Microbeads for Sampling and Mixing in a Complex Sample
Abstract
:1. Introduction
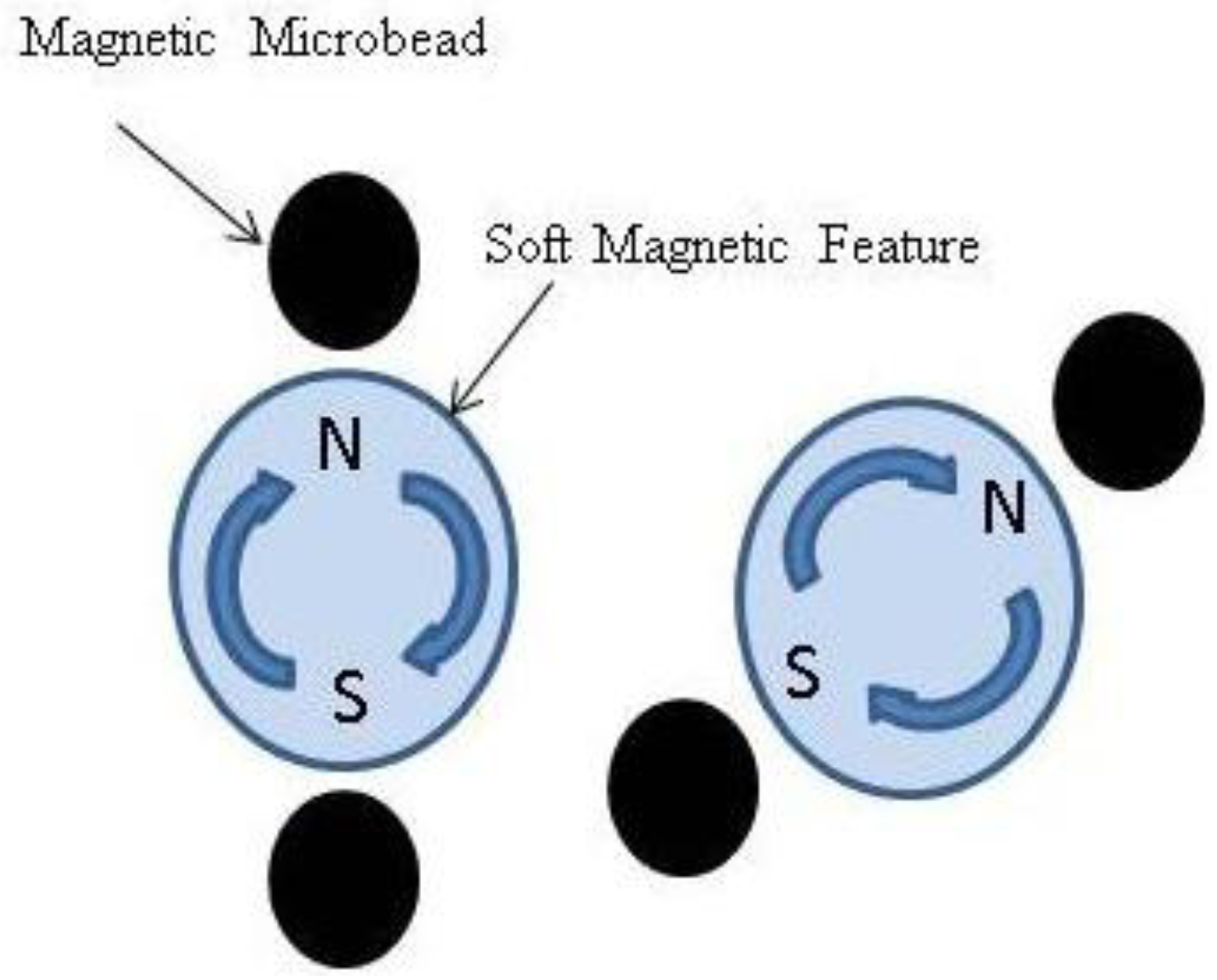
2. Experimental Section
2.1. Computational Modeling













 is the permeability of free space, B is the magnetic field vector, and
is the permeability of free space, B is the magnetic field vector, and



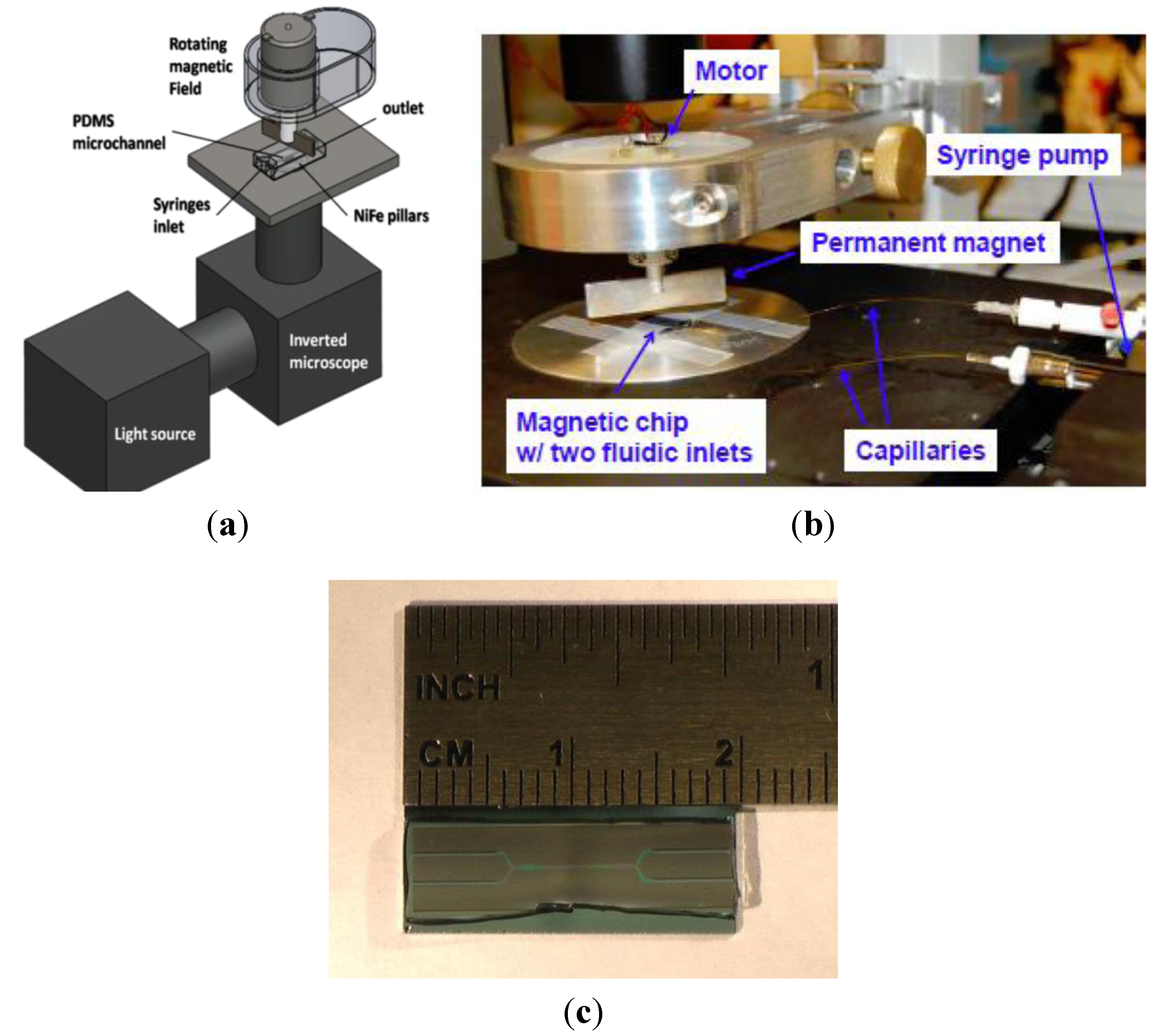
2.2. Current Device Fabrication and Characterization
2.2.1. PDMS Molding
2.2.2 Chip Fabrication
2.2.3. Chip Preparation
2.2.4. Device Assembly
2.2.5. Magnetic Bead Solution
2.2.6. Magnet and Motor Assembly
3. Results and Discussion
3.1. Computational Modeling of Mixing

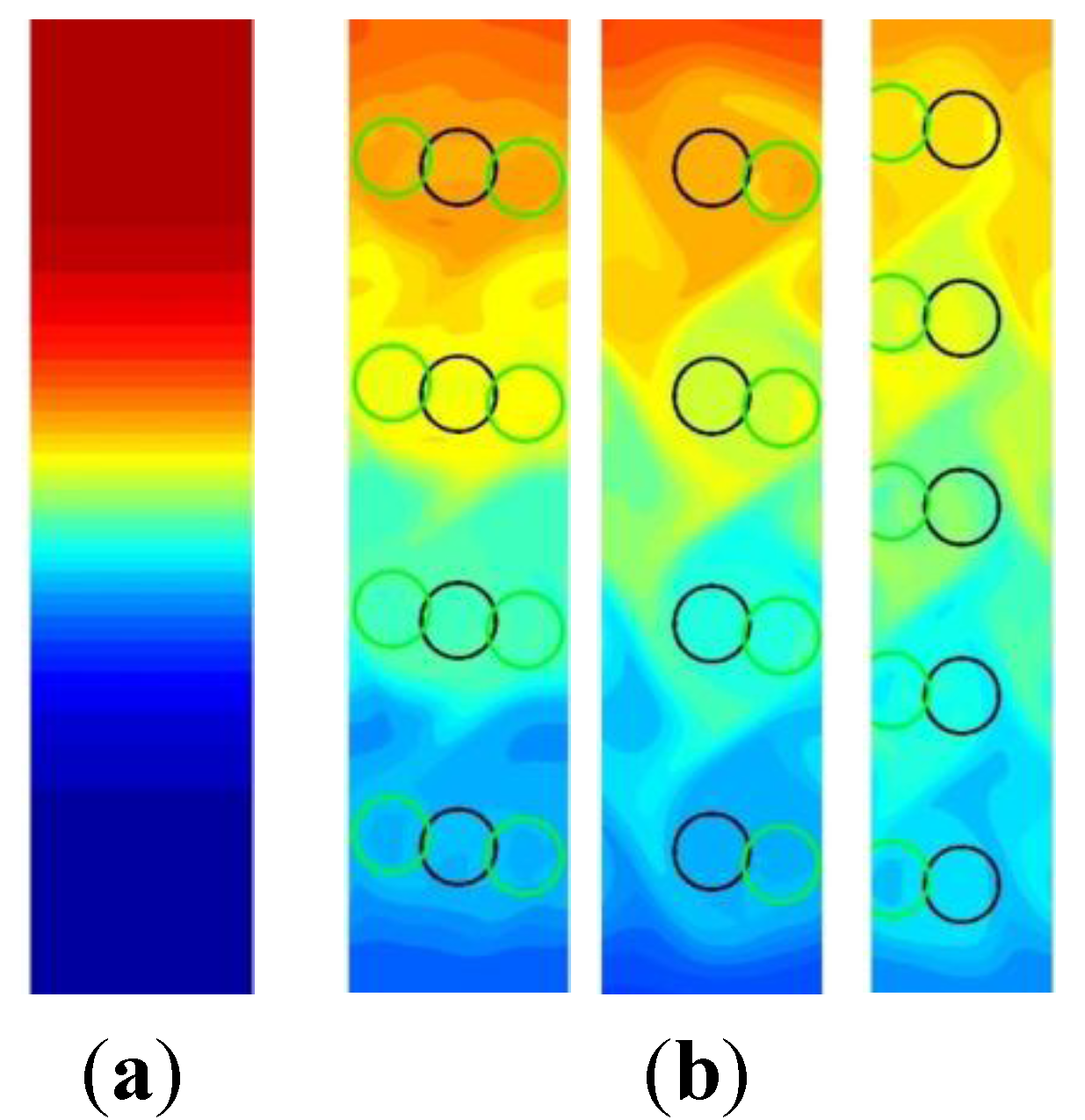
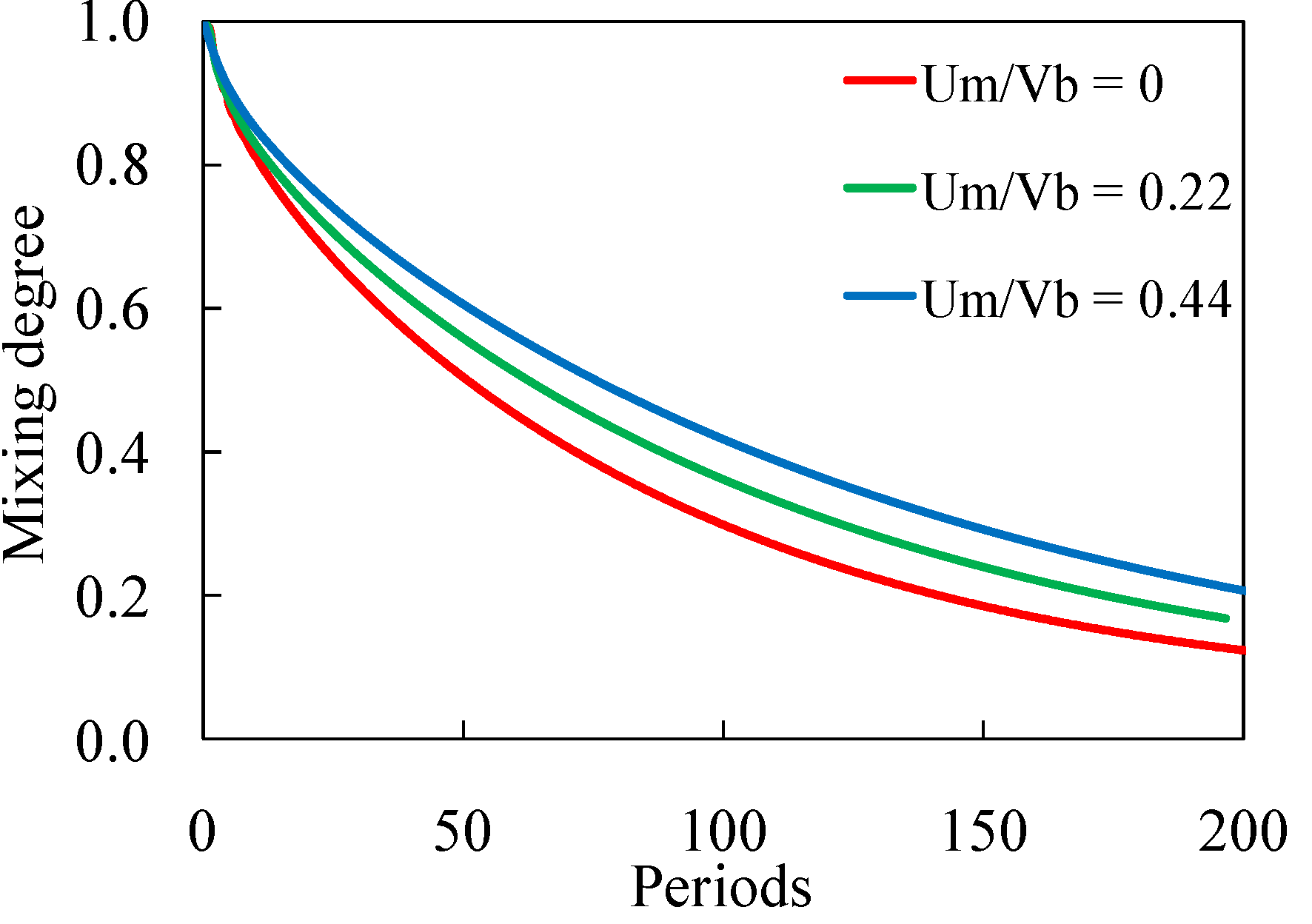
3.1.1. Bead Trajectories

3.2. Experimental Device Characterization
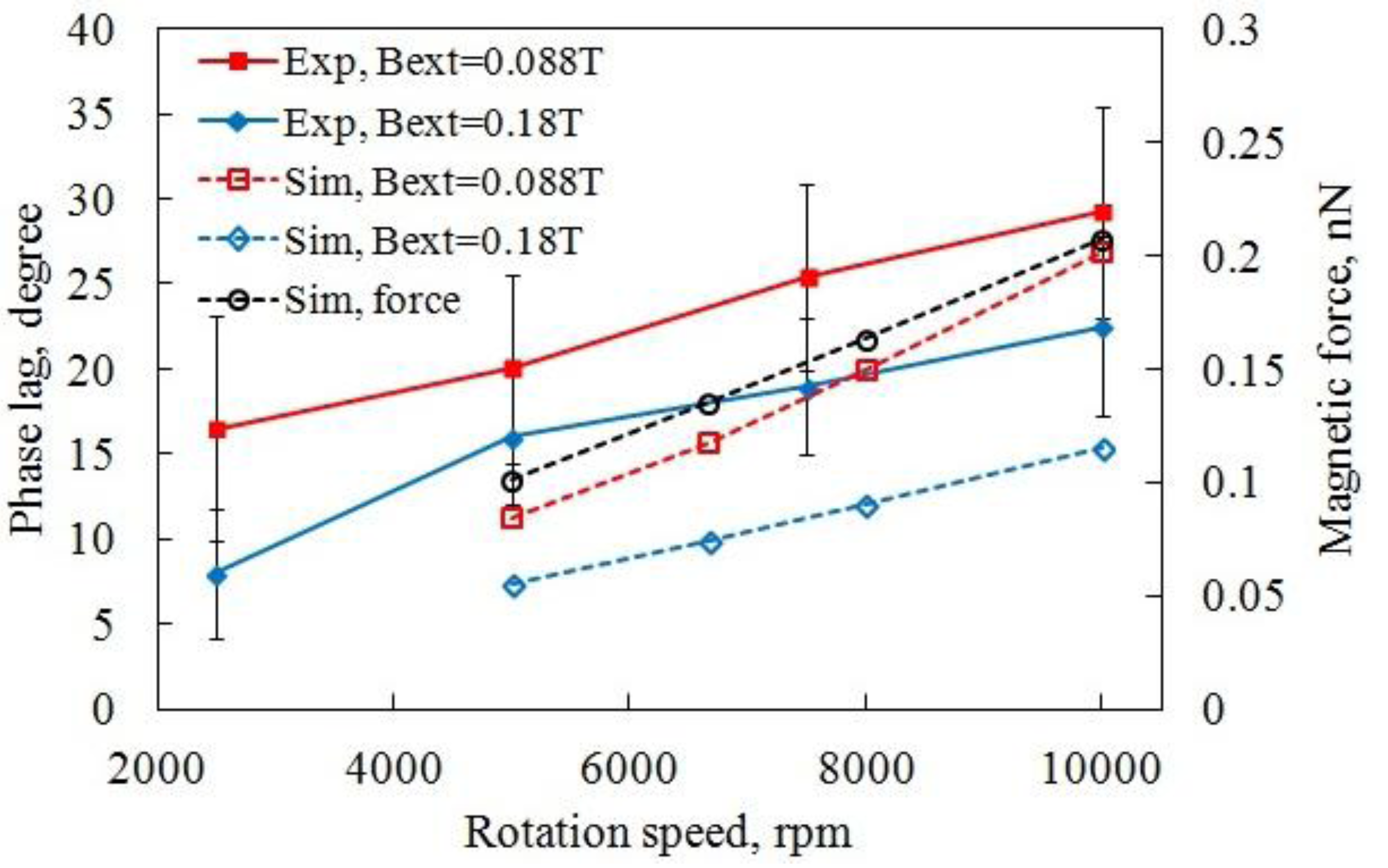
3.2.1. Dynamics of High Speed Rotation
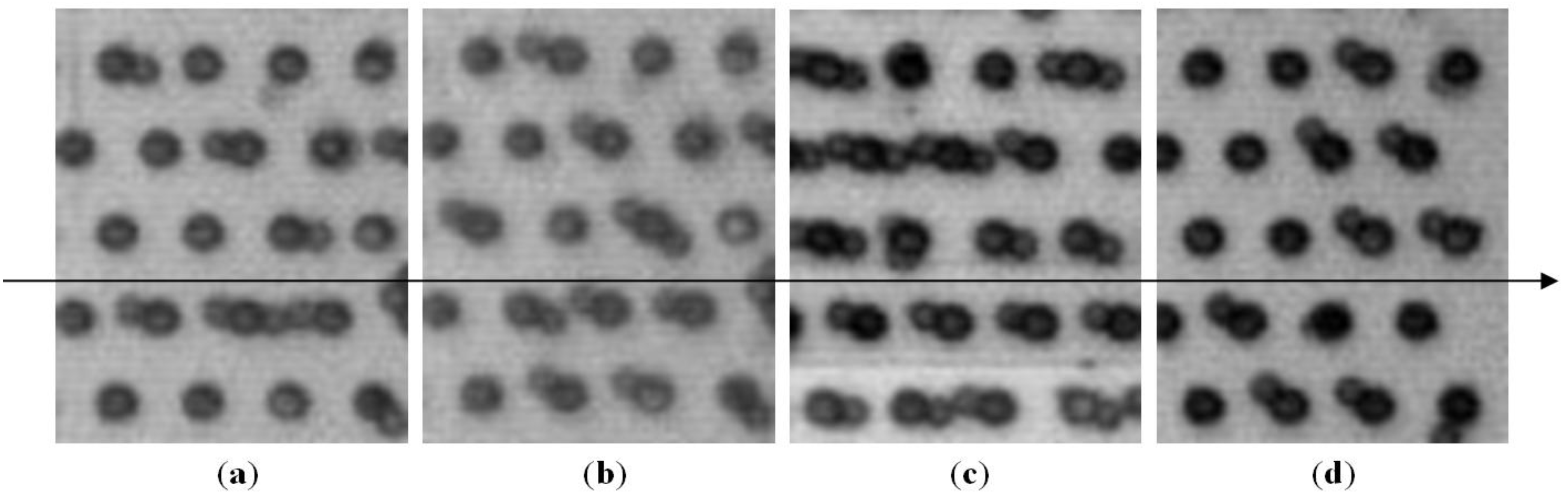

3.2.2. Microbeads Capture Capacity
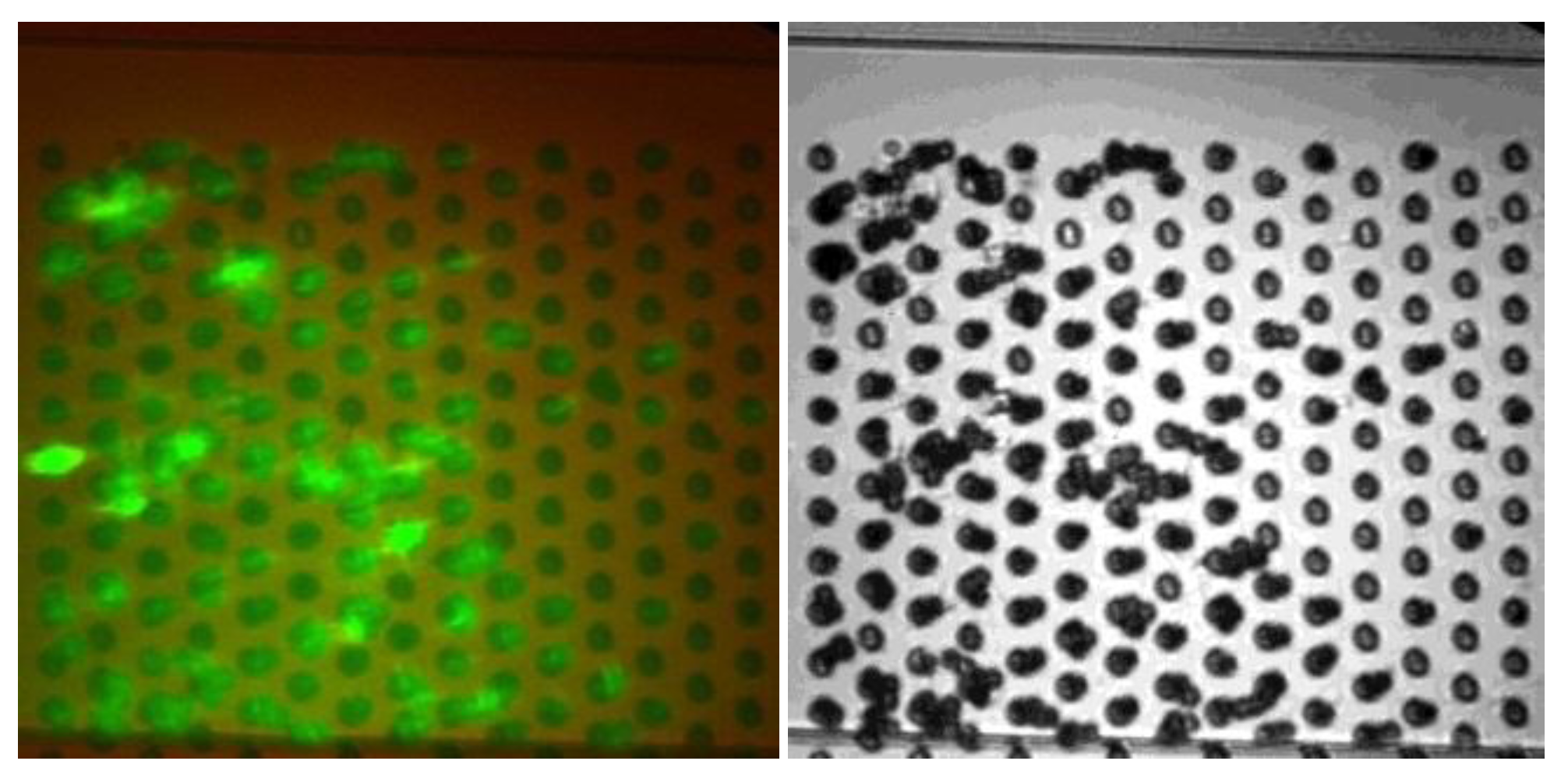
4. Conclusion and Future Work
Acknowledgments
References
- Dwivedi, H.P.; Jaykus, L.A. Detection of pathogens in foods: The current state-of-the-art and future directions. Crit. Rev. Microbiol. 2011, 37, 40–63. [Google Scholar] [CrossRef]
- Suh, Y.K.; Kang, S. A review on mixing in microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Ramadan, Q.; Samper, V.; Poenar, D.; Yu, C. Magnetic-based microfluidic platform for biomolecular separation. Biomed. Devices 2006, 8, 151–158. [Google Scholar]
- Deng, T.; Whitesides, G.M. Manipulation of magnetic microbeads in suspension using micromagnetic systems fabricated with soft lithography. Appl. Phys. Lett. 2001, 78, 1775–1777. [Google Scholar] [CrossRef]
- Lee, H.; Purdon, A.M.; Chu, V.; Westervelt, R.M. Controlled assembly of magnetic nanoparticles from magnetotactic bacteria using microelectromagntes arrays. Nano Lett. 2004, 4, 995–998. [Google Scholar] [CrossRef]
- Wirix-Speetjens, R.; de Boeck, J. On-chip magnetic particle transport by alternating magnetic field gradients. IEEE Trans. Magn. 2004, 40, 1944–1946. [Google Scholar] [CrossRef]
- Mao, W.; Peng, Z.; Hesketh, P.J.; Alexeev, A. Microfluidic mixing using an array of superparamagnetic beads. In Proceedings of the American Physical Society Meeting, Dallas, TX, USA, 21 March 2011.
- Alexeev, A.; Verberg, R.; Balzas, A.C. Modeling the motion of microcapsules on compliant polymeric surfaces. Macromolecules 2005, 38, 10244–10260. [Google Scholar] [CrossRef]
- Alexeev, A.; Verberg, R.; Balazs, A.C. Designing compliant substrates to regulate the motion of vesicles. Phys. Rev. Lett. 2006, 96. [Google Scholar] [CrossRef]
- Succi, S. The Lattice Boltzmann Equation for Fluids Dynamics and Beyond; Clarendon Press: Oxford, UK, 2001. [Google Scholar]
- Ladd, A.J.C.; Verberg, R. Lattice-Boltzmann simulations of particle-fluid suspensions. J. Stat. Phys. 2001, 104, 1191–1251. [Google Scholar] [CrossRef]
- Verberg, R.; Pooley, C.; Yeomans, J.; Balzas, A. Pattern formation in binary fluids confined between rough, chemically heterogeneous surfaces. Phys. Rev. Lett. 2004, 93, 184501–185504. [Google Scholar]
- Verberg, R.; Yeomans, J.M.; Balazs, A.C. Modeling the flow of fluid/particle mixtures in microchannels: Encapsulating nanoparticles within monodisperse droplets. J. Chem. Phys. 2005, 123, 224706–224714. [Google Scholar] [CrossRef]
- Swift, M.R.; Orlandini, E.; Osborn, W.; Yeomans, J. Lattice Boltzmann simulations of liquid-gas and binary fluid systems. Phys. Rev. E 1996, 54, 5041–5052. [Google Scholar] [CrossRef]
- Bouzidi, M.; Firdaouss, M.; Lallemand, P. Momentum transfer of a Boltzmann-lattice fluid with boundaries. Phys. Fluids 2001, 13, 3452–3459. [Google Scholar] [CrossRef]
- Alexeev, A.; Verberg, R.; Balazs, A.C. Modeling the interactions between deformable capsules rolling on a compliant surface. Soft Matter 2006, 2, 499–509. [Google Scholar] [CrossRef]
- Alexeev, A.; Verberg, R; Balazs, A.C. Patterned surfaces segregate compliant microcapsules. Langmuir 2007, 23, 983–987. [Google Scholar] [CrossRef]
- Masoud, H; Alexeev, A. Modeling magnetic microcapsules that crawl in microchannels. Soft Matter 2010, 6, 794–799. [Google Scholar] [CrossRef]
- Mao, W.; Alexeev, A. Hydrodynamic sorting of microparticles by size in ridged microchannels. Phys. Fluids. 2011. [Google Scholar] [CrossRef]
- Arata, J.P.; Alexeev, A. Designing microfluidic channel that separates elastic particles upon stiffness. Soft Matter 2009, 5, 2721–2724. [Google Scholar] [CrossRef]
- Furlani, E.P. Permanent Magnet and Electromechanical Devices: Materials, Analysis, and Applications; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Furlani, E.P. Analysis of particle transport in a magnetophoretic microsystem. J. Appl. Phys. 2006, 99, 024912–024922. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Ferrohydrodynamics; Dover Publications: Mineola, NY, USA, 1997. [Google Scholar]
- Peng, Z. Parallel Manipulation of Individual Magnetic Microbeads for Lab-on-a-Chip Applications. Ph.D. Thesis, School of Mechanical Engineering, Georgia Institute of Technology, Atlanta, GA, USA, 2011. [Google Scholar]
- Peng, Z.; Guo, W.; Cannon, J.L.; Hesketh, P.J. A magnetophoresis system for controlled transport and trapping of magnetic beads. In Proceedings of Transactions of microTAS 2010, Groningen, The Netherlands, 3–7 October 2010.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Owen, D.; Mao, W.; Alexeev, A.; Cannon, J.L.; Hesketh, P.J. Microbeads for Sampling and Mixing in a Complex Sample. Micromachines 2013, 4, 103-115. https://doi.org/10.3390/mi4010103
Owen D, Mao W, Alexeev A, Cannon JL, Hesketh PJ. Microbeads for Sampling and Mixing in a Complex Sample. Micromachines. 2013; 4(1):103-115. https://doi.org/10.3390/mi4010103
Chicago/Turabian StyleOwen, Drew, Wenbin Mao, Alex Alexeev, Jennifer L. Cannon, and Peter J. Hesketh. 2013. "Microbeads for Sampling and Mixing in a Complex Sample" Micromachines 4, no. 1: 103-115. https://doi.org/10.3390/mi4010103




