Effects of Micromachining Processes on Electro-Osmotic Flow Mobility of Glass Surfaces
Abstract
:1. Introduction
2. Material and Method
2.1. Micromachining of Glass Substrates
2.2. Measurement of EOF
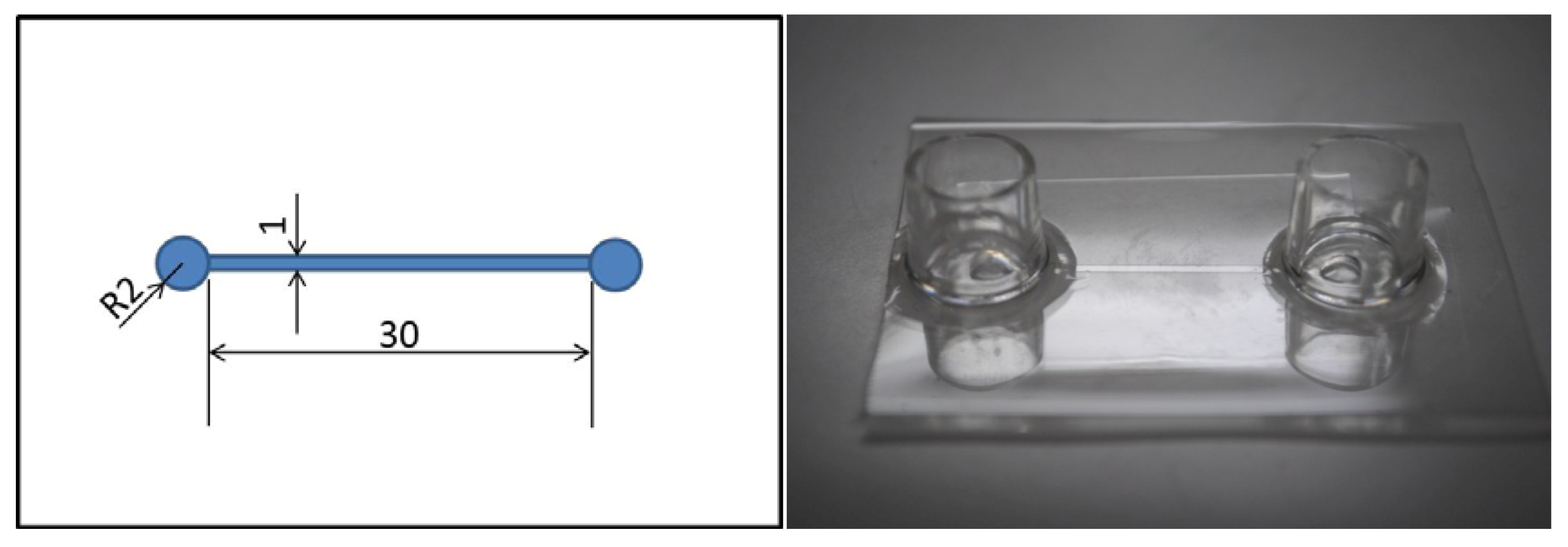
3. Experiments
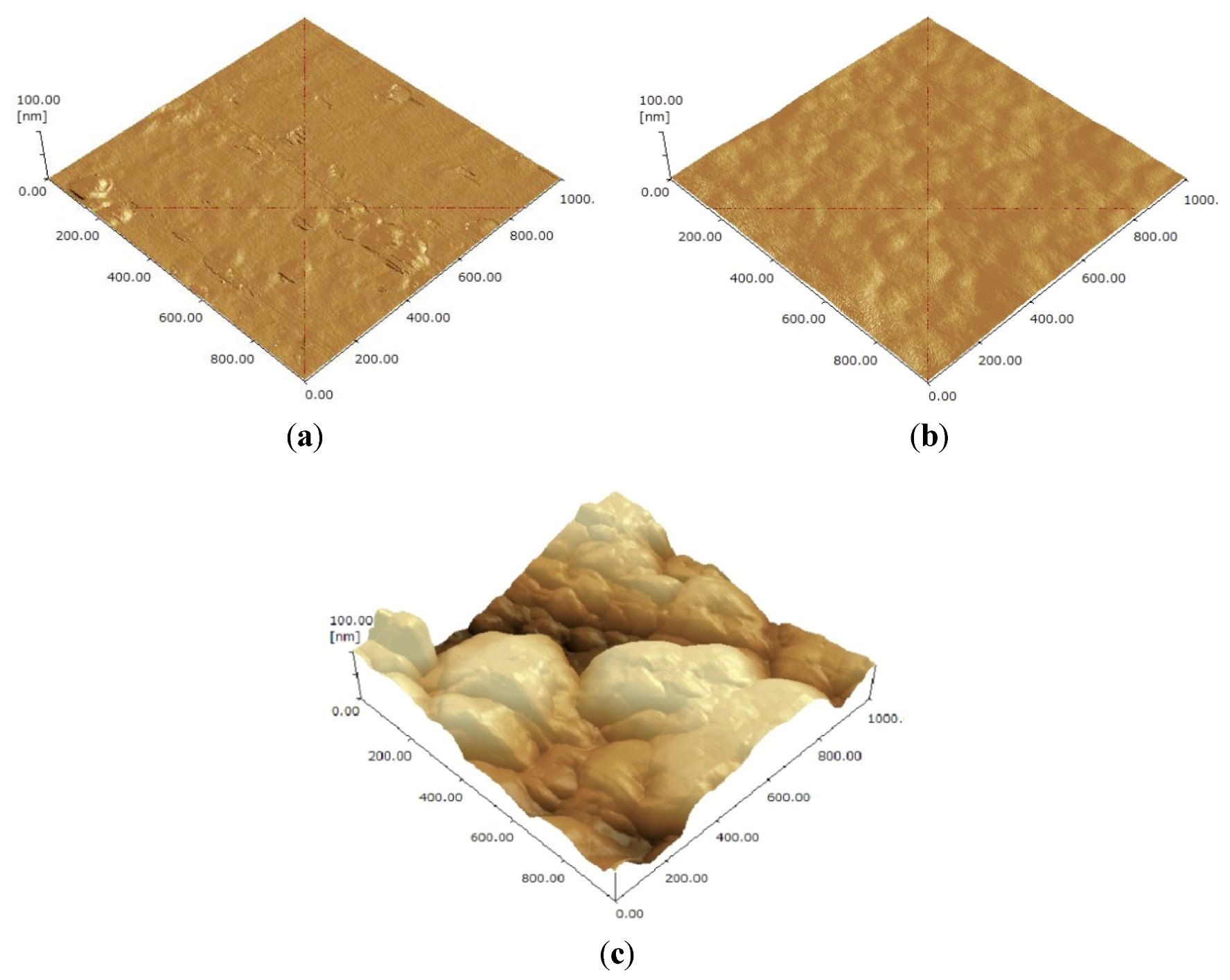
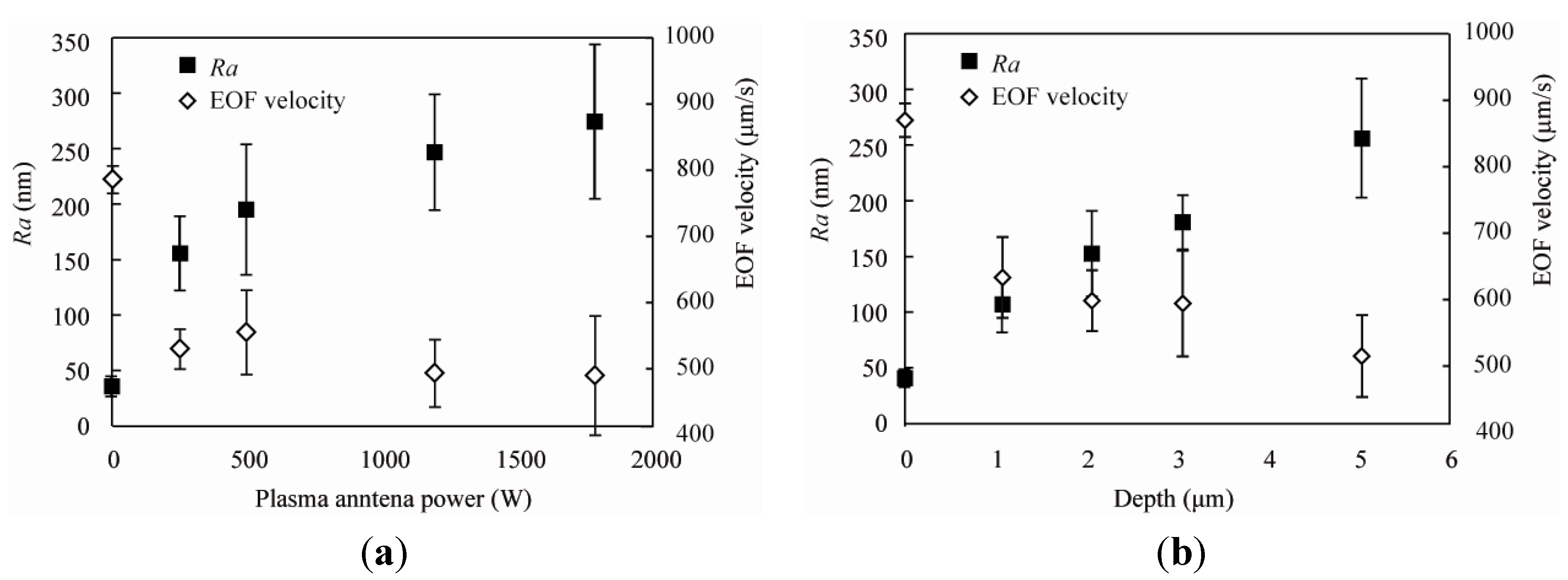
3.1. Surface Roughness after Glass Patterning
3.2. EOF Measurement

3.3. Effective Zeta Potential

 is the zeta potential, and E is the applied electric field strength [46].
is the zeta potential, and E is the applied electric field strength [46]. 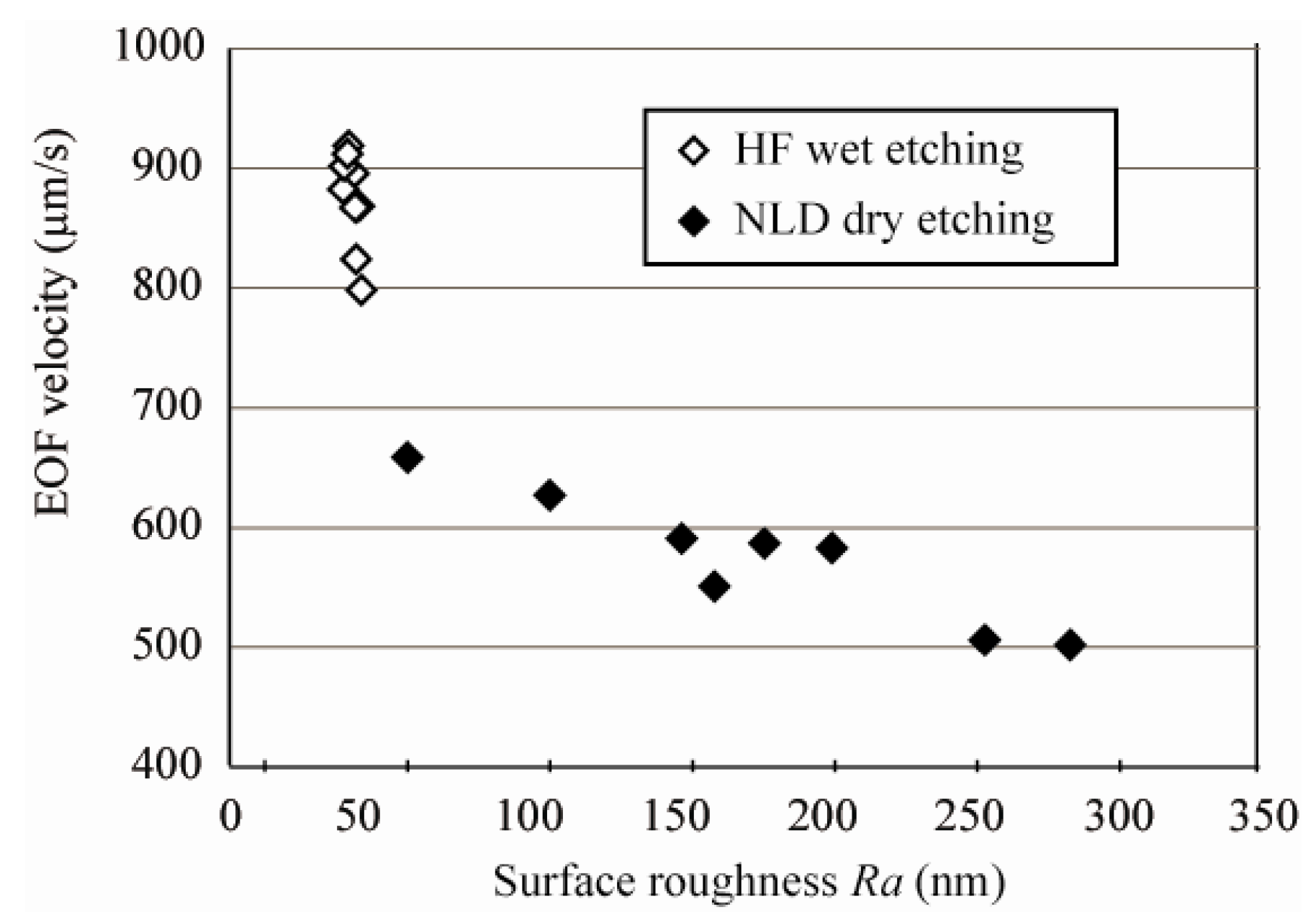
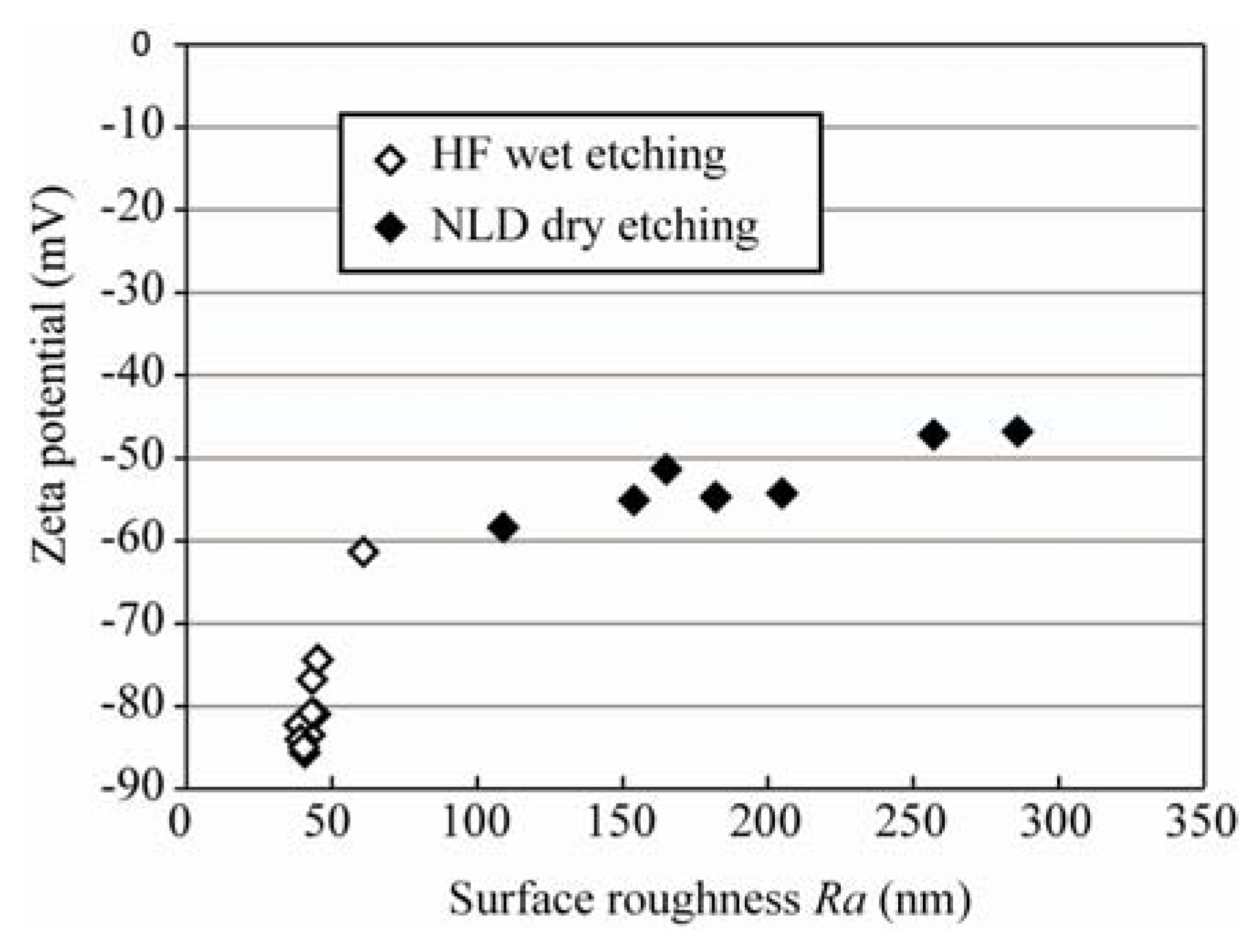
4. Conclusions
Acknowledgements
References
- Manz, A.; Graver, N.; Widmer, H.M. Miniaturized total chemical-analysis systems—a novel concept for chemical sensing. Sens. Actuators B 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Shoji, S. Fluids for sensor systems. Top. Curr. Chem. 1998, 194, 163–188. [Google Scholar] [CrossRef]
- Khandurina, J.; Guttman, A. Bioanalysis in microfluidic devices. J. Chromatogr. A 2002, 943, 159–183. [Google Scholar] [CrossRef]
- Chovan, T.; Guttman, A. Microfabricated devices in biotechnology and biochemical processing. Trends Biotechnol. 2002, 20, 116–122. [Google Scholar] [CrossRef]
- Huikko, K.; Kostiainen, R.; Kotiaho, T. Introduction to micro-analytical systems: Bioanalytical and pharmaceutical applications. Eur. J. Pharm. Sci. 2003, 20, 149–171. [Google Scholar]
- Sheehan, A.D.; Quinn, J.; Daly, S.; Dillon, P.; O’Kennedy, R. The development of novel miniaturized immuno-sensing devices: A review of a small technology with a large future. Anal. Lett. 2003, 36, 511–537. [Google Scholar]
- Sato, K.; Hibara, A.; Tokeshi, M.; Hisamoto, H.; Kitamori, T. Microchip-based chemical and biochemical analysis systems. Adv. Drug Deliv. Rev. 2003, 55, 379–391. [Google Scholar]
- Lee, S.J.; Lee, S.Y. Micro total analysis system (mu-TAS) in biotechnology. Appl. Microbiol. Biot. 2004, 64, 289–299. [Google Scholar]
- Sun, Y.; Kwok, Y.C. Polymeric microfluidic system for DNA analysis. Anal. Chim. Acta 2006, 556, 80–96. [Google Scholar]
- Gu, Y.; Miki, N. A microfilter utilizing a polyethersulfone porous membrane with nanopores. J. Micromech. Microeng. 2007, 17, 2308–2315. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, K.; Shimizu, T.; Yamato, M.; Okano, T.; Kitamori, T. Biological cells on microchips: New technologies and applications. Biosens. Bioelectron. 2007, 23, 449–458. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.H.; Hong, M.; Erramilli, S.; Mohanty, P. Surface-modified silicon nano-channel for urea sensing. Sens. Actuators 2008, 133, 593–598. [Google Scholar] [CrossRef]
- Suzuki, H.; Takeuchi, S. Microtechnologies for membrane protein studies. Anal. Bioanal. Chem. 2008, 391, 2695–2702. [Google Scholar] [CrossRef]
- Gu, Y.; Miki, N. Multilayered microfilter using a nanoporous PES membrane and applicable as the dialyzer of a wearable artificial kidney. J. Micromech. Microeng. 2009, 19. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Chen, H.; Kong, J. Integration of electrochemistry in micro-total analysis systems for biochemical assays: Recent developments. Talanta 2009, 80, 8–18. [Google Scholar] [CrossRef]
- Ota, H.; Yamamoto, R.; Deguchi, K.; Tanaka, Y.; Kazoe, Y.; Sato, Y.; Miki, N. Three-dimensional spheroid-forming lab-on-a-chip using micro-rotational flow. Sens. Actuators B 2010, 147, 359–365. [Google Scholar] [CrossRef]
- Liu, K.K.; Wu, R.G.; Chuang, Y.J.; Khoo, H.S.; Huang, S.-H.; Tseng, F.-G. Microfluidic systems for biosensing. Sensors 2010, 10, 6623–6661. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Cooper, J.M. Tumors on chips: Oncology meets microfluidics. Curr. Opin. Chem. Biol. 2010, 14, 556–567. [Google Scholar] [CrossRef]
- Ota, H.; Kodama, T.; Miki, N. Rapid formation of size-controlled three dimensional hetero-cell aggregates using micro-rotation flow for spheroid study. Biomicrofluidics 2011, 5. [Google Scholar] [CrossRef]
- Ota, H.; Miki, N. Microfluidic experimental platform for producing size-controlled three-dimensional spheroids. Sens. Actuators A 2011, 169, 266–273. [Google Scholar] [CrossRef]
- Shen, C.; Mokkapati, V.R.S.S.; Pham, H.T.M.; Sarro, P.M. Micromachined nanofiltration modules for lab-on-a-chip applications. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Wang, K.; Yue, S.; Wang, L.; Jin, A.; Gu, C.; Wang, P.; Wang, H.; Xu, X.; Wang, Y.; Niu, H. Nanofluidic channels fabrication and manipulation of DNA molecules. IEEE Proc. Nanobiotechnol. 2006, 153, 11–15. [Google Scholar] [CrossRef]
- Das, S.K.; Austin, M.D.; Akana, M.C.; Deshpande, P.; Cao, H.; Xiao, M. Single molecule linear analysis of DNA in nano-channel labeled with sequence specific fluorescent probles. Nucleic Acids Res. 2010, 38. [Google Scholar] [CrossRef]
- Liu, C.; Qu, Y.Y.; Luo, Y.; Fang, N. Recent advances in single-molecule detection on micro- and nano-fluidic devices. Electrophoresis 2011, 32, 3308–3318. [Google Scholar] [CrossRef]
- Kish, L.L.; Kameoka, J.; Granqvist, C.G.; Kish, L.B. Log-normal distribution of single molecule fluorescence bursts in micro/nano-fluidic channels. Appl. Phys. Lett. 2011, 99. [Google Scholar] [CrossRef]
- Abad, E.; Juarros, A.; Retolaza, A.; Merino, S.; Marie, R.; Kristensen, A. DNA analysis by single molecule stretching in nanofluidic biochips. Microelectron. Eng. 2011, 88, 300–304. [Google Scholar]
- Eun, D.S.; Shin, J.K.; Lee, J.H. Enhanced micro-PCR chip using TiO2nanofluidic heat-sink. Sens. Lett. 2009, 9, 276–282. [Google Scholar]
- Chen, P.; Feng, X.; Du, W.; Liu, B-F. Microfluidic chips for cell sorting. Front. Biosci. 2008, 13, 2464–2483. [Google Scholar] [CrossRef]
- Mawatari, K.; Tsukahara, T.; Sugii, Y.; Kitamori, T. Extended-nano fluidic systems for analytical and chemical technologies. Nanoscale 2010, 2, 1588–1595. [Google Scholar]
- Huang, M.; Yanik, A.A.; Chang, T.Y.; Altug, H. Sub-wavelength nanofluidics in photonic crystal sensors. Opt. Express 2009, 17, 24224–24233. [Google Scholar]
- Cao, H.; Yu, Z.N.; Wang, J.; Tegenfeldt, J.O.; Austin, R.H.; Chen, E.; Wu, W.; Chou, S.Y. Fabrication of 10 nm enclosed nanofluidic channels. Appl. Phys. Lett. 2002, 81, 174–176. [Google Scholar] [CrossRef]
- Pepin, A.; Youninou, P.; Studer, V.; Lebib, A.; Chen, Y. Nanoimprint lithography for the fabrication of DNA electrophoresis chips. Microelectron. Eng. 2002, 61, 927–932. [Google Scholar]
- Zheng, H.J.; Wang, Z.L.; Feinerman, A.D. Fabrication of micro/nano fluidic channels with sacrificial galvanic coupled metals. Nanotechnology 2006, 17, 3183–3188. [Google Scholar]
- Shao, P.E.; van Kan, A.; Wang, L.P.; Ansari, K.; Bettiol, A.A.; Watt, F. Fabrication of enclosed nanochannels in poly(methylmethacrylate) using proton beam writing and thermal bonding. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Kehagias, N.; Chansin, G.; Reboud, V.; Zelsmann, M.; Schuster, C.; Kubenz, M.; Reuther, F.; Gruetzner, G.; Sotomayer Torres, C.M. Embedded nano channels fabricated by non-selective reverse contact UV nanoimprint lithography technique. Microelectron. Eng. 2007, 84, 921–924. [Google Scholar]
- Wong, C.C.; Agarwal, A.; Balasubramanian, N.; Kwong, D.L. Fabrication of self-sealed circular nano/microfluidic channels in glass substrates. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Koller, D.M.; Galler, N.; Ditlbacher, H.; Hohenau, A.; Leitner, A.; Aussenegg, F.R.; Kren, J.R. Direct fabrication of micro/nano fluidic channels by electron beam lithography. Microelectron. Eng. 2009, 86, 1314–1316. [Google Scholar]
- Yang, R.; Lu, B.R.; Wang, J.; Xie, S-Q.; Chen, Y.; Hug, E.; Qu, X-P.; Liu, R. Fabrication of micro/nano fluidic channels by nanoimprint lithography and bonding using SU-8. Microelectron. Eng. 2009, 86, 1379–1381. [Google Scholar]
- Venkatakrishnan, K.; Jariwala, S.; Tan, B. Maskless fabrication of nano-fluidic channels by two-photon absorption (TPA) polymerization of SU-8 on glass substrate. Opt. Express 2009, 17, 2756–2762. [Google Scholar]
- Kim, S.K.; Cho, H.; Park, H.K.; Kim, J.H.; Chung, B.H. Fabrication of nanochannels by anisotropic wet etching on silicon-on-insulator wafers and their applications to DNA stretch. J. Nanosci. Nanotechnol. 2010, 10, 637–642. [Google Scholar]
- Fernandez-Cuesta, I.; Palmarelli, A.L.; Liang, X.G.; Zhang, J.; Dhuey, S.; Olynick, D.; Cabrini, S. Fabrication of fluidic devices with 30 nm nanochannels by direct imprinting. J. Vac. Sci. Technol. B 2011, 29. [Google Scholar] [CrossRef]
- Afanasiev, A.; Lahdesmaki, I.; Parviz, B.A. Fabrication and electrical characterization of integrated nano-scale fluidic channels. Microsyst. Technol. 2011, 17, 1511–1518. [Google Scholar]
- Hong, L.F.; Pan, T.R. Surface microfluidics fabricated by photopatternable superhydrophobic nanocomposite. Microfluid. Nanofluid. 2011, 10, 991–997. [Google Scholar]
- Hunter, R.J. Zeta potential in Colloid Science: Principles and Applications; Academic Press: New York, NY, US, 1981. [Google Scholar]
- Sze, A.; Erickson, D.; Ren, L. Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electrosmotic flow. J. Colloid Interf. Sci. 2003, 261, 402–410. [Google Scholar]
- Venditti, R.; Xuan, X.C.; Li, D.Q. Experimental characterization of the temperature dependence of zeta potential and its effect on electroosmotic flow velocity in microchannels. Microfluid. Nanofluid. 2006, 2, 493–499. [Google Scholar]
- Santiago, J.G.; Wereley, S.T.; Meinhart, C.D.; Beebe, D.J.; Adrian, R.J. A particle image velocimetry system for microfluidics. Exp. Fluids 1998, 25, 316–319. [Google Scholar]
- Sato, Y.; Hishida, K. Electrokinetic effects on motion of submicron particles in microchannel. Fluid Dyn. Res. 2006, 38, 787–802. [Google Scholar]
- Lindken, R.; Rossi, M.; Grosse, S.; Westerweel, J. Micro-Particle Image Velocimetry (mu PIV): Recent developments, applications, and guidelines. Lab Chip 2009, 9, 2551–2567. [Google Scholar] [CrossRef]
- Tatsumi, K.; Nishitani, K.; Fukuda, K.; Katsumoto, Y.; Nakabe, K. Measurement of electroosmotic flow velocity and electric field in microchannels by micro-particle image velocimetry. Meas. Sci. Technol. 2010, 21. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, C.K.; Kihm, K.D. Optically sliced micro-PIV using confocal laser scanning microscopy (CLSM). Exp. Fluids 2004, 37, 105–119. [Google Scholar]
- Lima, R.; Wada, S.; Tsubota, K.; Yamaguchi, T. Confocal micro-PIV measurements of three-dimensional profiles of cell suspension flow in a square microchannel. Meas. Sci. Technol. 2006, 17, 797–808. [Google Scholar] [CrossRef]
- Park, J.S.; Kihm, K.D. Use of confocal laser scanning microscopy (CLSM) for depthwise resolved microscale-particle image velocimetry (µ-PIV). Opt. Lasers Eng. 2006, 44, 208–223. [Google Scholar]
- Ichiyanagi, M.; Sato, Y.; Hishida, K. Optically sliced measurement of velocity and pH distribution in microchannel. Exp. Fluids 2007, 43, 425–435. [Google Scholar]
- Kinoshita, H.; Kaneda, S.; Fujii, T.; Oshima, M. Three-dimensional measurement and visualization of internal flow of a moving droplet using confocal micro-PIV. Lab Chip 2007, 7, 338–346. [Google Scholar]
- Ichiyanagi, M.; Sasaki, S.; Sato, Y.; Hishida, K. Micro-PIV/LIF measurements on electrokinetically-driven flow in surface modified microchannels. J. Micromech. Microeng. 2009, 19. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S. Advanced particle-based velocimetry techniques for microscale flows. Microfluid. Nanofluid. 2009, 6, 577–588. [Google Scholar]
- Oishi, M.; Kinoshita, H.; Fujii, T.; Oshima, M. Simultaneous measurement of internal and surrounding flows of a moving droplet using multicolour confocal micro-particle image velocimetry (micro-PIV). Meas. Sci. Technol. 2011, 22. [Google Scholar] [CrossRef]
- Williams, S.J.; Park, C.; Wereley, S.T. Advances and applications on microfluidic velocimetry techniques. Microfluid. Nanofluid. 2010, 8, 709–726. [Google Scholar]
- Zettner, C.M.; Yoda, M. Particle velocity field measurements in a near-wall flow using evanescent wave illumination. Exp. Fluids 2003, 34, 115–121. [Google Scholar]
- Banerjee, A.; Kihm, K.D. Three-dimensional tracking of nanoparticles using R-TIRFM technique. J. Heat Trans. 2004, 126. [Google Scholar] [CrossRef]
- Kazoe, Y.; Sato, Y. Effect of ion motion on zeta-potential distribution at microchannel wall obtained from nanoscale laser-induced fluorescence. Anal. Chem. 2007, 79, 6727–6733. [Google Scholar]
- Kazoe, Y.; Miyakawa, S.; Miki, N.; Sato, Y. Fluorescence imaging technique of surface electrostatic potential using evanescent wave illumination. Appl. Phys. Lett. 2009, 92. [Google Scholar] [CrossRef]
- Min, Y.U.; Kim, K.C. Hybrid micro-/nano-particle image velocimetry for 3D3C multi-scale velocity field measurement in microfluidics. Meas. Sci. Technol. 2011, 22. [Google Scholar] [CrossRef]
- Joly, L.; Ybert, C.; Trizac, E.; Bocquet, L. Liquid friction on charged surfaces: From hydrodynamic slippage to electrokinetics. J. Chem. Phys. 2006, 125. [Google Scholar] [CrossRef]
- Lim, J.M.; Chun, M.S. Curvature-induced secondary microflow motion in steady electro-osmotic transport with hydrodynamic slippage effect. Phys. Fluids 2011, 23. [Google Scholar] [CrossRef]
- Miki, N.; Spearing, S.M. Effect of nanoscale surface roughness on the bonding energy of direct-bonded silicon wafers. J. Appl. Phys. 2003, 94, 6800–6806. [Google Scholar]
- Ayón, A.A.; Braff, R.; Lin, C.C.; Sawin, H.H.; Schmidt, M.A. Characterization of a time multiplexed inductively coupled plasma etcher. J. Electrochem. Soc. 1999, 146, 339–349. [Google Scholar]
- Miki, N.; Teo, C.J.; Ho, L.C.; Zhang, X. Enhancement of rotordynamic performance of high-speed micro-rotors for power MEMS applications by precision deep reactive ion etching. Sens. Actuators A 2003, 104, 263–267. [Google Scholar]
- Chen, W.; Itoh, M.; Hayashi, T.; Uchida, T. Dry etch process in magnetic neutral loop discharge plasma. Jpn. J. Appl. Phys. 1998, 37, 332–336. [Google Scholar]
- Chen, W.; Morikawa, Y.; Itoh, M.; Hayashi, T.; Sugita, K.; Shindo, H.; Uchida, T. Very uniform and high aspect ratio anisotropy SiO2 etching process in magnetic neutral loop discharge plasma. J. Vac. Sci. Technol. A 1999, 17, 2546–2550. [Google Scholar]
- Chen, W.; Sugita, K.; Morikawa, Y.; Yasunami, S.; Hayashi, T.; Uchida, T. Application of magnetic neutral loop discharge plasma in deep silica etching. J. Vac. Sci. Technol. A 2001, 19, 2936–2940. [Google Scholar]
- Uchida, T.; Hamaguchi, S. Magnetic neutral loop discharge (NLD) plasmas for surface processing. J. Phys. D 2008, 41. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koga, Y.; Kuriyama, R.; Sato, Y.; Hishida, K.; Miki, N. Effects of Micromachining Processes on Electro-Osmotic Flow Mobility of Glass Surfaces. Micromachines 2013, 4, 67-79. https://doi.org/10.3390/mi4010067
Koga Y, Kuriyama R, Sato Y, Hishida K, Miki N. Effects of Micromachining Processes on Electro-Osmotic Flow Mobility of Glass Surfaces. Micromachines. 2013; 4(1):67-79. https://doi.org/10.3390/mi4010067
Chicago/Turabian StyleKoga, Yosuke, Reiko Kuriyama, Yohei Sato, Koichi Hishida, and Norihisa Miki. 2013. "Effects of Micromachining Processes on Electro-Osmotic Flow Mobility of Glass Surfaces" Micromachines 4, no. 1: 67-79. https://doi.org/10.3390/mi4010067




