Recreational Exposure during Algal Bloom in Carrasco Beach, Uruguay: A Liver Failure Case Report
Abstract
:1. Introduction
2. Results
2.1. Environmental Conditions of the Carrasco and Malvín Beaches in the Summer Season, 2014–2015
2.2. Patient Condition and Differential Diagnose
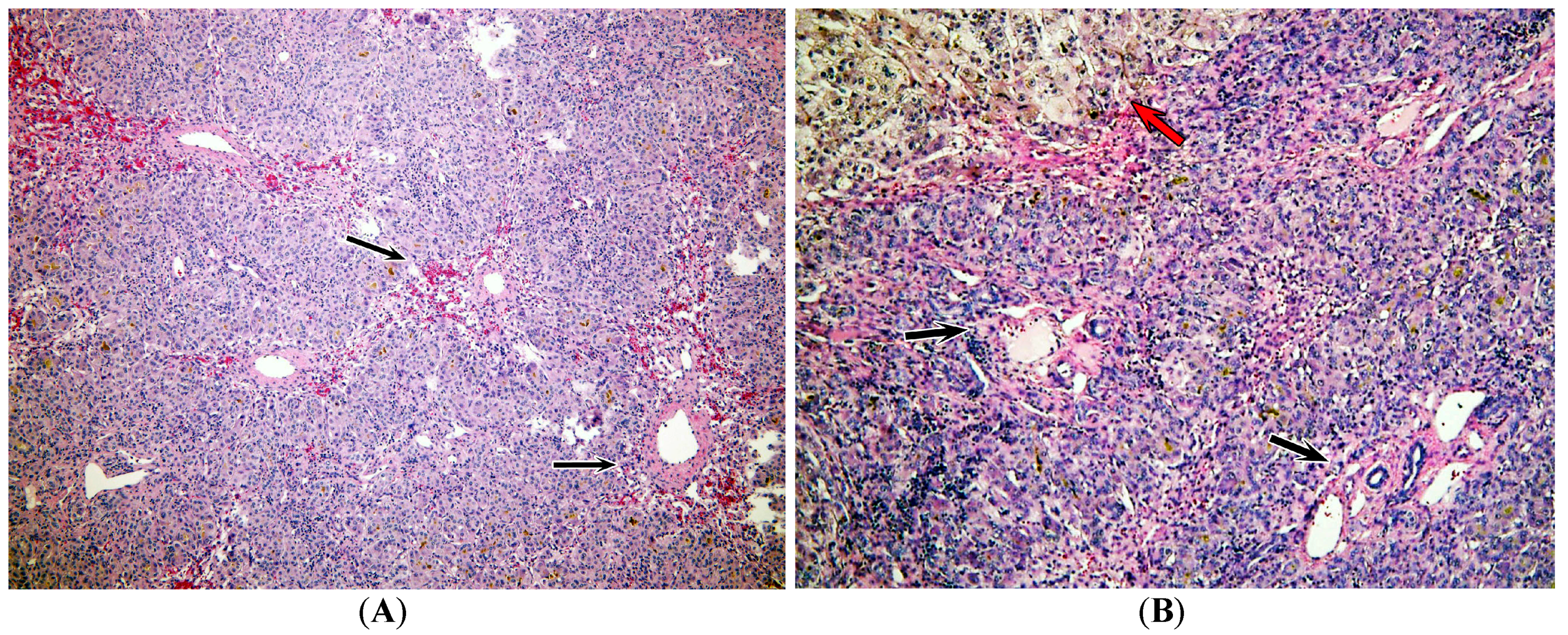
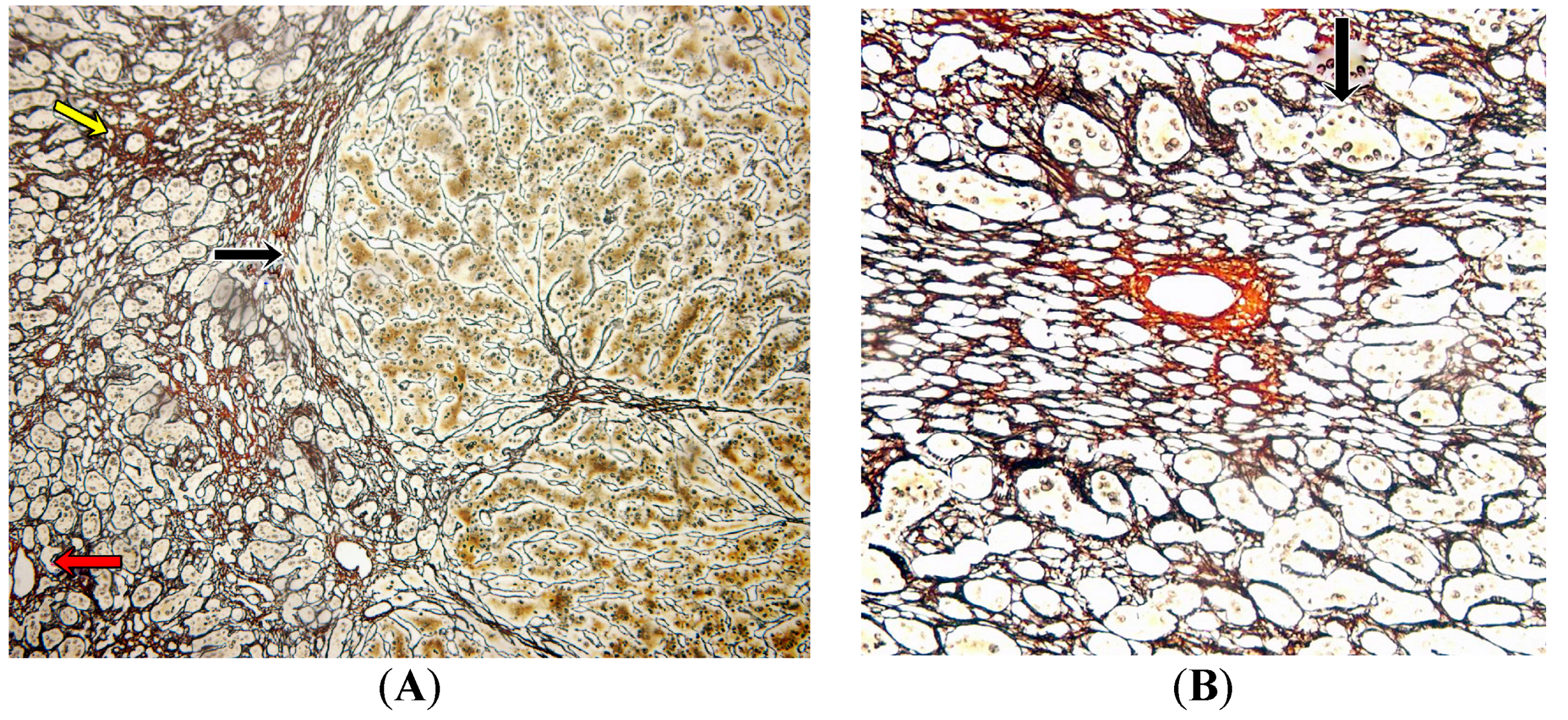
2.3. Pathology Analysis of Explanted Liver
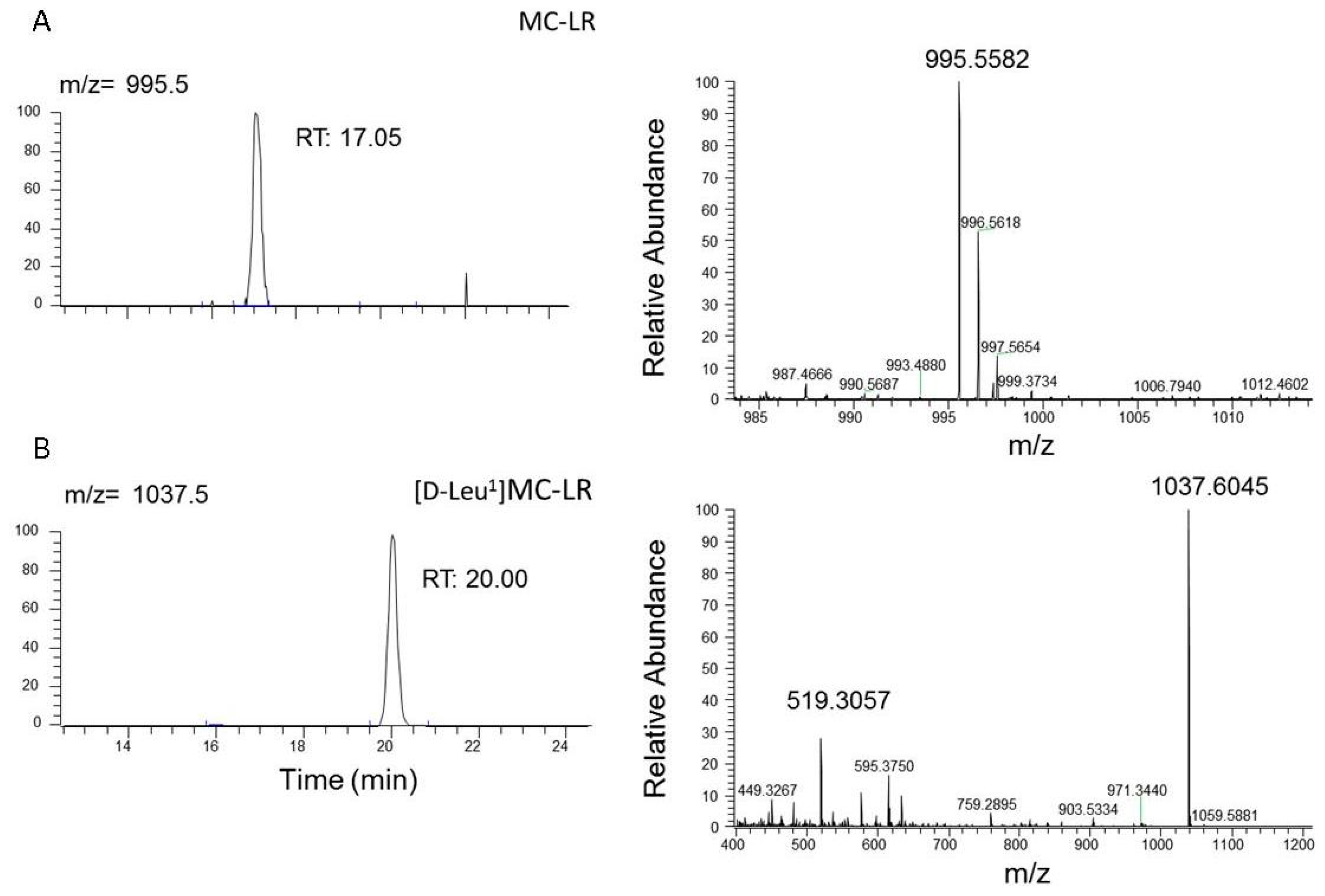
2.4. Microcystins Analysis in the Explanted Liver
2.5. Patient’s Condition after Liver Transplant
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Environmental Conditions of the Uruguayan Coast
5.2. Medical Treatment and Clinical Biomarkers
5.3. Pathology Studies
5.4. Toxicological Studies
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hallegraeff, G. A review of harmful algal blooms and their apparent global increase. Phycologia 1992, 32, 79–99. [Google Scholar] [CrossRef]
- Pearl, H.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 1st ed.; World Health Organization: Geneva, Switzerland, 1999; pp. 55–161. ISBN 0-419-23930-8. [Google Scholar]
- Matthiensen, A.; Beattie, K.A.; Yunes, J.S.; Kaya, K.; Codd, G.A. [D-Leu1]Microcystin-LR, from the cyanobacterium Microcystis RST 9501 and from a Microcystis bloom in the Patos Lagoon estuary, Brazil. Phytochemistry 2000, 55, 383–387. [Google Scholar] [CrossRef]
- De León, L.; Yunes, J. First report of a Microcystis aeruginosa toxic bloom in La Plata River. Environ. Toxicol. 2001, 16, 110–112. [Google Scholar] [CrossRef]
- Sedan, D.; Andrinolo, D. Cianobacterias y cianotoxinas, Efectos en la salud humana, Casos informados y primeros acercamientos al estudio epidemiológico. In Cianobacterias Como Determinantes Ambientales de la Salud, 1st ed.; Giannuzzi, L., Ed.; Departamento de Salud Ambiental, Dirección Nacional de Determinantes de la Salud e Investigación, Ministerio de Salud de la Nación: Buenos Aires, Argentina, 2011; pp. 59–70. ISBN 978-950-38-0118-5. [Google Scholar]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.S.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.M.; Antunes, M.B.; de Melo Filho, D.A.; Lyra, T.M.; Barreto, V.S.; et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N. Engl. J. Med. 1998, 338, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.F.O.; An, J.S.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human Fatalities from Cyanobacteria: Chemical and Biological Evidence for Cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J. Epidemic of intestinal disorders in Charleston (West Virginia) occurring simultaneously with unprecedented water supply conditions. Am. J. Public Health 1931, 21, 198–200. [Google Scholar]
- Zilberg, B. Gastroenteritis in Salisbury European children—A five year study. Cent. Afr. J. Med. 1966, 12, 164–168. [Google Scholar] [PubMed]
- Lippy, E.C.; Erb, J. Gastrointestinal illness at Sewickley, PA. J. Am. Water Works Assoc. 1976, 68, 606–610. [Google Scholar]
- Teixera, M.; Costa, M.; Carvalho, V.; Pereira, M.; Hage, E. Gastroenteritis epidemic in the area of the Itaparica Dam, Bahia, Brazil. Bull. Pan Am. Health Organ. 1993, 27, 244–253. [Google Scholar]
- Annadotter, H.; Cronberg, G.; Lawton, L.; Hansson, H.-B.; Göthe, U.; Skulberg, O.M. An extensive outbreak of gastroenteritis associated with the toxic cyanobacterium planktothrix agardhii (oscillatoriales, cyanophyceae) in scania, South Sweden. In Cyanotoxins; Chorus, I., Ed.; Springer: Berlin, Germany, 2001; pp. 200–208. [Google Scholar]
- Giannuzzi, L.; Sedan, D.; Echenique, R.; Andrinolo, D. An Acute Case of Intoxication with Cyanobacteria and Cyanotoxins in Recreational Water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Gammie, A.J.; Hollinrake, K.; Codd, G.A. Pneumonia associated with cyanobacteria. Br. Med. J. 1990, 300, 1440–1441. [Google Scholar] [CrossRef]
- Amstrong, B. The epidemiology of cancer in the people of China. Int. J. Epidemiol. 1980, 9, 305–315. [Google Scholar] [CrossRef]
- Yu, S.-J. Primary prevention of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1995, 10, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.G.; Codd, G.A. Cyanobacterial toxins and human health. Rev. Med. Microbiol. 1994, 5, 256–264. [Google Scholar] [CrossRef]
- Stewart, I.; Webb, P.; Schluter, P.; Fleming, L.; Burns, J., Jr.; Gantar, M.; Backer, L.; Shaw, G. Epidemiology of recreational exposure to freshwater cyanobacteria—An international prospective cohort study. BMC Public Health 2006, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.; Carmichael, W.W.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Hill, V.R.; Kieszak, S.M.; et al. Recreational exposure to low concentrations of Microcystins during an algal bloom in a small lake. Mar. Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Monitoring Program for Beach and Coastal Waters of the Department of Montevideo, April 2014–March 2015. Available online: http://www.montevideo.gub.uy/sites/default/files/Informe%20Anual%20de%20Calidad%20de%20Agua%20de%20la%20Costa%20de%20Montevideo%202014-2015.pdf (accessed on 25 July 2017).
- Andrinolo, D.; Pereira, P.; Giannuzzi, L.; Aura, C.; Massera, S.; Caneo, M.; Caixach, J.; Braco, M.; Echenique, R. Ocurrence of Microcystis Aeruginosa and Microcystins in Rio de La Plata River (Argentina). Acta Toxicol. Argent. 2007, 15, 8–14. [Google Scholar]
- Backer, L.C. Cyanobacterial Harmful Algal Blooms (CyanoHABs): Developing a Public Health Response. Lake Reserv. Manag. 2002, 18, 20–31. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Soares, R.M.; Servaites, J.C.; Delgado, A.G.; Magalhães, V.F.; Carmichael, W.W.; Azevedo, S.M.F.O. Sublethal Microcystin Exposure and Biochemical Outcomes among Hemodialysis Patients. PLoS ONE 2013, 8, e69518. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.A. Autoimmune Hepatitis: Histopathology. Clin. Liver Dis. 2014, 3, 19–23. [Google Scholar] [CrossRef]
- Rosso, L.; Sedan, D.; Kolman, M.; Caixach, J.; Flores, C.; Oteiza, J.M.; Salerno, G.; Echenique, R.; Giannuzzi, L.; Andrinolo, D. Microcystis aeruginos strain [D-Leu1] Mcyst-LR producer, from Buenos Aires province, Argentina. J. Coast. Life Med. 2014, 2, 287–296. [Google Scholar] [CrossRef]
- Ouahid, Y.; Zaccaro, M.C.; Zulpa, G.; Storni, M.; Stella, A.M.; Bossio, J.C.; Tanuz, M.; Del Campo, F.F. A single microcystin in a toxic Microcystis bloom from the river Río de la Plata (Argentina). Int. J. Environ. Anal. Chem. 2009, 91, 1–12. [Google Scholar] [CrossRef]
- Qi, Y.; Rosso, L.; Sedan, D.; Giannuzzi, L.; Andrinolo, D.; Volmer, D.A. Seven new microcystin variants discovered from a native Microcystis aeruginosa strain—Unambiguous assignment of product ions by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Chu, F.S. Kinetics of Distribution of Microcystin LR in Serum and Liver Cytosol of Mice: An Immunochemical Analysis. J. Agric. Food Chem. 1994, 42, 1035–1040. [Google Scholar] [CrossRef]
- Sedan, D.; Giannuzzi, L.; Rosso, L.; Marra, C.A.; Andrinolo, D. Biomarkers of prolonged exposure to microcystin-LR in mice. Toxicon 2013, 68, 9–17. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Waste Water, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Brena, B.M.; Díaz, L.; Sienra, D.; Ferrari, G.; Ferraz, N.; Hellman, U.; Gonzalez-Sapienza, G.; Last, J.A. ITREOH Building of Regional Capacity to Monitor Recreational Water: Development of a Non-commercial Microcystin ELISA and Its Impact on Public Health Policy. Int. J. Occup. Environ. Health 2006, 12, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Culling, C.F.A. Handbook of Histological Techniques, 3rd ed.; Butterworths: London, UK, 1975. [Google Scholar]
- Gordon, H.; Sweet, H.H. A simple method for the silver impregnation of reticulin. Am. J. Pathol. 1936, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Caixach, J. An integrated strategy for rapid and accurate determination of free and cell-bound microcystins and related peptides in natural blooms by liquid chromatography-electrospray-high resolution mass spectrometry and matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry using both positive and negative ionization modes. J. Chromatogr. A 2015, 1407, 76–89. [Google Scholar] [CrossRef] [PubMed]

| Parameter | On Admission | Pre-Liver Transplant | Post-Liver Transplant | 8 Months after Transplant |
|---|---|---|---|---|
| TB 1 (mg·dL−1) | 9.4 | 10.8 | 0.3 | 0.3 |
| DB 2 (mg·dL−1) | 4.8 | 4.8 | 0.1 | 0.1 |
| AST 3 (UI·L−1) | 1268 | 79 | 31 | 30 |
| ALT 4 (UI·L−1) | 1386 | 81 | 60 | 50 |
| INR 5 | 3.5 | 3.25 | 0.99 | 0.92 |
| PT 6 (%) | 26 | 14 | 93 | 100 |
| AM 7 (µg·dL−1) | 257 | 291 | 34 | 50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, F.; Sedan, D.; D’Agostino, D.; Cavalieri, M.L.; Mullen, E.; Parot Varela, M.M.; Flores, C.; Caixach, J.; Andrinolo, D. Recreational Exposure during Algal Bloom in Carrasco Beach, Uruguay: A Liver Failure Case Report. Toxins 2017, 9, 267. https://doi.org/10.3390/toxins9090267
Vidal F, Sedan D, D’Agostino D, Cavalieri ML, Mullen E, Parot Varela MM, Flores C, Caixach J, Andrinolo D. Recreational Exposure during Algal Bloom in Carrasco Beach, Uruguay: A Liver Failure Case Report. Toxins. 2017; 9(9):267. https://doi.org/10.3390/toxins9090267
Chicago/Turabian StyleVidal, Flavia, Daniela Sedan, Daniel D’Agostino, María Lorena Cavalieri, Eduardo Mullen, María Macarena Parot Varela, Cintia Flores, Josep Caixach, and Dario Andrinolo. 2017. "Recreational Exposure during Algal Bloom in Carrasco Beach, Uruguay: A Liver Failure Case Report" Toxins 9, no. 9: 267. https://doi.org/10.3390/toxins9090267





