Self-Aligned Interdigitated Transducers for Acoustofluidics
Abstract
:1. Introduction
2. Results and Discussion
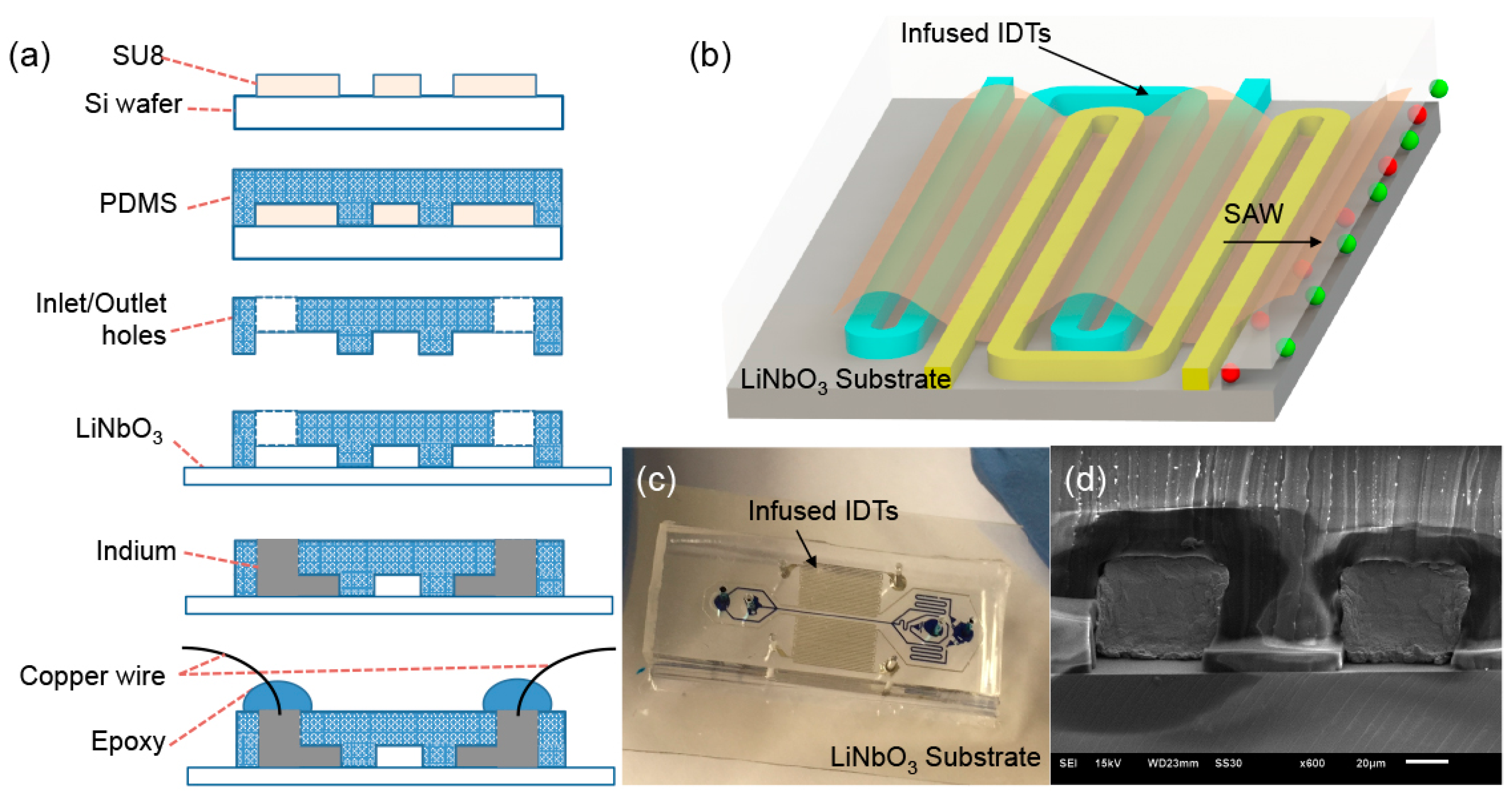
2.1. Design and Fabrication of Self-Aligned SAW-Based Acoustofluidic Device
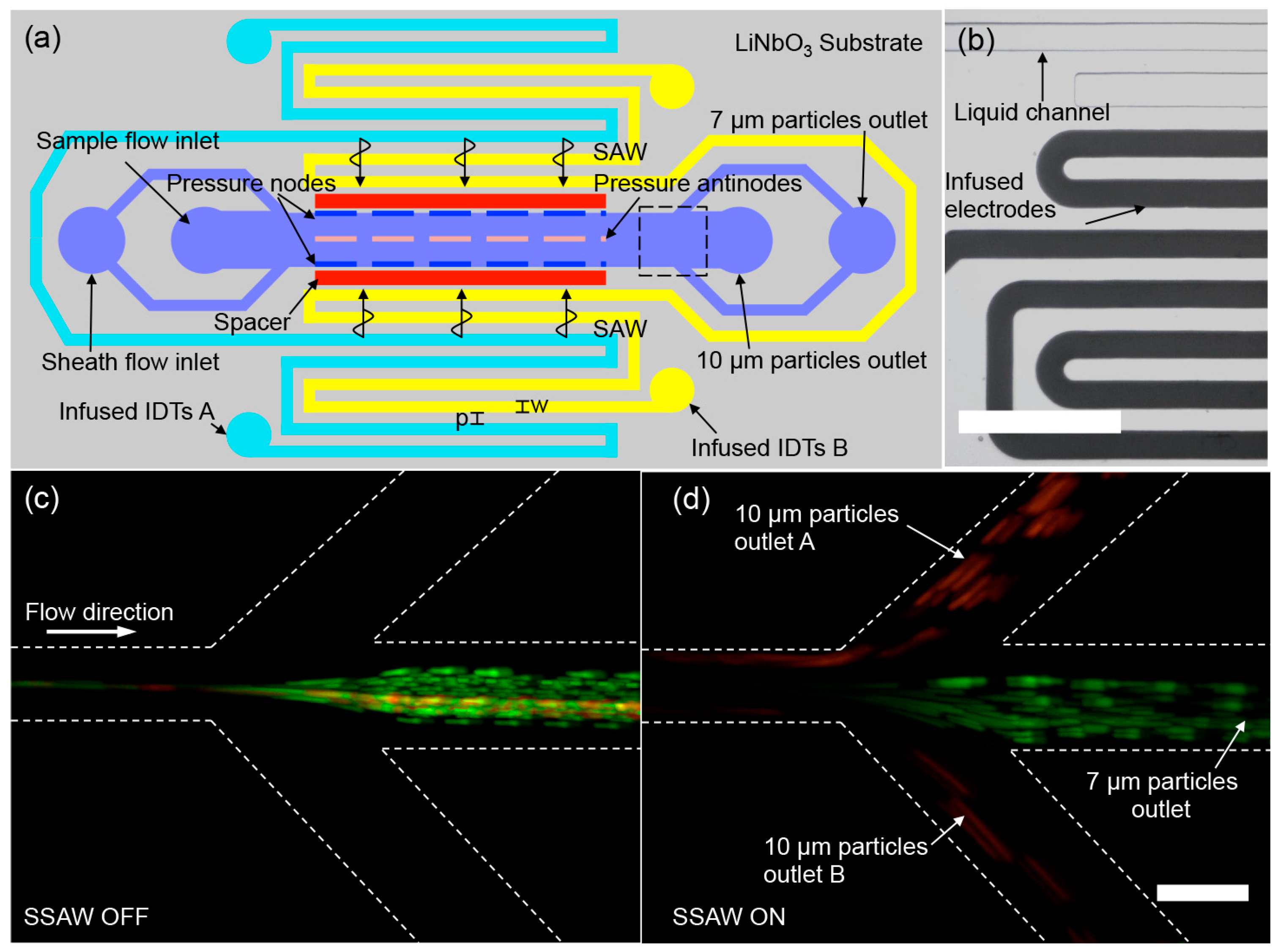
2.2. Size-Based Particle Separation in a Standing SAW Field
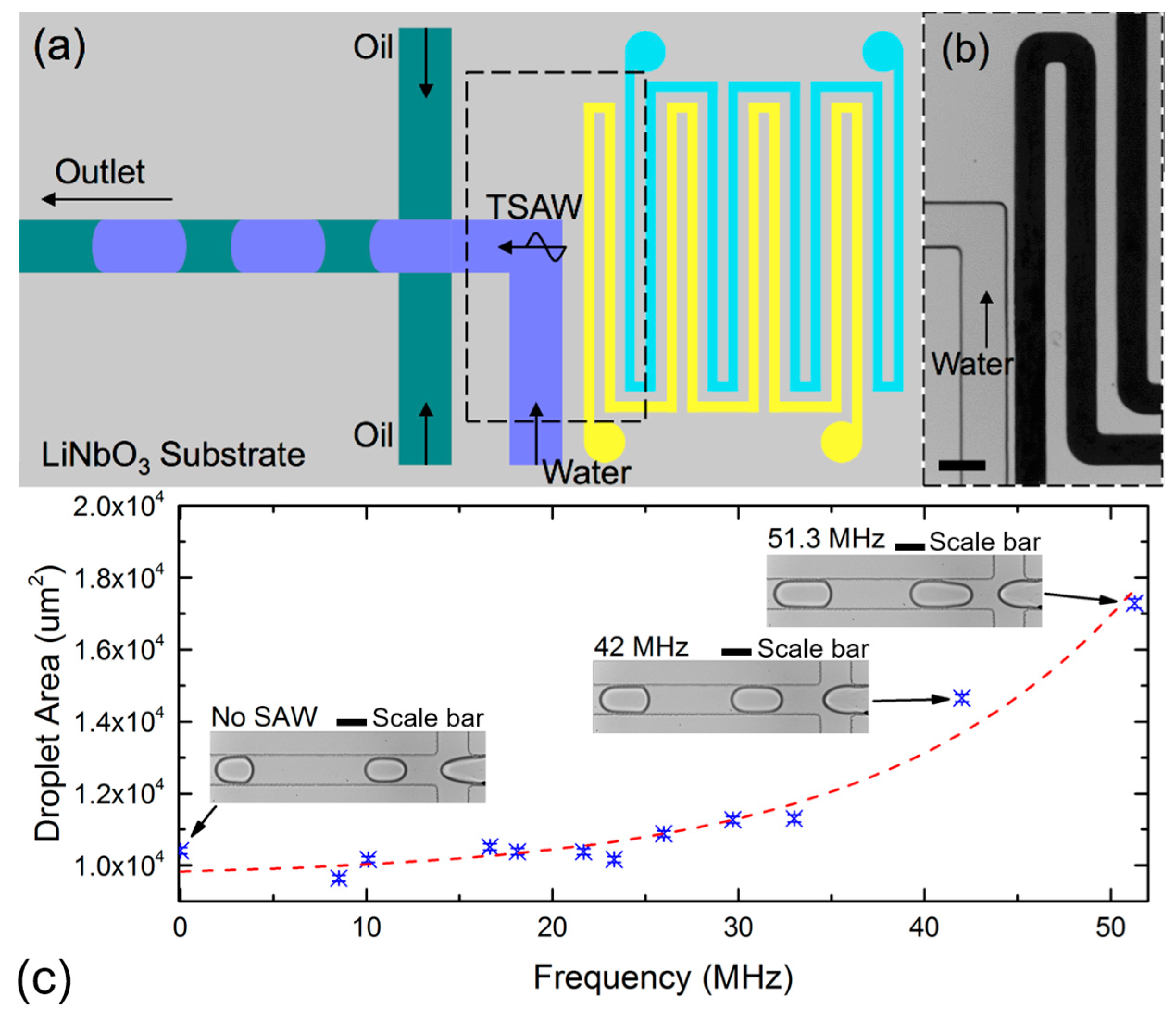
2.3. Active Control of Droplet Generation Using a Traveling SAW
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Destgeer, G.; Sung, H.J. Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves. Lab Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef] [PubMed]
- Lenshof, A.; Magnusson, C.; Laurell, T. Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems. Lab Chip 2012, 12, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Biophysics: Using sound to move cells. Nat. Methods 2015, 12, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Antfolk, M.; Antfolk, C.; Lilja, H.; Laurell, T.; Augustsson, P. A single inlet two-stage acoustophoresis chip enabling tumor cell enrichment from white blood cells. Lab Chip 2015, 15, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Collins, D.J.; Ai, Y. Detachable Acoustofluidic System for Particle Separation via a Traveling Surface Acoustic Wave. Anal. Chem. 2016, 88, 5316–5323. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Alan, T.; Neild, A. The particle valve: On-demand particle trapping, filtering, and release from a microfabricated polydimethylsiloxane membrane using surface acoustic waves. Appl. Phys. Lett. 2014, 105, 033509. [Google Scholar] [CrossRef]
- Collins, D.J.; Ma, Z.; Ai, Y. Highly Localized Acoustic Streaming and Size-Selective Submicrometer Particle Concentration Using High Frequency Microscale Focused Acoustic Fields. Anal. Chem. 2016, 88, 5513–5522. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Morahan, B.; Garcia-Bustos, J.; Doerig, C.; Plebanski, M.; Neild, A. Two-dimensional single-cell patterning with one cell per well driven by surface acoustic waves. Nat. Commun. 2015, 6, 8686. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Devendran, C.; Ma, Z.; Ng, J.W.; Neild, A.; Ai, Y. Acoustic tweezers via sub–time-of-flight regime surface acoustic waves. Sci. Adv. 2016, 2, e1600089. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.R.; Wilson, R.; Shaffer, S.A.; Goodlett, D.R.; Cooper, J.M. Surface Acoustic Wave Nebulization of Peptides As a Microfluidic Interface for Mass Spectrometry. Anal. Chem. 2010, 82, 3985–3989. [Google Scholar] [CrossRef] [PubMed]
- Shilton, R.J.; Travagliati, M.; Beltram, F.; Cecchini, M. Nanoliter-Droplet Acoustic Streaming via Ultra High Frequency Surface Acoustic Waves. Adv. Mater. 2014, 26, 4941–4946. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Marrone, B.L. Droplet translocation by focused surface acoustic waves. Microfluid. Nanofluid. 2012, 13, 715–722. [Google Scholar] [CrossRef]
- Destgeer, G.; Cho, H.; Ha, B.H.; Jung, J.H.; Park, J.; Sung, H.J. Acoustofluidic particle manipulation inside a sessile droplet: Four distinct regimes of particle concentration. Lab Chip 2016, 16, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Destgeer, G.; Ha, B.; Park, J.; Sung, H.J. Lamb Wave-Based Acoustic Radiation Force-Driven Particle Ring Formation Inside a Sessile Droplet. Anal. Chem. 2016, 88, 3976–3981. [Google Scholar] [CrossRef] [PubMed]
- Reboud, J.; Bourquin, Y.; Wilson, R.; Pall, G.S.; Jiwaji, M.; Pitt, A.R.; Graham, A.; Waters, A.P.; Cooper, J.M. Shaping acoustic fields as a toolset for microfluidic manipulations in diagnostic technologies. Proc. Natl. Acad. Sci. USA 2012, 109, 15162–15167. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Tan, S.H.; Gañán-Calvo, A.M.; Tor, S.B.; Loh, N.H.; Nguyen, N.-T. Active droplet generation in microfluidics. Lab Chip 2016, 16, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Schmid, L.; Franke, T. Acoustic modulation of droplet size in a T-junction. Appl. Phys. Lett. 2014, 104, 133501. [Google Scholar] [CrossRef]
- Schmid, L.; Franke, T. SAW-controlled drop size for flow focusing. Lab Chip 2013, 13, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kang, Y.; Ai, Y. Radiation dominated acoustophoresis driven by surface acoustic waves. J. Colloid Interface Sci. 2015, 455, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Sanders, C.K.; Marrone, B.L. Separation of Escherichia coli Bacteria from Peripheral Blood Mononuclear Cells Using Standing Surface Acoustic Waves. Anal. Chem. 2013, 85, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.R.; Friend, J.R.; Yeo, L.Y. Simple, low cost MHz-order acoustomicrofluidics using aluminium foil electrodes. Lab Chip 2014, 14, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Puttaswamy, S.V.; Xue, P.; Kang, Y.; Ai, Y. Simple and low cost integration of highly conductive three-dimensional electrodes in microfluidic devices. Biomed. Microdevices 2015, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.-D.; Guo, W.; Leniart, M.; Chong, Z.Z.; Tan, S.H. AC electric field induced droplet deformation in a microfluidic T-junction. Lab Chip 2016, 16, 2982–2986. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hernández, E.; García-Sánchez, P.; Alzaga-Gimeno, J.; Tan, S.H.; Baret, J.-C.; Ramos, A. AC electrified jets in a flow-focusing device: Jet length scaling. Biomicrofluidics 2016, 10, 043504. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hernández, E.; García-Sánchez, P.; Tan, S.H.; Gañán-Calvo, A.M.; Baret, J.-C.; Ramos, A. Breakup length of AC electrified jets in a microfluidic flow-focusing junction. Microfluid. Nanofluid. 2015, 19, 787–794. [Google Scholar] [CrossRef]
- Tan, S.H.; Maes, F.; Semin, B.; Vrignon, J.; Baret, J.-C. The Microfluidic Jukebox. Sci. Rep. 2014, 4, 4787. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Semin, B.; Baret, J.-C. Microfluidic flow-focusing in AC electric fields. Lab Chip 2014, 14, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, C.S. A conductive liquid-based surface acoustic wave device. Lab Chip 2016, 16, 3750–3755. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Nguyen, N.-T. Generation and manipulation of monodispersed ferrofluid emulsions: The effect of a uniform magnetic field in flow-focusing and T-junction configurations. Phys. Rev. E 2011, 84, 036317. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Nguyen, N.-T.; Chua, Y.C.; Kang, T.G. Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 2010, 4, 032204. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-H.; Murshed, S.S.; Nguyen, N.-T.; Wong, T.N.; Yobas, L. Thermally controlled droplet formation in flow focusing geometry: Formation regimes and effect of nanoparticle suspension. J. Phys. D Appl. Phys. 2008, 41, 165501. [Google Scholar] [CrossRef]
- Hashimoto, K. Ultrasonic Transducers: Materials and Design for Sensors, Actuators and Medical Applications; Woodhead Publishing: Cambridge, UK, 2012; p. 331. [Google Scholar]
- Petersson, F.; Nilsson, A.; Holm, C.; Jönsson, H.; Laurell, T. Continuous separation of lipid particles from erythrocytes by means of laminar flow and acoustic standing wave forces. Lab Chip 2005, 5, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.W., IV; Johnson, L.M.; Gao, L.; López, G.P. Elastomeric negative acoustic contrast particles for capture, acoustophoretic transport, and confinement of cells in microfluidic systems. Langmuir 2014, 30, 3923–3927. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guo, J.; Liu, Y.J.; Ai, Y. The patterning mechanism of carbon nanotubes using surface acoustic waves: The acoustic radiation effect or the dielectrophoretic effect. Nanoscale 2015, 7, 14047–14054. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Huang, H.; Stratton, Z.; Huang, Y.; Huang, T.J. Continuous particle separation in a microfluidic channelvia standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Peng, Z.; Lin, S.-C.S.; Geri, M.; Li, S.; Li, P.; Chen, Y.; Dao, M.; Suresh, S.; Huang, T.J. Cell separation using tilted-angle standing surface acoustic waves. Proc. Natl. Acad. Sci. USA 2014, 111, 12992–12997. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Alan, T.; Neild, A. Particle separation using virtual deterministic lateral displacement (vDLD). Lab Chip 2014, 14, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Tor, S.B.; Gañán-Calvo, A.M.; Chong, Z.J.; Loh, N.H.; Nguyen, N.-T.; Tan, S.H. Automated droplet measurement (ADM): an enhanced video processing software for rapid droplet measurements. Microfluid. Nanofluid. 2016, 20, 1–14. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Tor, S.B.; Loh, N.H.; Wong, T.N.; Gañán-Calvo, A.M.; Tan, S.H.; Nguyen, N.-T. Acoustofluidic control of bubble size in microfluidic flow-focusing configuration. Lab Chip 2015, 15, 996–999. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Teo, A.J.T.; Tan, S.H.; Ai, Y.; Nguyen, N.-T. Self-Aligned Interdigitated Transducers for Acoustofluidics. Micromachines 2016, 7, 216. https://doi.org/10.3390/mi7120216
Ma Z, Teo AJT, Tan SH, Ai Y, Nguyen N-T. Self-Aligned Interdigitated Transducers for Acoustofluidics. Micromachines. 2016; 7(12):216. https://doi.org/10.3390/mi7120216
Chicago/Turabian StyleMa, Zhichao, Adrian J. T. Teo, Say Hwa Tan, Ye Ai, and Nam-Trung Nguyen. 2016. "Self-Aligned Interdigitated Transducers for Acoustofluidics" Micromachines 7, no. 12: 216. https://doi.org/10.3390/mi7120216






