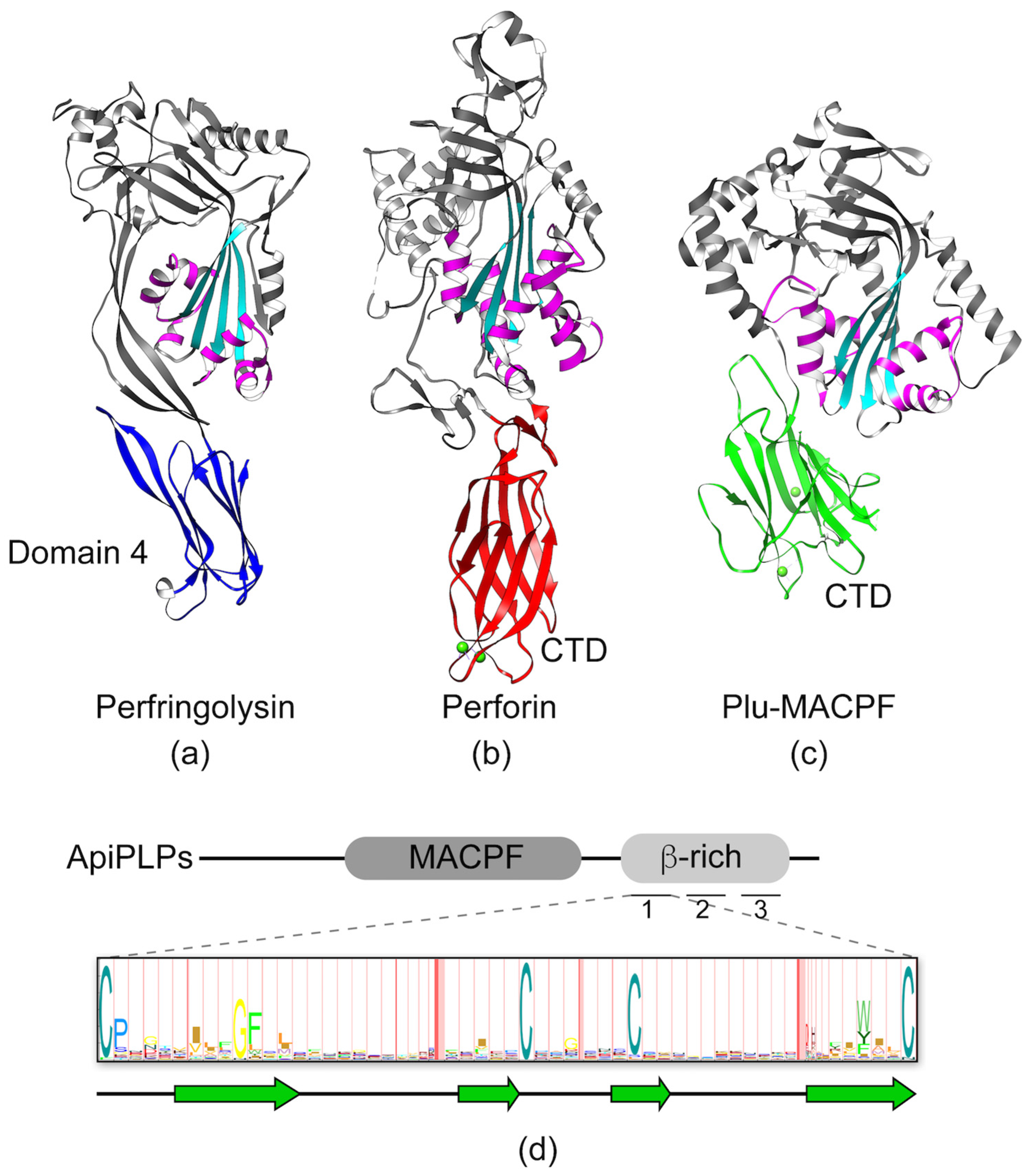
| Protein | Parasites | Type of PFP | Toxoplasma Stage/Function | Plasmodium Stage/Function |
|---|---|---|---|---|
| SPECT1 | Plasmodium spp. | unknown | NA | sporozoite/cell traversal |
| CelTOS | Aconoidasida | α 4 | NA | sporozoite and ookinete/cell traversal |
| PLP1/SPECT2 | Apicomplexa 1 | β | tachyzoite/egress | sporozoite/cell traversal, merozoite/egress |
| PLP2 | Apicomplexa 1 | β | sexual stages/unknown | male gametocyte/egress, merozoite/egress |
| PLP3//MAOP | Apicomplexa 2 | β | NA | ookinete/cell traversal |
| PLP4 | Apicomplexa 3 | β | NA | ookinete/cell traversal |
| PLP5 | Apicomplexa 3 | β | NA | ookinete/cell traversal |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, A.J.; Carruthers, V.B. Structural Features of Apicomplexan Pore-Forming Proteins and Their Roles in Parasite Cell Traversal and Egress. Toxins 2017, 9, 265. https://doi.org/10.3390/toxins9090265
Guerra AJ, Carruthers VB. Structural Features of Apicomplexan Pore-Forming Proteins and Their Roles in Parasite Cell Traversal and Egress. Toxins. 2017; 9(9):265. https://doi.org/10.3390/toxins9090265
Chicago/Turabian StyleGuerra, Alfredo J., and Vern B. Carruthers. 2017. "Structural Features of Apicomplexan Pore-Forming Proteins and Their Roles in Parasite Cell Traversal and Egress" Toxins 9, no. 9: 265. https://doi.org/10.3390/toxins9090265






