Zearalenone (ZEN) and Its Influence on Regulation of Gene Expression in Carp (Cyprinus carpio L.) Liver Tissue
Abstract
:1. Introduction
2. Results
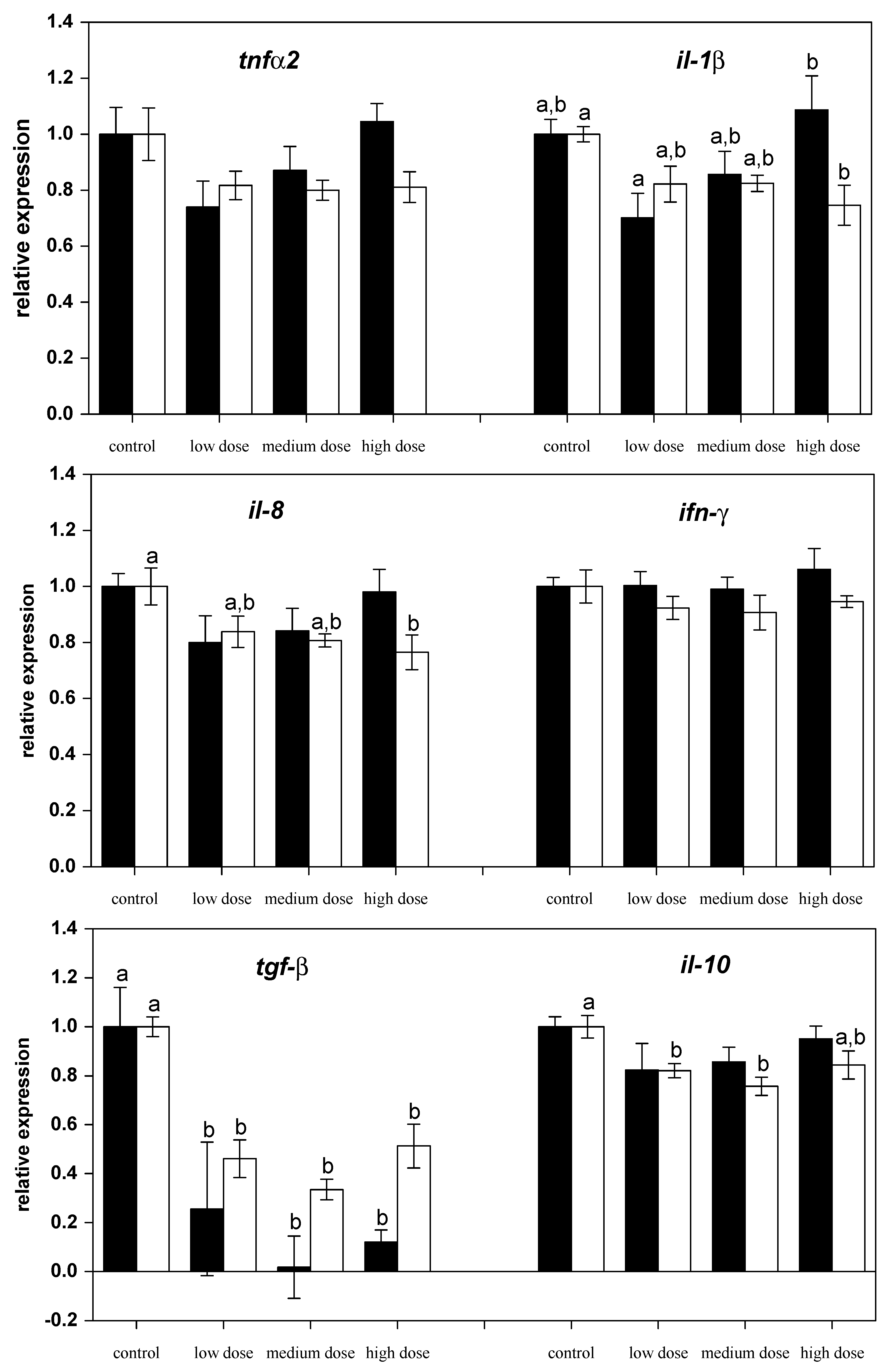
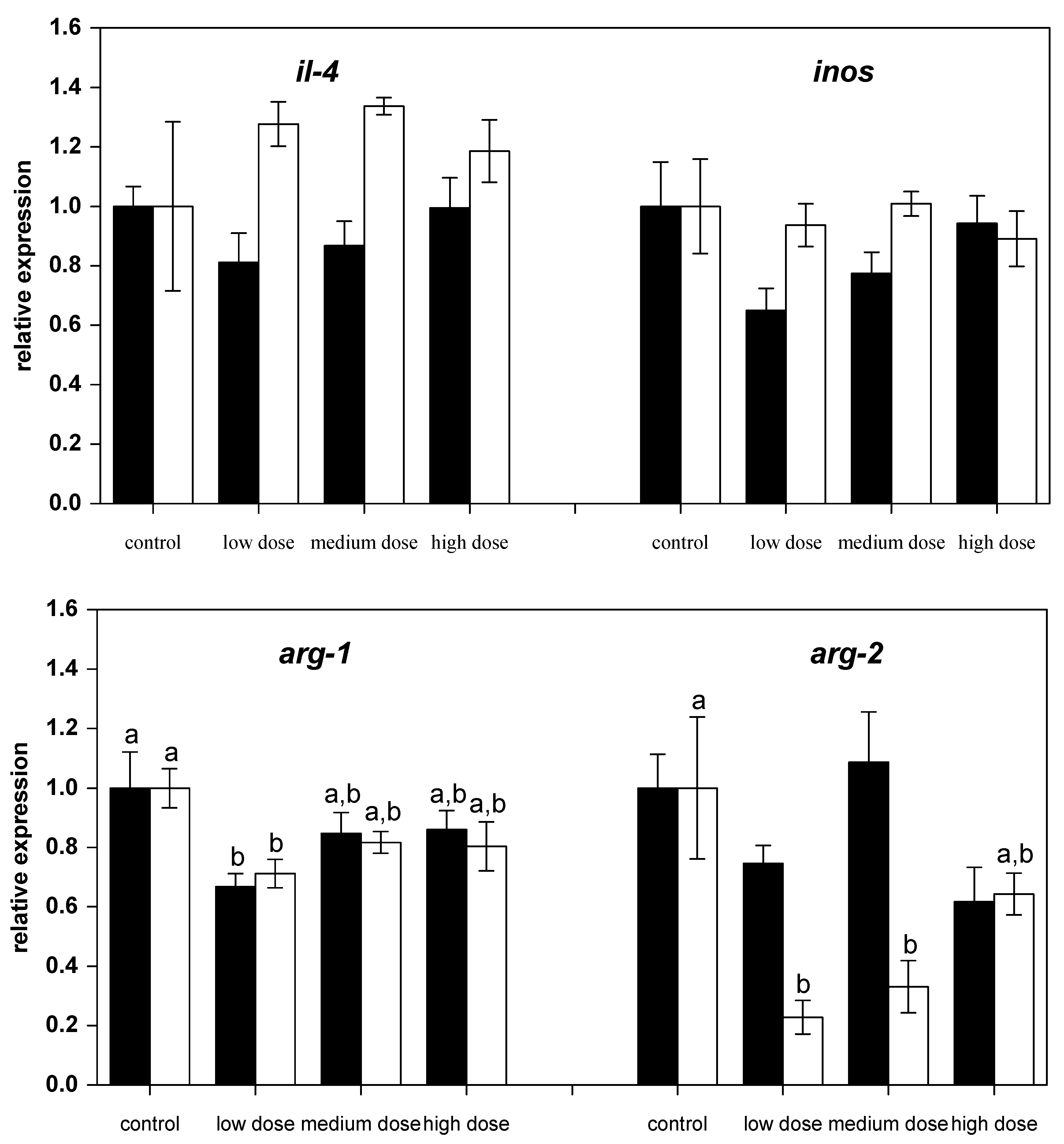
2.1. Effects on mRNA Expression of Immune Genes
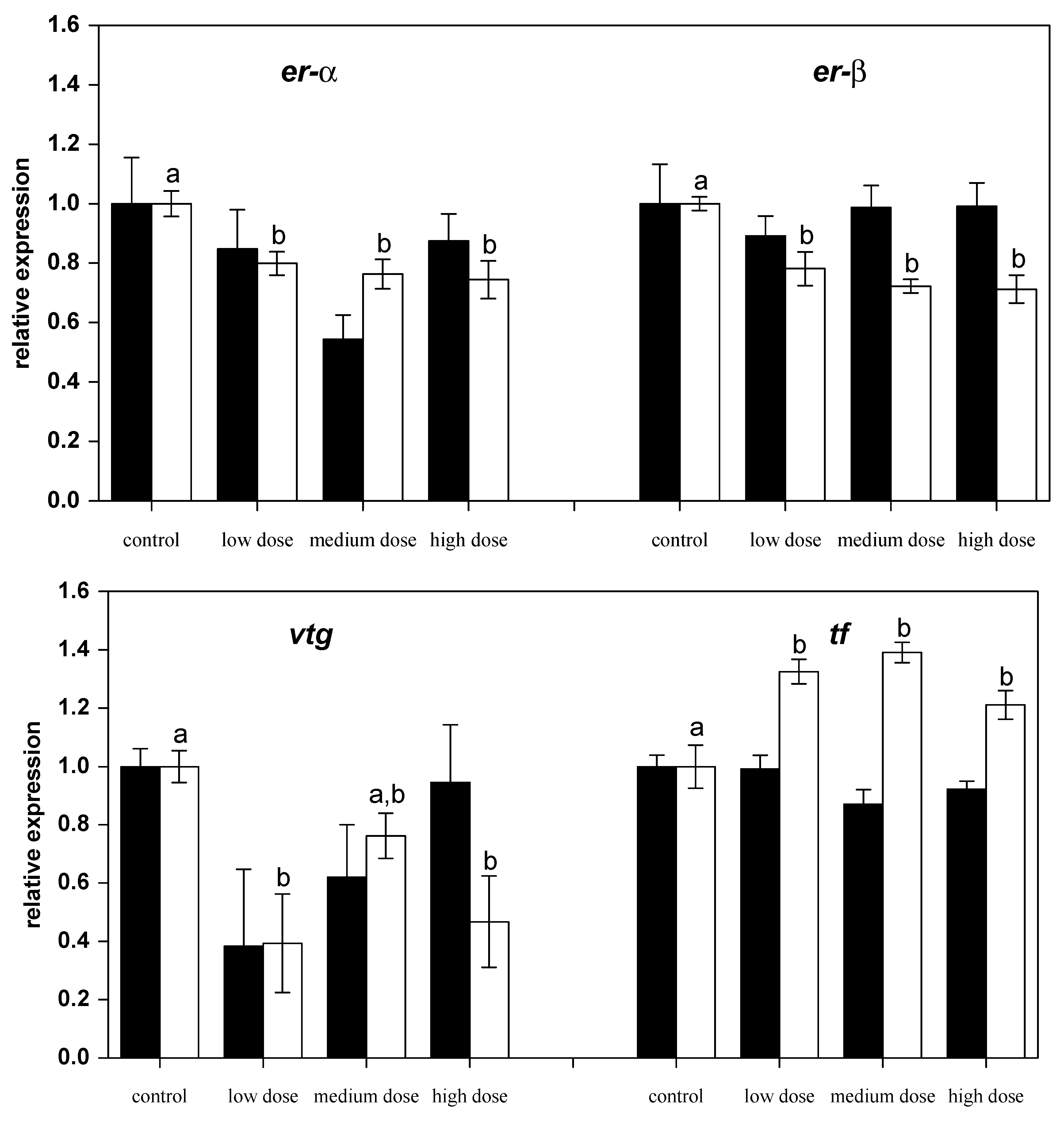
2.2. Effects on mRNA Expression of Estrogen-Sensitive Genes
2.3. Effects on mRNA Expression of Anti-Oxidative Genes
2.4. Effects on mRNA Expression of Lysosomal Genes
3. Discussion
3.1. Immunotoxicity of ZEN and Anti-Oxidative Responses
3.2. Estrogenic Effects of ZEN
3.3. Effects of ZEN on Expression of Lysosomal Genes
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Preparation of Feeds
5.3. Exposure of Fish
5.4. Gene Expression Analyses
5.5. Statistics
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Santos, G.A.; Rodrigues, I.; Naehrer, K.; Encarnacao, P. Mycotoxins in aquaculture: Occurrence in feed components and impact on animal performance. Aquac. Eur. 2010, 35, 6–10. [Google Scholar]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Dänicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed. An initial study. Toxins 2013, 5, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, M.; Pardo, A.; Pose, G. Mycotoxigenic fungi and natural co-occurrence of mycotoxins in rainbow trout (Oncorhynchus mykiss) feeds. Toxins 2015, 7, 4595–4609. [Google Scholar] [CrossRef] [PubMed]
- Johns, S.M.; Denslow, N.D.; Kane, M.D.; Watanabe, K.H.; Orlando, E.F.; Sepulveda, M.S. Effects of estrogens and antiestrogens on gene expression of fathead minnow (Pimephales promelas) early life stages. Environ. Toxicol. 2009, 26, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.; Thorpe, K.L.; Bucheli, T.D.; Wettstein, F.E.; Burkhardt-Holm, P. Short-term exposure to the environmentally relevant estrogenic mycotoxin zearalenone impairs reproduction in fish. Sci. Total Environ. 2010, 409, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Noser, J.; Wettstein, F.E.; Burkhardt-Holm, P. Unravelling the mechanisms involved in zearalenone-mediated toxicity in permanent fish cell cultures. Toxicon 2014, 88, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Kersten, S.; Valenta, H.; Dänicke, S.; Burkhardt-Holm, P.; Junge, R. Effects of dietary exposure to zearalenone (ZEN) on carp (Cyprinus carpio L.). Toxins 2015, 7, 3465–3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietsch, C.; Junge, R.; Burkhardt-Holm, P. Immunomodulation by zearalenone (ZEN) in carp (Cyprinus carpio L.). BioMed Res. Int. 2015, 2015, 420702. [Google Scholar] [CrossRef] [PubMed]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health Part B 2000, 3, 109–143. [Google Scholar]
- Marin, D.E.; Taranu, I.; Burlacu, R.; Tudor, D.S. Effects of zearalenone and its derivatives on the innate immune response of swine. Toxicon 2010, 56, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Burlacu, R.; Manda, G.; Motiu, M.; Neagoe, I.; Dragomir, C.; Stancu, M.; Calin, L. Effects of zearalenone and its derivatives on porcine immune response. Toxicol. In Vitro 2011, 25, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Abbès, S.; Ben Salah-Abbès, J.; Sharafi, H.; Noghabi, K.A.; Oueslati, R. Interaction of Lactobacillus plantarum MON03 with Tunisian montmorillonite clay and ability of the composite to immobilize zearalenone in vitro and counteract immunotoxicity in vivo. Immunopharmacol. Immunotoxicol. 2012, 34, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Deng, J.L.; Xu, S.W.; Peng, X.; Zuo, Z.C.; Cui, H.M.; Wang, Y.; Ren, Z.H. Effects of zearalenone on IL-2, IL-6, and IFN-gamma mRNA levels in the splenic lymphocytes of chickens. Sci. World J. 2012, 2012, 567327. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef] [PubMed]
- Byung-Kook, C.; Joon-Hyung, C.; Sang-Hee, J.; Hyo-Sook, S.; Seong-Wan, S.; Young-Keun, Y.; Hwan-Goo, K. Zearalenone affects immune-related parameters in lymphoid organs and serum of rats vaccinated with porcine parvovirus vaccine. Toxicol. Res. 2012, 28, 279–288. [Google Scholar]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laing, K.J.; Zou, J.J.; Wang, T.; Bols, N.; Hirono, I.; Aoki, T.; Secombes, C.J. Identification and analysis of an interleukin 8-like molecule in rainbow trout Oncorhynchus mykiss. Dev. Comp. Immunol. 2002, 26, 433–444. [Google Scholar] [CrossRef]
- Sangrador-Vegas, A.; Lennington, J.B.; Smith, T.J. Molecular cloning of an IL-8-like CXC chemokine and tissue factor in rainbow trout (Oncorhynchus mykiss) by use of suppression subtractive hybridization. Cytokine 2002, 17, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Grayfer, L.; Walsh, J.G.; Belosevic, M. Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor alpha. Dev. Comp. Immunol. 2008, 32, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Grayfer, L.; Garcia-Garcia, E.; Belosevic, M. Comparison of macrophage antimicrobial responses induced by type II interferons of the goldfish (Carassius auratus L.). J. Biol. Chem. 2010, 285, 23537–23547. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Ochoa, J.B. Arginine availability, arginase, and the immune response. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.P.; Grody, W.W.; Cederbaum, S.D. Comparative properties of arginases. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 107–132. [Google Scholar] [CrossRef]
- Joerink, M.; Savelkoul, H.F.J.; Wiegertjes, G.F. Evolutionary conservation of alternative activation of macrophages: Structural and functional characterization of arginase 1 and 2 in carp (Cyprinus carpio L.). Mol. Immunol. 2006, 43, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Joerink, M.; Forlenza, M.; Ribeiro, C.M.S.; de Vries, B.J.; Savelkoul, H.F.J.; Wiegertjes, G.F. Differential macrophage polarisation during parasitic infections in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2006, 21, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Haddad, G.; Hanington, P.C.; Wilson, E.C.; Grayfer, L.; Belosevic, M. Molecular and functional characterization of goldfish (Carassius auratus L.) transforming growth factor beta. Dev. Comp. Immunol. 2008, 32, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Grayfer, L.; Hodgkinson, J.W.; Hitchen, S.J.; Belosevic, M. Characterization and functional analysis of goldfish (Carassius auratus L.) interleukin-10. Mol. Immunol. 2011, 48, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Saag, P.T.V.D.; Burg, B.V.D.; Gustafsson, J.-Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Arukwe, A.; Grotmol, T.; Haugen, T.B.; Knudsen, F.R.; Goksøyr, A. Fish model for assessing the in vivo estrogenic potency of the mycotoxin zearalenone and its metabolites. Sci. Total Environ. 1999, 236, 153–161. [Google Scholar] [CrossRef]
- Bucheli, T.D.; Erbs, M.; Hartmann, N.; Vogelsang, S.; Wettstein, F.E.; Forrer, H.R. Estrogenic mycotoxins in the environment. Mitteilungen aus Lebensmitteluntersuchung und Hygiene 2005, 96, 386–403. [Google Scholar]
- Moens, L.N.; van der Ven, K.; Van Remortel, P.; Del-Favero, J.; De Coen, W.M. Gene expression analysis of estrogenic compounds in the liver of common carp (Cyprinus carpio) using a custom cDNA microarray. J. Biochem. Mol. Toxicol. 2007, 21, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Jurecka, P.; Wiegertjes, G.F.; Rakus, K.Ł.; Pilarczyk, A.; Irnazarow, I. Genetic resistance of carp (Cyprinus carpio L.) to Trypanoplasma borreli: Influence of transferrin polymorphisms. Vet. Immunol. Immunopathol. 2009, 127, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture; Food and Agriculture Organization: Rome, Italy, 2012. [Google Scholar]
- Ding, X.; Lichti, K.; Staudinger, J.L. The mycoestrogen zearalenone induces CYP3A through activation of the pregnane X receptor. Toxicol. Sci. 2006, 91, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Yin, S.; Jia, Z.; Shan, A. Biochemical changes and oxidative stress induced by zearalenone in the liver of pregnant rats. Hum. Exp. Toxicol. 2015, 34, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ruh, M.F.; Bi, Y.; Cox, L.; Berk, D.; Howlett, A.C.; Bellone, C.J. Effect of environmental estrogens on IL-1β promoter activity in a macrophage cell line. Endocrine 1998, 9, 207–211. [Google Scholar] [CrossRef]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef] [PubMed]
- Salah-Abbès, J.B.; Abbès, S.; Houas, Z.; Abdel-Wahhab, M.A.; Oueslati, R. Zearalenone induces immunotoxicity in mice: Possible protective effects of radish extract (Raphanus sativus). J. Pharm. Pharmacol. 2008, 60, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Salah-Abbès, J.B.; Abbès, S.; Abdel-Wahhab, M.A.; Oueslati, R. Raphanus sativus extract protects against zearalenone induced reproductive toxicity, oxidative stress and mutagenic alterations in male Balb/c mice. Toxicon 2009, 53, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, P.; Li, D.; Tang, R.; Lei, H.; Zhao, Y. Time-dependent oxidative stress responses of crucian carp (Carassius auratus) to intraperitoneal injection injection of extracted microcystins. Bull. Environ. Contam. Toxicol. 2009, 82, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Shull, S.; Heintz, N.H.; Periasamy, M.; Manohar, M.; Janssen, Y.M.W.; Marsh, J.P.; Mossman, B.T. Differential regulation of antioxidant enzymes in response to oxidants. J. Biol. Chem. 1991, 266, 24398–24403. [Google Scholar] [PubMed]
- Röhrdanz, E.; Kahl, R. Alterations of antioxidant enzyme expression in response to hydrogen peroxide. Free Radic. Biol. Med. 1998, 24, 27–38. [Google Scholar] [CrossRef]
- Röhrdanz, E.; Obertrifter, B.; Ohler, S.; Tran-Thi, Q.H.; Kahl, R. Influence of adriamycin and paraquat on antioxidant enzyme expression in primary rat hepatocytes. Arch. Toxicol. 2000, 74, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Malek, R.L.; Sajadi, H.; Abraham, J.; Grundy, M.A.; Gerhard, G.S. The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp. Biochem. Physiol. 2004, 138C, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kammer, A.R.; Orczewska, J.I.; O’Brien, K.M. Oxidative stress is transient and tissue specific during cold acclimation of threespine stickleback. J. Exp. Biol. 2011, 214, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 2013, 58, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Obremski, K. Changes in Th1 and Th2 cytokine concentrations in ileal Peyer’s patches in gilts exposed to zearalenone. Pol. J. Vet. Sci. 2014, 17, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.; Gras, M.; Marin, D.E.; Tabuc, C.; Taranu, I. Zearalenone induces alterations of hepatic immune responses by modulation of pro-inflammatory cytokines and matrix metalloproteinase gene expression. In Proceedings of the 6th International Workshop on Immunonutrition Nutrition Society (2013), 72 (OCE1), E2, Palma de Mallorca, Spain, 15–17 October 2012. [Google Scholar]
- Katsu, Y.; Lange, A.; Miyagawa, S.; Urushitani, H.; Tatarazako, N.; Kawashima, Y.; Tyler, C.R.; Iguchi, T. Cloning, expression and functional characterization of carp, Cyprinus carpio, estrogen receptors and their differential activations by estrogens. J. Appl. Toxicol. 2013, 33, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mitak, M.; Gojmerac, T.; Cvrtila, D.; Cvetnić, Ž. Effect of atrazine and zearalenone on the number of receptor binding sites for 3H-estradiol in the rat uterus cytosol. Vet. Med. 2002, 47, 12–16. [Google Scholar]
- Menuet, A.; Le Page, Y.; Torres, O.; Kern, L.; Kah, O.; Pakdel, F. Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of ERalpha, ERbeta1 and ERbeta2. J. Mol. Endocrinol. 2004, 32, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Brion, F.; Le Page, Y.; Piccini, B.; Cardoso, O.; Tong, S.-K.; Chung, B.-C.; Kah, O. Screening estrogenic activities of chemicals or mixtures in vivo using transgenic (cyp19a1b-GFP) zebrafish embryos. PLoS ONE 2012, 7, e36069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Q.; Zhang, Z.; Lin, P.; Lei, L.; Wang, A.; Jin, Y. Endoplasmic reticulum stress cooperates in zearalenone-induced cell death of RAW 264.7 macrophages. Int. J. Mol. Sci. 2015, 1, 19780–19795. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W.; Wieczorek, H. The V-type H+ ATPase: Molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006, 209, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Dröse, S.; Altendorf, K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 1997, 200, 1–8. [Google Scholar]
- Bandani, A.R.; Amiri, B.; Butt, T.M.; Gordon-Weeks, R. Effects of efrapeptin and destruxin, metabolites of entomogenous fungi, on the hydrolytic activity of a vacuolar type ATPase identified on the brush border membrane vesicles of Galleria mellonella midgut and on plant membrane bound hydrolytic enzymes. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1510, 367–377. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, E.; Orchard, I.; Lange, A.B. Effects of the cyclopeptide mycotoxin destruxin A on the Malpighian tubules of Rhodnius prolixus (Stål). Toxicon 2010, 55, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin D—Many functions of one aspartic protease. Crit. Rev. Oncol. 2008, 68, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Vanacore, R.; Goicoechea, O.; Amthauer, R. Intestinal alkaline phosphatase of the fish Cyprinus carpio: Regional distribution and membrane association. J. Exp. Zool. 1997, 279, 347–355. [Google Scholar] [CrossRef]
- Rai, A.K.; Saikia, P.; Mech, B. Histochemical localization of alkaline phosphatase activity during cutaneous wound healing in a catfish under acid stress. Int. J. Sci. Res. Publ. 2013, 3, 1–9. [Google Scholar]
- Molina, R.; Moreno, I.; Pichardo, S.; Jos, A.; Moyano, R.; Monterde, J.G.; Cameán, A. Acid and alkaline phosphatase acitivies and pathological changes induced in Tilapia fish (Oreochromis sp.) exposed subchronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon 2005, 46, 725–735. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Recommendation (2006/576/EC) of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229/7–L229/9. [Google Scholar]
- Allen, N.K.; Mirocha, C.J.; Weaver, G.; Aakhus-Allen, S.; Bates, F. Effects of dietary zearalenone on finishing broiler chickens and young turkey poults. Poult. Sci. 1981, 60, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, K.H. The effect of zearalenone on growth rate, organ weight and muscle fibre composition in growing rats. Basic Clin. Pharmacol. Toxicol. 1982, 51, 154–158. [Google Scholar] [CrossRef]

| Primers | Sense Sequence (5′-3′) | Anti-Sense Sequence (5′-3) |
|---|---|---|
| ifnγ1 | TGAGCTTAAAGAATGTGTGGCCCAA | ACTCCATATGTGACGGCTTTTGGT |
| tnfα-2 | AGGACCAAGACGTCGTGCAT | GGCTTGTAGCTGCCGTAGGA |
| il-1β il-4 il-8 | ACCTTGCAGTGCACCATTTGTGA TTTCTGGGCTGTCTGGTGCCAA TTATTTTGCTTTCAGGAATGAGTCTTAG | TGTTGCTCTGCACCATAGTCTT TTTCTTGTCAGTACGGAAATGCTCA CACACTCTCTATGTGTTTTCCAATGC |
| il-10 | GAACGAGATCCTGCGCTTTT | TTGAGTGCAAGTGGTCCTTCTG |
| tgf-β inos | AATCCTTTACTACATCGGAAAAACGCC AACAGGTCTGAAAGGGAATCC | TGTTGGACAGTTGTTCGATTTTGGGC CATTATCTCTCATGTCCAGAGTCTCTTCT |
| arg-1 | TGAGGAGCTTCAGCGGATTAC | CCTATTATTCCCACGCAGTGATG |
| arg-2 | GGAGACCTGGCCTTCAAGCATCT | CTGATTGGCACGTCCAACT |
| er-α er-β2 vtg tf cat mn-sod cu-sod cathep v-atp-ase alkphos b2m | GGAGAATGTGTCGAGGGGATGGCT TACAGTCCCGCTCTGCTGGGTTAC TTCTGTTGGCACACCAGTC AGCAAGTGCAAAGCYTCTTCTGGA AGGAAACAACACCCCCATCT TCAAGCGTGACTTTGGCTCA AGCTGGTGAAAATGGGGTTGC TCCATGTCTTTGCACTGCTG GTGCAGAAATCAAAAGATGTGATGGAC TTAAATGGGCCAAAGATGCAGGC TTCAGGTGTACAGCCATTTTCCCG | TCCAGCATGCACTGCACCATGAAG GTGGCGTAAGTTCTGCCCAACTCC AGCCAGGAACTTCTCCTTGATG TGAAAGCCCCATCATAGCCA AAACAGAAGCGCATCCCTGAT TGACATCTTCTCCTTCATCTTCTG CAGCATTTTGTCCACAATGTCAA TCATTTCTTCTCCATGTGCCTCT ATGGCGAAGTTGTCTGCACTGT TGTTGTTGTGACGATTCCCACTGA TGTTCTCTTTTCCGTACTCTCCG |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietsch, C. Zearalenone (ZEN) and Its Influence on Regulation of Gene Expression in Carp (Cyprinus carpio L.) Liver Tissue. Toxins 2017, 9, 283. https://doi.org/10.3390/toxins9090283
Pietsch C. Zearalenone (ZEN) and Its Influence on Regulation of Gene Expression in Carp (Cyprinus carpio L.) Liver Tissue. Toxins. 2017; 9(9):283. https://doi.org/10.3390/toxins9090283
Chicago/Turabian StylePietsch, Constanze. 2017. "Zearalenone (ZEN) and Its Influence on Regulation of Gene Expression in Carp (Cyprinus carpio L.) Liver Tissue" Toxins 9, no. 9: 283. https://doi.org/10.3390/toxins9090283





