Gravitational Field-Flow Fractionation Devices Fabricated via a Hot Embossing/Thermal Bonding Method
Abstract
:1. Introduction
2. Experiment
2.1. Material and Template

2.2. Experimental Setup

2.3. Hot Embossing Process

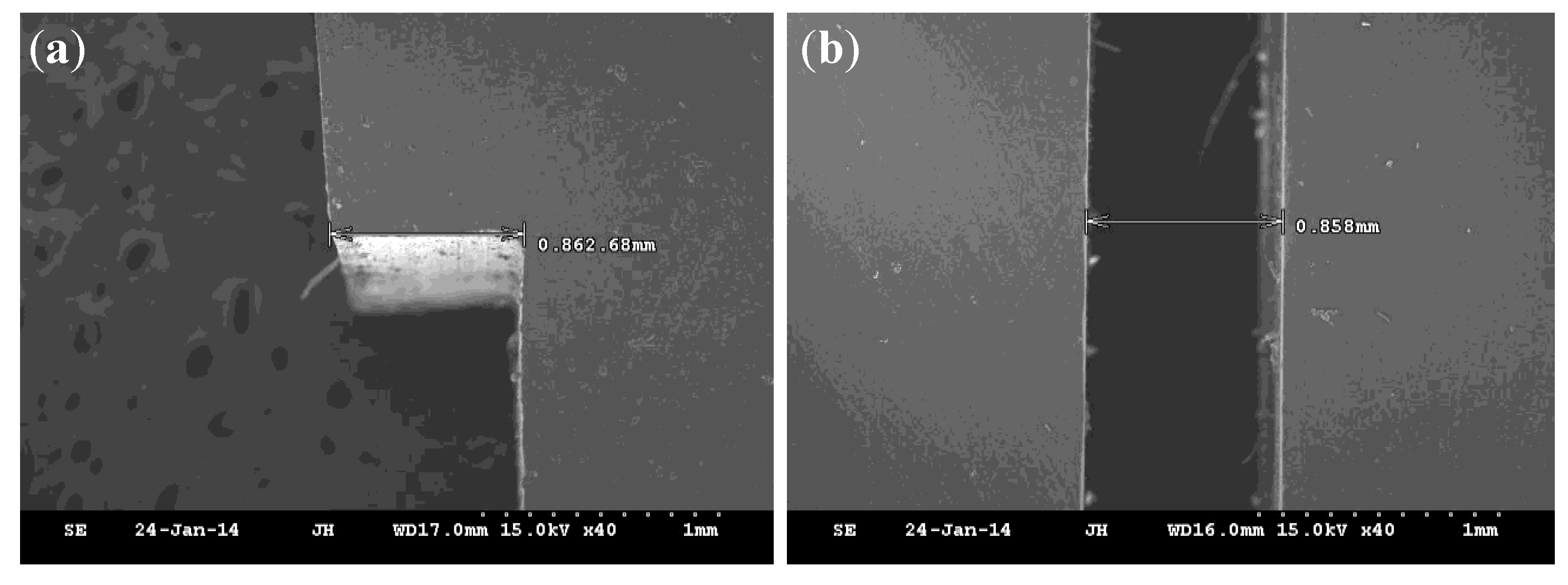
2.4. The Channel Dimension Measurement
2.5. Hot Bonding Process

2.6. Bonding Performance Analysis
2.6.1. Separation Channel Characterization Test
2.6.2. Burst Pressure Test
2.7. Separation Experiment
2.7.1. Operating Principle and Basic Structure

2.7.2. Experiment Setup

3. Results and Discussion
3.1. Influence of Hot Embossing Parameters
3.1.1. Effect of Hot Embossing Temperature


3.1.2. Effect of Hot Embossing Pressure

3.2. Influence of Thermal Bonding Parameters



3.3. Gravitational Field Flow Fractionation Characteristic Test and Burst Pressure Test


3.4. Fractionation Test Results


4. Conclusions
Author Contributions
Conflicts of Interest
References
- Urbánková, E.; Vacek, A.; Nováková, N.; Matulík, F.; Chmelík, J. Investigation of red blood cell fractionation by gravitational field-flow fractionation. J. Chromatogr. B Biomed. Sci. Appl. 1992, 583, 27–34. [Google Scholar] [CrossRef]
- Lattuada, D.; Roda, B.; Pignatari, C.; Magni, R.; Colombo, F.; Cattaneo, A.; Zattoni, A.; Cetin, I.; Reschiglian, P.; Bolis, G. A tag-less method for direct isolation of human umbilical vein endothelial cells by gravitational field-flow fractionation. Anal. Bioanal. Chem. 2013, 405, 977–984. [Google Scholar] [CrossRef]
- Ibrahim, T.; Battu, S.; Cook-Moreau, J.; Cardot, P. Instrumentation of hollow fiber flow field flow fractionation for selective cell elution. J. Chromatogr. B 2012, 901, 59–66. [Google Scholar] [CrossRef]
- Roda, B.; Reschiglian, P.; Alviano, F.; Lanzoni, G.; Bagnara, G.P.; Ricci, F.; Buzzi, M.; Tazzari, P.L.; Pagliaro, P.; Michelini, E.; et al. Gravitational field-flow fractionation of human hemopoietic stem cells. J. Chromatogr. A 2009, 1216, 9081–9087. [Google Scholar]
- Benincasa, M.-A.; Moore, L.R.; Williams, P.S.; Poptic, E.; Carpino, F.; Zborowski, M. Cell sorting by one gravity SPLITT fractionation. Anal. Chem. 2005, 77, 5294–5301. [Google Scholar] [CrossRef]
- Mazanec, K.; Dycka, F.; Bobalova, J. Monitoring of barley starch amylolysis by gravitational field flow fractionation and MALDI-TOF MS. J. Sci. Food Agric. 2011, 91, 2756–2761. [Google Scholar] [CrossRef]
- Chmelík, J.; Mazanec, K.; Bohačenko, I.; Psota, V. Relationship between the ratio of large and small starch granules determined by gravitational field-flow fractionation and malting quality of barley varieties. J. Liquid Chromatogr. Relat. Technol. 2007, 30, 1289–1301. [Google Scholar] [CrossRef]
- Chmelík, J.; Krumlová, A.; Budinská, M.; Kruml, T.; Psota, V.; Bohac̆enko, I.; Mazal, P.; Vydrová, H. Comparison of size characterization of barley starch granules determined by electron and optical microscopy, low angle laser light scattering and gravitational field-flow fractionation. J. Inst. Brew. 2001, 107, 11–17. [Google Scholar] [CrossRef]
- Psota, V.; Bohačenko, I.; Hartmann, J.; Budinská, M.; Chmelík, J. Comparison of the GFFF and LALLS methods for the measurement of starch granule size distribution in spring barley caryopses. J. Inst. Brew. 2002, 108, 200–203. [Google Scholar] [CrossRef]
- Janoušková, J.; Budinská, M.; Plocková, J.; Chmelı́k, J. Optimization of experimental conditions for the separation of small and large starch granules by gravitational field-flow fractionation. J. Chromatogr. A 2001, 914, 183–187. [Google Scholar] [CrossRef]
- Contado, C.; Reschiglian, P.; Faccini, S.; Zattoni, A.; Dondi, F. Continuous split-flow thin cell and gravitational field-flow fractionation of wheat starch particles. J. Chromatogr. A 2000, 871, 449–460. [Google Scholar] [CrossRef]
- Contado, C.; Hoyos, M. SPLITT Cell Analytical Separation of Silica Particles. Non-Specific Crossover effects: does the shear-induced diffusion play a role? Chroma 2007, 65, 453–462. [Google Scholar] [CrossRef]
- Reschiglian, P.; Melucci, D.; Torsi, G.; Zattoni, A. Standardless method for quantitative particle-size distribution studies by gravitational field-flow fractionation.Application to silica particles. Chroma 2000, 51, 87–94. [Google Scholar] [CrossRef]
- Pazourek, J.; Chmelík, J. Characterization of chromatographic silica gel support particles by gravitational field-flow fractionation. J. Microcolumn Sep. 1997, 9, 611–617. [Google Scholar] [CrossRef]
- Park, M.R.; Chum, Y.S.; Kang, D.Y.; Yu, S.K.; Choi, S.H.; Lee, K.H.; Lee, S. Effect of reaction parameters on size distribution of emulsion-polymerized polystyrene latex beads studied by gravitational flow-flow fractionation (GrFFF). J. Liquid Chromatogr. Relat. Technol. 2009, 32, 909–922. [Google Scholar] [CrossRef]
- Meng, H.; Caldwell, K.D.; Calvin Giddings, J. Examination of residues from coal liquefaction by steric field-flow fractionation. Fuel Process. Technol. 1984, 8, 313–320. [Google Scholar] [CrossRef]
- Roda, B.; Reschiglian, P.; Zattoni, A.; Tazzari, P.; Buzzi, M.; Ricci, F.; Bontadini, A. Human lymphocyte sorting by gravitational field-flow fractionation. Anal. Bioanal. Chem. 2008, 392, 137–145. [Google Scholar] [CrossRef]
- Roda, B.; Casolari, S.; Reschiglian, P.; Mirasoli, M.; Simoni, P.; Roda, A. Hybrid gravitational field-flow fractionation using immunofunctionalized walls for integrated bioanalytical devices. Anal. Bioanal. Chem. 2009, 394, 953–961. [Google Scholar] [CrossRef]
- Magliulo, M.; Roda, B.; Zattoni, A.; Michelini, E.; Luciani, M.; Lelli, R.; Reschiglian, P.; Roda, A. An innovative, flow-assisted, noncompetitive chemiluminescent immunoassay for the detection of pathogenic bacteria. Clin. Chem. 2006, 52, 2151–2155. [Google Scholar] [CrossRef]
- Park, M.R.; Kang, D.Y.; Chmelik, J.; Kang, N.; Kim, J.S.; Lee, S. Different elution modes and field programming in gravitational field-flow fractionation: Effect of channel angle. J. Chromatogr. A 2008, 1209, 206–211. [Google Scholar]
- Plocková, J.; Chmelík, J. Different elution modes and field programming in gravitational field-flow fractionation: Field programming using density and viscosity gradients. J. Chromatogr. A 2006, 1118, 253–260. [Google Scholar] [CrossRef]
- Casolari, S.; Roda, B.; Mirasoli, M.; Zangheri, M.; Patrono, D.; Reschiglian, P.; Roda, A. Gravitational field-flow fractionation integrated with chemiluminescence detection for a self-standing point-of-care compact device in bioanalysis. Analyst 2013, 138, 211–219. [Google Scholar] [CrossRef]
- Messaud, F.A.; Sanderson, R.D.; Runyon, J.R.; Otte, T.; Pasch, H.; Williams, S.K.R. An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Progr. Polym. Sci. 2009, 34, 351–368. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef]
- Lin, M.-C.; Yeh, J.-P.; Chen, S.-C.; Chien, R.-D.; Hsu, C.-L. Study on the replication accuracy of polymer hot embossed microchannels. Int. Commun. Heat Mass Transf. 2013, 42, 55–61. [Google Scholar] [CrossRef]
- Yi, S.; Yien Chian, K.; Nam-Trung, N. Low-pressure, high-temperature thermal bonding of polymeric microfluidic devices and their applications for electrophoretic separation. J. Micromech. Microeng 2006, 16, 1681. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, G.; Guo, Y.; Tian, Y. Study of PMMA thermal bonding. Microsyst. Technol. 2007, 13, 403–407. [Google Scholar]
- Kricka, L.J.; Fortina, P.; Panaro, N.J.; Wilding, P.; Alonso-Amigo, G.; Becker, H. Fabrication of plastic microchips by hot embossing. Lab Chip 2002, 2, 1–4. [Google Scholar]
- Chen, Z.; Gao, Y.; Lin, J.; Su, R.; Xie, Y. Vacuum-assisted thermal bonding of plastic capillary electrophoresis microchip imprinted with stainless steel template. J. Chromatogr. A 2004, 1038, 239–245. [Google Scholar]
- Li, J.M.; Liu, C.; Qiao, H.C.; Zhu, L.Y.; Chen, G.; Dai, X.D. Hot embossing/bonding of a poly(ethylene terephthalate) (PET) microfluidic chip. J. Micromech. Microeng. 2008, 18, 015008. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.Y.; Venkatraman, S.S.; Ma, L.L. Low-temperature (below Tg) thermal bonding of COC microfluidic devices using UV photografted HEMA-modified substrates: High strength, stable hydrophilic, biocompatible surfaces. J. Mater. Chem. 2011, 21, 15031–15040. [Google Scholar] [CrossRef]
- Umbrecht, F.; Müller, D.; Gattiker, F.; Boutry, C.M.; Neuenschwander, J.; Sennhauser, U.; Hierold, C. Solvent assisted bonding of polymethylmethacrylate: Characterization using the response surface methodology. Sens. Actuators A Phys. 2009, 156, 121–128. [Google Scholar]
- Sood, V. An Experimental Study on Thermal Bonding Effects of PMMA Based Micro-devices Using Hot Embossing; ProQuest: Ann Arbor, MI, USA, 2008. [Google Scholar]
- Hsu, Y.-C.; Chen, T.-Y. Applying Taguchi methods for solvent-assisted PMMA bonding technique for static and dynamic μ-TAS devices. Biomed. Microdevices 2007, 9, 513–522. [Google Scholar] [CrossRef]
- Tran, H.H.; Wu, W.; Lee, N.Y. Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens. Actuators B Chem. 2013, 181, 955–962. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, K. Gravitational Field-Flow Fractionation Devices Fabricated via a Hot Embossing/Thermal Bonding Method. Micromachines 2014, 5, 139-153. https://doi.org/10.3390/mi5020139
Yang K. Gravitational Field-Flow Fractionation Devices Fabricated via a Hot Embossing/Thermal Bonding Method. Micromachines. 2014; 5(2):139-153. https://doi.org/10.3390/mi5020139
Chicago/Turabian StyleYang, Kaijun. 2014. "Gravitational Field-Flow Fractionation Devices Fabricated via a Hot Embossing/Thermal Bonding Method" Micromachines 5, no. 2: 139-153. https://doi.org/10.3390/mi5020139



