1. Introduction
Cell lysis has been a research topic for many years [
1,
2], and is especially used to extract intracellular compounds out of cells for analysis in fields such as metabolomics, genomics or proteomics. The rupture of the cell membrane can be performed by different means including chemical [
3,
4], electrical [
5,
6], mechanical [
7,
8] and optical [
9,
10]. The current benchmark in cell lysis is chemical lysis due to its easy implementation, which requires no dedicated equipment but only mixing with chemical reagents such as the CelLytic kit from Sigma-Aldrich. However, the use of reagents to dissolve the cell membrane can interfere with cytosol analysis performed after lysis, such as mass spectrometry, and makes this technique not always applicable [
11]. Additionally, the chemical lysis process can take up to 30 min to complete, as is the case for the CelLytic kit.
In contrast, electrical lysis does not use reagents and therefore does not affect cytosol analysis. Furthermore, the electrical breakdown of a cell membrane happens in fractions of a second which in principle makes the technique faster than chemical lysis. The cell membrane can be disrupted relatively easy solely by the application of an electric field [
5]. Since the electric field is directly proportional to the voltage applied across the electrodes and inversely proportional to the distance between such electrodes, narrow gaps between electrodes are desired to minimize the voltage needed. Microfabrication techniques now make it possible to position electrodes very close to each other, therefore allowing for the creation of electrical fields up to kV/cm, strong enough to porate the cell membrane by dielectric breakdown, using voltages as low as tens of volts. Furthermore, the use of microelectrodes allows for the creation of miniaturized devices for cell lysis that feature low dead volumes, portability and can contain more modules, such as separation and detection [
6], other than cell lysis. Previous work on cell lysis using miniaturized devices include that from Lu
et al. [
12], Wang
et al. [
11] and by some of us [
13]. However, the throughput achieved so far in electrical lysis in microfluidic lab-on-a-chip devices is not enough to provide a sufficient amount of intracellular compounds to perform multiple analyses downstream, such as mass spectrometry, capillary electrophoresis or polymerase chain reaction. Common values reported in the literature are around 1 microliter per minute [
11,
12,
13,
14,
15,
16]. Recently, the use of a new electrical lysis device presented by Shahini and colleagues led to a throughput of up to 20 mL/h (333 µL/min), thanks mainly to a coating of carbon nanotubes on the 2D lysis electrodes which effectively introduces high intensity electric field gradients on the electrode surface [
17].
Microfluidic devices regularly feature channels with cross sections in the range of tens of micrometers. While these dimensions work well for certain applications, they are not suited for high throughput processing simply because the channel is too small to process high volumes of fluid rapidly. It is a must to increase these dimensions to hundreds of micrometers to significantly improve throughput. By doing so, we achieved a lysis throughput up to 600 µL/min at high cell density (108 cells per mL) with 90% efficiency. The channel cross-section used in this work is 100-µm high and 2-mm wide, and contains an array of 3D carbon electrodes, which are as tall as the channel height. The total channel volume is around 4 μL. To the best of our knowledge this is the highest throughput with such efficiency when using electrical lysis performed on-chip. The system has been evaluated with yeast cells and mammalian cells. The efficiency of extraction of intracellular components from mammalian cells is also compared to chemical lysis.
2. Device Design and Fabrication
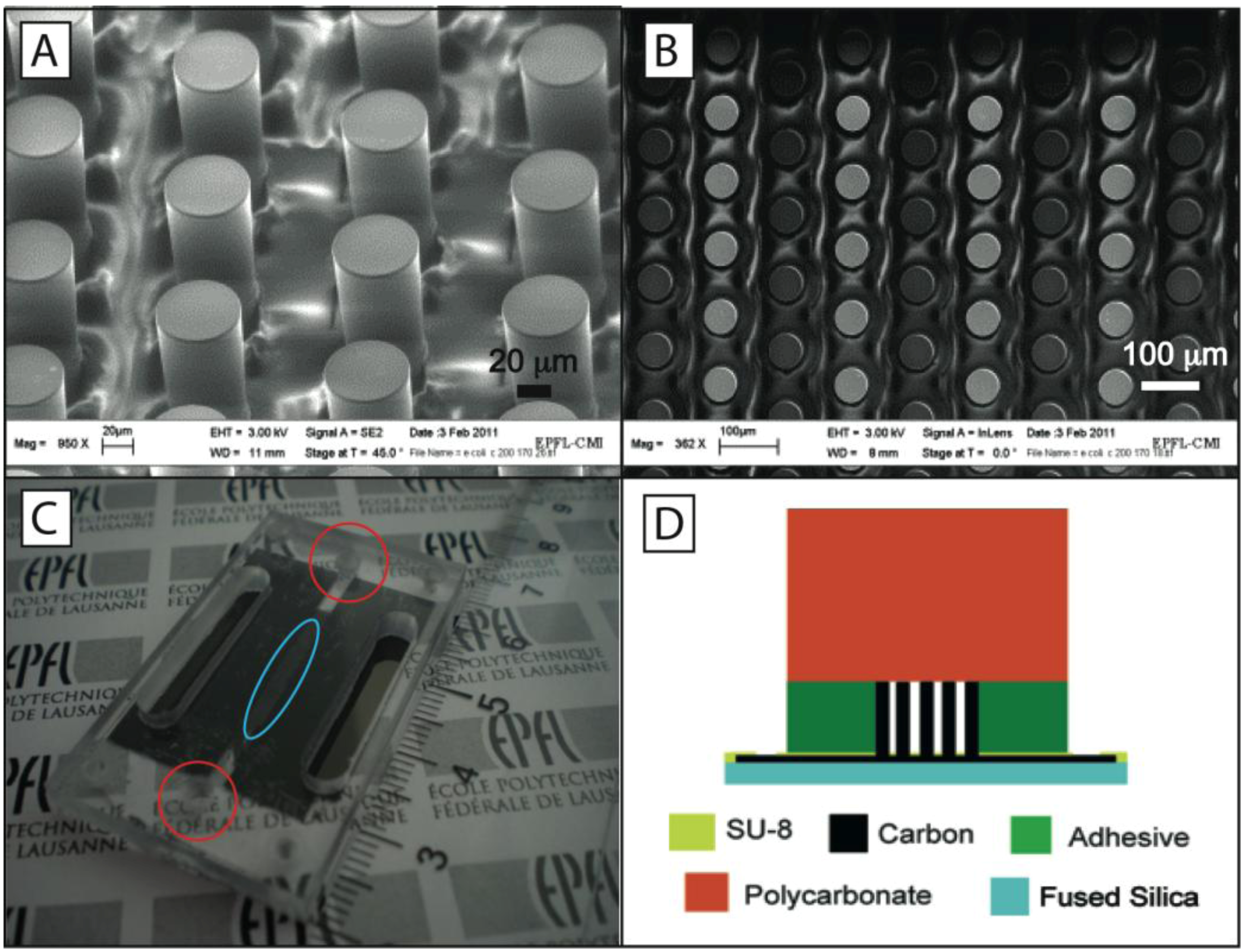
The device used here features 3D glass-like carbon microelectrodes derived by the pyrolysis, heating to high temperatures in an inert atmosphere, of a previously shaped organic polymer in a process known as Carbon MEMS (C-MEMS). The use of 3D carbon electrodes instead of metal electrodes brings many advantages. For example, carbon electrodes have a wider electrochemical stability window than gold and platinum and are highly biocompatible. Furthermore, the fabrication of 3D carbon structures is significantly easier and cheaper than that of precious metals. The complete fabrication process of the device has been detailed elsewhere [
18]. Briefly, a SU-8 precursor structure is fabricated on a transparent fused silica substrate and then carbonized by heating to 900 °C in a nitrogen atmosphere (
Figure 1(A)). Columns of 3D carbon electrodes are electrically connected such that every other column has the same polarity (
Figure 1(B)). A non-pyrolysed SU8 layer ensures electrical insulation on top of the paths connecting the pillars. The height of the electrodes is around 100 μm. Both the electrode diameter and spacing between electrodes are equal to 58 μm. The microfluidic network is made separate from the carbon electrodes and consists of a stack of pressure-sensitive double-sided adhesive (PSA) and polycarbonate (PC). A microchannel is first cut from a 100-μm thick PSA and is then aligned between inlet and outlet holes previously drilled in a PC substrate. An actual experimental device and its cross section are shown in
Figure 1(C,D), respectively.
Figure 1.
(A) Isometric view of the carbon electrodes; (B) Top view of carbon electrode array, every other column is connected to a different electric pole, i.e., gray circles are connected to the same electrical pole. The average separation between electrodes is 58 μm; (C) Actual device used for experiments. Inlet and outlet to and from the channel are shown in the two circles. The carbon electrode array is shown in the ellipse; (D) Schematic of the device cross-section.
Figure 1.
(A) Isometric view of the carbon electrodes; (B) Top view of carbon electrode array, every other column is connected to a different electric pole, i.e., gray circles are connected to the same electrical pole. The average separation between electrodes is 58 μm; (C) Actual device used for experiments. Inlet and outlet to and from the channel are shown in the two circles. The carbon electrode array is shown in the ellipse; (D) Schematic of the device cross-section.
3. Materials and Methods
Saccharomyces cerevisiae (Baker’s yeast, Migros, Switzerland) are suspended at a density of 108 cells per ml in a phosphate buffer saline (PBS) diluted 100 times in deionized water. The viability of the yeast cells injected into the chip remained at least 95% in all experiments as quantified by propidium iodide staining protocol. Bovine serum albumin (BSA) at 0.1% is added to reduce cell adhesion to the microchannel. The conductivity of the media is around 15 mS/m. T-cell acute lymphoblastic leukemia (T-ALL) cells from line RPMI8402 are transfected to express luciferase intracellularly. They are suspended at a density of 2 × 106 cells/mL in a 100-fold PBS dilution in a 300 mM sucrose solution for osmotic balance. Both PBS and BSA are obtained from Sigma-Aldrich. A sample volume of 100 µL containing cells are injected into the device at a flow rate controlled using a syringe pump (PhD2000, Harvard Apparatus).
Yeast cells pass through the lysis region and are collected from the chip to evaluate their viability using a propidium iodide (PI) staining protocol. A 100 μL plug of diluted PBS is passed through immediately after the sample to take the cells out of the dead volume of the chip. This two-fold dilution is taken into account for the calculation of the results. A similar protocol for sample injection and retrieval is used for mammalian cells. However, in this case the cell lysate recovered from the chip is analyzed using a luminescence assay to measure the extracted luciferase. A volume of 100 μL from the chip is placed in a 96-well plate where the dual luciferase kit (E1910, Promega) is added. The plate is then inserted in a Tecan 500 plate reader to read out luciferase concentration. The obtained results are compared to chemical lysis, which is performed on 100 μL of sample during 10 min at room temperature in the passive lysis buffer included in the luciferase kit (E1910, Promega).
4. Results and Discussion
The results of electrical lysis experiments on yeast cells are summarized in
Table 1. The lysis efficiency, defined as the percentage of dead cells after application of a lysing electric field, depends on the lysis voltage applied between electrodes and on the rate of sample flow through the electrode. A cell must be exposed to a strong-enough electric field for a minimum time to be lysed. The frequency of the lysis voltage is chosen at 10 kHz to optimize cell lysis efficiency while avoiding bubble formation at the electrodes. At an initial voltage of 52 V
pp the lysis efficiency is above 80% when using flow rates up to 50 μL /min. Unfortunately, such low flow rates are not desired because of low throughput and because they can lead to particle sedimentation in the channel, as previously detailed by one of us in a similar device [
19]. Indeed, the measured cell loss likely due to sedimentation increases from almost 0% at flows of 100 μL /min up to around 40% at flows of 20 μL /min. The cell lysis efficiency remains high when implementing higher flow rates as long as the voltage is increased accordingly; almost all cells injected at 100 μL /min when using a voltage of 65 V
pp are still lysed. If higher flow rates are required, the lysis voltage must be further increased. Following such rationale, the use of 130 V
pp leads to a 90% efficiency in cell lysis at 600 μL /min, corresponding to 60 millions cells lysed per second. The use of voltages higher than 130 V
pp led to breakdown of the sample and origination of bubbles inside the channel. Consequently, the implementation of faster flows at this voltage limit at 130 V
pp leads to a gradual decrease on lysis efficiency as shown in the last row of
Table 1.
Table 1.
Lysis efficiency and transit times inside the chip for different values of applied voltage and flow rate. The lysis efficiency is defined as the percentage of cells lysed during the experiment, as obtained by propidium iodide staining. The concentration of yeast cells in the sample is 108 cells/mL.
Table 1.
Lysis efficiency and transit times inside the chip for different values of applied voltage and flow rate. The lysis efficiency is defined as the percentage of cells lysed during the experiment, as obtained by propidium iodide staining. The concentration of yeast cells in the sample is 108 cells/mL.
| Lysis voltage | Flow rate (l/min) | Transit time inside chip | Lysis efficiency (%) |
|---|
| 52 Vpp | 10 | 24 s | 89% |
| 52 Vpp | 50 | 4.8 s | 84% |
| 52 Vpp | 200 | 1.2 s | 42% |
| 65 Vpp | 100 | 2.4 s | 98% |
| 65 Vpp | 600 | 400 ms | 3% |
| 130 Vpp | 600 | 400 ms | 90% |
| 130 Vpp | 2000 | 120 ms | 5% |
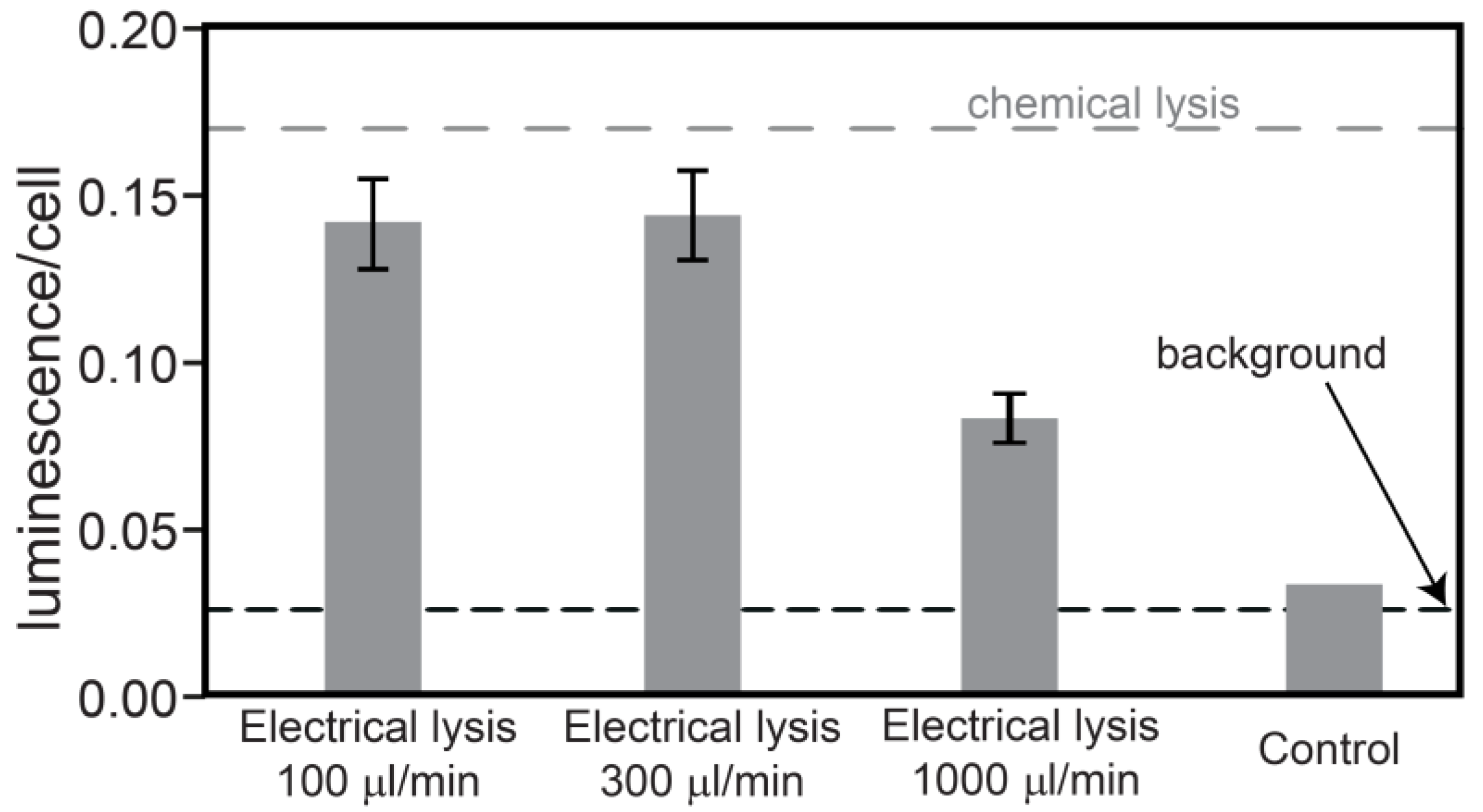
Incorporation of propidium iodide inside the cells does not necessarily prove that the entire cell interior is released, but at least proves that the membrane has been permeabilized. The device ability to extract intracellular compounds for further analysis has been evaluated with mammalian cells. T-cell acute lymphoblastic leukemia (T-ALL) cells from lines RPMI8402 are transfected to express luciferase intracellularly. Mammalian cells do not require as high electrical fields as yeast cells to be lysed because they are bigger and breakdown at lower trans-membrane potentials [
20]. The lysis voltage applied here was only 44 V
pp at 10 kHz. The results of the luminescence assay to detect intracellular compounds at flow rates of 100, 300 and 1,000 μL/min are shown in
Figure 2. The measured amount of luminescence is divided by the number of cells placed in the luminescence meter to provide an estimation of the luciferase extraction in arbitrary units. Flow rates of 100 and 300 μL/min give similar results, around 0.15. In comparison, chemical lysis, also performed by us, gives a luminescence per cell equal to 0.17 (dashed grey line). This difference can be explained by the fact that there is still a small percentage of cells not being lysed by our device, especially cells flowing on the edges of the channel, which are exposed to a lower electrical field. At flow rates of 1 mL/min the luminescence per cell drops significantly suggesting inefficient extraction of intracellular compounds. The dashed black line represents the background for living cells. The last column shows a control experiment obtained by flowing the cells through a non-polarized electrode array. The luminescence level is comparable to the background level, indicating no content extraction in that case.
The use of electrical lysis over chemical processes offers the main advantage of being able to recover relatively intact cytosol for downstream analysis. The device presented here advances the state-of-the-art in electrical lysis in terms of throughput and offers a powerful tool for cell lysis in lab-on-a-chip applications. Important advantages include high throughput and extraction of large amounts of intracellular compounds. The enabling improvements over existent devices are the use of large channels and the incorporation of 3D electrodes, as tall as the channel, penetrating the bulk of the sample.
The use of carbon electrodes instead of metal ones allows for relatively easy and inexpensive fabrication of very tall electrodes that are separated by very narrow gaps [
19]. In this way, the throughput is improved by increasing the channel cross-section, but the low voltage needed for cell lysis is maintained thanks to the close gap between electrodes. Indeed, the throughput achieved by this device, often the limitation of electrical lysis devices, is sufficient to consider electrical lysis as an advantageous alternative to chemical lysis. For example, a sample volume of milliliters can be treated in only a few minutes, faster than the reaction time required to chemically lyse similar volumes, while providing enough intracellular content for multiple analyses. Besides the low dead volume, another significant advantage of this device over bulk chemical lysis is that the delay between lysis and cytosol analysis can be minimized, as the total time including lysis and sample transport is smaller. This is very critical as extracted compounds degrade with time. This delay could be further reduced by integration of the analysis in the same microdevice. The ability to lyse cells at high densities is also essential as it guarantees that the concentration of extracted compounds can be measured accurately using post-lysis analyses.
Figure 2.
Measured luminescence divided by number of cells, giving an indication of the luciferase extraction. The values correspond to the average of 3 electrical lysis experiments, error bars referring to the standard deviation. The last column is a control experiment without applied voltage. The grey line corresponds to chemical lysis experiments, whereas the black line is the background signal for living cells.
Figure 2.
Measured luminescence divided by number of cells, giving an indication of the luciferase extraction. The values correspond to the average of 3 electrical lysis experiments, error bars referring to the standard deviation. The last column is a control experiment without applied voltage. The grey line corresponds to chemical lysis experiments, whereas the black line is the background signal for living cells.
In the context of bioreactors used in the pharmaceutical industry, lysis speed is an important factor to be able to regularly monitor the cell batch. A faster throughput allows more frequent monitoring (if the analysis systems situated afterwards are not limiting it), leading to a better optimization of the production process.